3.1. Flow Properties
Powder mixtures must be evaluated to determine the dosage of the formulations using standardized parameters, such as the angle of repose (°) and Carr’s index, that are widely used by the pharmaceutical industry [
6].
EZ raw material (EZ-RM) had an angle of repose of >40°, a Carr’s index of 35.51% ± 2.92% and a Hausner ratio of 1.55 ± 0.07, indicating poor fluidity and compressibility (
Table 1) owing to the small particle size of the EZ. The solid dispersion of EZ (SD) showed an improvement in the angle of repose compared to EZ-RM, but presented poor values for the Carr’s index and Hausner ratio. The micellar systems MS-I (1:0.75) and MS-II (1:0.75) showed poor flowability, although with a slight improvement in the angle of repose, which was probably due to the presence of the croscarmellose carrier (
Table 1). Poor powder flow properties in solid dispersions of the drug with a small particle size have been observed previously [
7].
High percentages of microcrystalline cellulose (MC) are needed to improve the flow properties of the EZ formulations. The SD showed significant improvements (
p < 0.05) in the angle of repose, Carr’s index and Hausner ratio for the EZ/MC (10/100) proportions compared to the 10/50 EZ/MC ratio. The MS-I and MS-II micellar systems with different manufacturing processes showed slight improvements in flow properties. The results for the different EZ: Kolliphor
® RH40 ratios 1:0.25, 1:0.5 and 1:0.75 exhibited no significant differences (
p > 0.05) for both MS-I and MS-II micellar systems. The formulations MS-I (1:0.75) and MS-II (1:0.75) with the 10/50 EZ/MC ratio had slight improvements in flow properties. However, the 10/100 EZ/MC ratio showed good results with angles of repose of 22.67 ± 2.31° and 17.67 ± 2.08° for MS-I (1:0.75) and MS-II (1:0.75), respectively. A similar improvement was observed for Carr’s index and Hausner ratio in both MS-I and MS-II micellar systems with different manufacturing processes. These results suggest that MS-I and MS-II with 10/100 EZ/MC ratios are necessary to improve the flow properties of EZ formulations [
7]. Similar amounts of excipients have previously been added to an ultra-fine dispersion of EZ obtained by spray-drying to obtain a free-flowing powder and prevent the agglomeration of EZ particles [
6].
3.2. Solubility Study
The low solubility of EZ in simulated intestinal medium at pH 4.5 (1.99 ± 0.24 µg/mL) requires a combination of different technological resources to increase its solubility. The physical mixture of EZ (PM) showed a slight improvement in solubility, and the solid dispersions of EZ (SD) presented 2.63-fold increases compared to EZ-RM. This improvement in SD was related to the water absorption and swelling properties of the croscarmellose and MC, which favored the dispersion and wettability of the EZ. The drug-carrier interactions in the solid dispersion (e.g., hydrogen bonding) and its wettability could explain these increases in the solubility of EZ [
4].
A microscopic study of the residual precipitate at the end of the solubility studies showed an agglomeration of EZ crystals for EZ-RM and PM samples. However, the SD formulation showed less agglomeration of EZ particles with smaller crystals. These results confirmed an increase in the amorphous form of EZ present in SD which improves solubility. Similar increases in the solubility of poorly soluble drugs have been observed with various hydrophilic cellulose polymers [
13,
16].
The micellar systems with Kolliphor
® RH40 MS-I and MS-II showed improvements in the solubility studies. These formulations presented higher solubility values compared to SD (
Figure 1). The use of Kolliphor
® RH40 as a surfactant significantly improves the solubility of EZ with 3.82 and 5.85-fold increases for MS-I (1:0.25) and MS-II (1:0.25), respectively, compared to EZ-RM. The 1:0.75 EZ:surfactant ratio produced the highest EZ solubility values, with similar 12.46 and 12.72-fold increases for MS-I (1:0.75) and MS-II (1:0.75), respectively, compared to EZ-RM.
The solubility results with SD and micellar systems with low surfactant ratios MS-I (1:0.25) and MS-II (1:0.25) produced a fast precipitation process related to slower micelle formation at pH 4.5. The solubility differences between MS-I (1:0.25) and MS-II (1:0.25) may be related to the different elaboration process for MS-I and MS-II. However, there was no difference between MS-I and MS-II with the ratios 1:0.5 and 1:0.75. The high solubility results observed with the highest surfactant ratios in MS-I (1:0.75) and MS-II (1:0.75) indicated a more rapid micelle formation process at this pH [
17]. The differences between the formulations with ratios 1:0.25, 1:0.5 and 1:0.75 may be related to an improvement in the interaction of the surfactant with the croscarmellose chains and ezetimibe particles, MS-I (1:0.25) may present a slower micelle formation process and increase EZ precipitation, thus reducing its solubility values [
16]. The microscopic study of the residual solids of these formulations at the end of the solubility showed significant decrease (
p < 0.05) in EZ crystalline precipitate with a lower amount of agglomerated particles for MS-I (1:0.75) and MS-II (1:0.75) compared to MS-I (1:0.25) and MS-II (1:0.25).
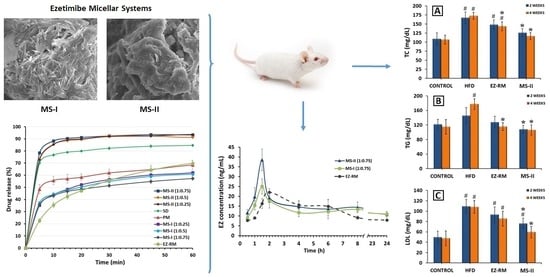
3.3. In Vitro Drug Release
EZ-RM had a slow dissolution profile in these studies, with 35.54% ± 1.55% and 64.23% ± 2.70% at 10 and 45 min respectively (
Figure 2). Similar slow dissolution profiles for EZ have previously been reported [
5]. The dissolution rate of the EZ physical mixture (PM 1:2.5) with the addition of MC showed a significant 1.57-fold increase (
p < 0.05) at 10 min and a similar percentage of dissolution at 45 min (64.76% ± 3.58%) compared to EZ-RM. The presence of hydrophilic excipients such as croscarmellose and microcrystalline cellulose improves wettability and reduces the interfacial tension between EZ and the dissolution medium, resulting in a relatively higher dissolution rate [
3]. The solid dispersion (SD) produces a significant improvement in dissolution profiles during the initial times with a significant 2.16-fold increase (
p < 0.05) at 10 min and a 1.31-fold increase at 45 min, compared to EZ-RM. SD showed higher dissolution profiles compared to MS-I formulations, but lower profiles than MS-II micellar systems. The different manufacturing process of the MS-I formulations increases recrystallization and decreases the dissolution profiles. The presence of a higher amount of EZ’s amorphous form in SD and the incorporation of the hydrophilic excipient MC improves wettability and increases its dissolution profiles.
Ternary solid dispersions MS-I (1:0.25), MS-I (1:0.5) and MS-I (1:0.75) had similar dissolution percentages (44.02% ± 1.26%, 44.32% ± 1.69% and 43.21% ± 1.94%, respectively) at 10 min. The MS-I formulations had a lower dissolution profile than the PM (1:2.5), although their dissolution rates were higher than EZ-RM (
Figure 2). It is important to note that the dissolution profile in the micellar systems MS-I (1:0.25), (1:0.5) and (1:0.75) does not change as the surfactant ratio increases. The addition of microcrystalline cellulose (MC) before the drying process produces a partial dispersion of the EZ within the MC, and a slow diffusion out of the matrix was observed [
18].
The micellar systems of EZ MS-II showed a significant increase in dissolution profiles compared to the other EZ formulations (
Figure 2). The similar dissolution rates of MS-II (1:0.25) and MS-II (1:0.5) may be due to the formation of a submicellar structure [
3]. A previous study showed that submicellar or micellar systems of Kolliphor
® RH40 increase the solubility of poorly soluble drugs [
9]. However, the micellar system MS-II (1:0.75) showed a slight increase in dissolution percentages compared to MS-II (1:0.25) and MS-II (1:0.5), which may be related to the significant improvement observed in the solubility studies (
Figure 1). The MS-II (1:0.75) formulation also showed a significant 2.48-fold increase (
p < 0.05) at 10 min and a 1.46-fold increase at 45 min compared to EZ-RM. The increase in the ratio of Kolliphor
® RH40 enhances the mobility of the amorphous form of EZ, and improves the formation of micellar systems and the solubility of EZ at pH 4.5. This may be due to the presence of surfactant on the surface of the hydrophobic EZ particles that could reduce their aggregation during dissolution [
5].
3.4. Powder X-ray Diffraction (PXRD)
PXRD studies are used to determine changes in the crystallinity of EZ and MC for the micellar systems MS-I and MS-II.
Figure 3 compares the X-ray diffraction patterns of EZ-RM, PM and formulations SD, MS-I and MS-II.
EZ-RM shows sharp peaks corresponding to the crystal structure with major diffraction angles at 16.32 °, 18.93 °, 20.11° and 23.69° 2θ (
Figure 3), related to anhydrous EZ [
19]. PXRD patterns of MC show two semi-crystalline halos between 13–18° and 18–25° 2θ, and the croscarmellose presented a semi-crystalline halo with a diffraction intensity between 16–25° 2θ [
9]. A previous study with different proportions of EZ/croscarmellose and EZ/MC allowed us to select peaks at 16.32 °, 18.96° and 20.11° 2θ to analyze the decrease in the crystallinity of the EZ in the different formulations. The comparison of MC with PM and SD formulations in
Figure 3 showed MC halos between 13–18° and 18–21° 2θ with a low diffraction intensity of the MC, which did not modify the intensity of the EZ peaks. PM showed characteristic diffraction peaks of EZ at 16.32 °, 18.96° and 20.17° 2θ. The study of EZ at 18.96° and 20.17° 2θ showed a decrease in crystallinity index as a result of a dilution effect of the croscarmellose.
The SD presented a decrease in diffraction intensity peaks at 16.33°, 18.94° and 20.19° 2θ. The EZ patterns showed significant decreases in crystallinity compared to EZ-RM. The preparation of an SD formulation improves the interaction between EZ molecules and croscarmellose chains, which increases the amorphous form of EZ. The MC did not show changes in its crystallinity during its elaboration process. MS-I ternary solid dispersions revealed significant changes in diffraction patterns for EZ and MC. These micellar systems showed decreases in intensity in the EZ peaks at 18.93° and 20.11° 2θ respectively. The MS-I formulations (1:0.75) presented a decrease in intensity peaks related to the higher percentages of Kolliphor
® RH40. Lower drug crystallinity has been observed previously in a solid dispersion with a surfactant [
10]. Moreover, all the MS-I micellar systems had a broad halo with a higher crystalline shape between 21–25° 2θ, corresponding to the semi-crystalline MC halo. The MC showed a significant increase in crystallinity index during the MS-I preparation. This change was attributed to a significant surfactant/polymer interaction.
The ternary solid dispersion with the addition of MC after the drying process, MS-II (1:0.25), showed a decrease in the intensity peaks of the EZ at 18.96° and 20.17° 2θ compared to MS-I (1:0.25). This micellar system also showed no changes in the crystallinity index of the MC halo (18–25° 2θ) compared to the SD formulation. These results indicated that the MS-II process is more suitable for decreasing the crystallinity of the EZ without recrystallizing the MC in the formulation. Several studies have attributed a decrease in crystallinity in PXRD to similar surfactant interactions with different excipients [
10].
MS-II (1:0.5) and MS-II (1:0.75) showed the greatest decreases in diffraction patterns for peaks at 18.93° and 20.11° 2θ. These results are related to a high proportion of the amorphous form of EZ. The presence of high proportions of Kolliphor
® RH40 during the preparation process of the MS-II improved the inclusion of EZ molecules in the croscarmellose chains and decreased the EZ crystallinity. Amorphous forms of EZ in surfactant systems have been observed previously [
2]. All the MS-II micellar systems exhibited a semi-crystalline halo (21–25° 2θ) of MC, which had a similar or lower crystalline shape compared to the MC raw material, indicating that the addition of MC after the drying process avoided the surfactant/polymer interaction observed in MS-I.
3.5. Differential Scanning Calorimetry (DSC)
Figure 4 shows the differences in DSC curves between EZ raw material (EZ-RM), physical mixture (PM), solid dispersions (SD) and two types of micellar systems with Kolliphor
® RH40 (MS-I and MS-II).
The EZ raw material (EZ-RM) presented a sharp endothermic peak at 162.24 °C with an enthalpy of fusion of 77.02 J/g (
Figure 4B) [
13]. The MC showed a broad endothermic peak at 183.66 °C. Physical mixtures (PM) showed lower melting temperatures in the first endothermic peak (150.34 °C) corresponding to the interaction of EZ/croscarmellose, and a second broad endothermic peak at 173.16 °C due to the croscarmellose/MC interaction (
Figure 4A). The croscarmellose chains facilitate the mobility of the EZ molecules and reduce the crystallinity of the first endothermic peak [
9]. The temperature increase in the second endothermic peak of PM confirms that croscarmellose chains interact with MC chains.
The SD formulation showed a decrease in the first endothermic peak at 148.73 °C with an EZ crystallinity around 22%. The presence of this amount of the amorphous form of EZ is related with the decrease observed in PXRD, and could explain the increase in the EZ dissolution profile. This solid dispersion showed a second peak at 171.03 °C, which indicates an interaction between the croscarmellose and MC. The micellar system MS-I (1:0.25) showed a decrease in the temperature of the endothermic peak of EZ/croscarmellose (142.03 °C) and a crystallinity around 14%, corresponding to an EZ interaction with the croscarmellose (
Figure 4A). The second endothermic peak of MS-I (1:0.25) at 153.76 °C showed a significant increase in the crystallinity of MC compared to PM and SD formulations, which may be due to the addition of MC before the drying process. The crystallized MC hinders the interaction between the EZ and the surfactant. Similar effects on chain mobility have been observed with other cellulose derivatives with surfactants [
3].
The first endothermic peak in the formulations MS-I (1:0.5) and MS-I (1:0.75) presented significant decreases in the crystallinity of the EZ (13% and 12% respectively), and an increase in the temperatures of the endothermic peaks of MC (155.58 °C and 160.13 °C respectively) in comparison with MS-I (1:0.25) (
Figure 4A). The absence of a first endothermic peak in
Figure 4A indicates the presence of EZ in a practically amorphous form. These first endothermic peaks at 143.11 °C and 142.79 °C could only be observed after magnification (20 mW heat flow). These results suggest that a greater surfactant ratio increases the surface tension and mobility of EZ molecules and the amorphous form of EZ. Significant decreases were observed in the crystallinity of the second endothermic peaks for MS-I (1:0.5) and MS-I (1:0.75), respectively. High surfactant ratios improve the mobility of MC chains and decrease the crystallinity of the MC. The partial recrystallization of MC observed in the PXRD and DSC studies for MS-I (1:0.25), MS-I (1:0.5) and MS-I (1:0.75) may be due to the low dissolution profiles of these formulations. A similar result was previously described in MC granules containing irbersartan nanocrystals [
20].
In DSC studies, the MS-II micellar systems showed a significant decrease in crystallinity compared to the MS-I formulations. MS-II (1:0.25) showed a first endothermic peak at 147.50 °C (
Figure 4B) with a low crystallinity percentage around 10%, caused by the EZ/croscarmellose interactions. The MS-II (1:0.25) also exhibited a second endothermic peak at 167.53 °C for the MC/croscarmellose chains. The high decrease in EZ crystallinity in MS-II was related to an increased mobility of EZ molecules within the croscarmellose carrier, improved by the surfactant interaction. These results agree with the partial amorphization of EZ observed in PXRD. Similar decreases in the crystallinity of EZ have been obtained in micro and nanoparticles of poorly soluble drugs [
21].
The thermographs of the MS-II (1:0.5) and MS-II (1:0.75) micellar systems did not show the first endothermic peak of the EZ in
Figure 4B. After a magnification of these thermographs (5 mW heat flow), this first endothermic peak could be observed at 145.53 °C and 146.79 °C, respectively, with the lowest EZ crystallinity percentages for both micellar systems (around 6% and 3% respectively). One possible explanation for the amorphous form of EZ could be the high surfactant ratios, which facilitate the dispersion and mobility of croscarmellose chains, favouring stronger intermolecular interactions between EZ/croscarmellose and inhibiting the recrystallization of EZ [
22]. MS-II (1:0.5) and MS-II (1:0.75) also presented a second broad endothermic peak at 168.31 °C and 166.97 °C. The low crystallinity percentages were due to the increased mobility of the MC/croscarmellose chains. The development of amorphous forms of EZ and the decrease in carrier crystallinity in MS-II (1:0.75) led to a significant improvement in EZ dissolution. Similar improvements in dissolution profiles were obtained by developing formulations containing the amorphous form of the different drugs produced [
5,
16].
3.6. SEM Characterization
Figure 5 shows the microphotographs of EZ raw material (EZ-RM), physical mixture (PM-EZ) and micellar systems MS-I (1:0.75) and MS-II (1:0.75). Small spherical particles of EZ-RM with a size between 0.5–2 µm can be seen in
Figure 5A, which also shows some aggregations of the EZ particles. This morphology is common in hydrophobic drug microparticles and was previously observed in other studies [
4,
13].
The PM (1:2.5) had large irregularly-shaped particles (between 50 and 75 µm) formed by the aggregation of croscarmellose and MC fibres (
Figure 5B). This SEM image reveals small crystals of EZ on the surface of the croscarmellose and MC aggregations.
Significant differences in particle morphology are observed depending on the preparation process. The ternary solid dispersion MS-I (1:0.75) shows a matrix structure corresponding to the aggregation of the croscarmellose carrier with the MC fibres, with a film of surfactant on the surface (
Figure 5C). Almost none of the small EZ crystals were observed on the surface of the carrier. However, a new morphological structure can be seen during the MS-I formation process, with crystals between 10 and 20 µm caused by the partial crystallization of MC during the drying process. This matrix structure could be related to the sustained release profile observed in dissolution studies. The partial crystallization of the MC was confirmed by the increase in its endothermic peak in MS-I (DSC studies). The presence of crystalline particles on the surface of micellar systems has been described previously [
4,
23].
The micellar systems show major changes on the surface of the croscarmellose and MC aggregation. MS-II (1:0.75) revealed a homogeneous film of surfactant on the surface of the croscarmellose with the EZ particles trapped inside (
Figure 5D). EZ molecules were identified in different samples by energy-dispersive X-ray microanalysis [
24]. The interaction enhancement between EZ and croscarmellose by the addition of the surfactant has been shown in these SEM studies. The elasticity of the surfactant film improves the aggregation process between the large elongated MC fibres and the croscarmellose carrier particles. The absence of small acicular MC crystals was attributed to the addition of the MC after the drying process. These results may be related to the decrease in the semicrystalline peak observed in the PXRD and DSC studies, with an increase in the proportion of surfactant in the micellar system. Previous studies showed that high proportions of surfactant improve the wettability and dispersion of the particles in the dissolution medium [
9].
3.7. Pharmacokinetic Study
EZ conjugates after oral administration to form the active glucuronide metabolite (EZ glucuronide). EZ-glucuronide represents 80%–90% of the total amount of EZ in plasma (total EZ), while only 10% or 20% is free EZ. EZ-glucuronide has been reported to be more effective than EZ [
1,
5,
12]. Both drugs exhibit complex pharmacokinetic profiles mainly due to repetitive enterohepatic kinetics [
1,
5].
EZ-glucuronide can be reformulated to EZ with the enzyme β-glucuronidase, which allows an analysis of all the EZ in blood (EZ-glucuronide and free-EZ) as total EZ [
5]. To assess the improvement in pharmacokinetic behaviour, the plasma concentration-time curve profiles of total EZ after the oral administration of the optimized micellar system MS-II (1:0.75) were compared with EZ-RM and micellar system MS-I (1:0.75), as shown in
Figure 6.
In this pharmacokinetic study, a direct relationship was observed between the increase in dissolution profiles and their pharmacokinetic profiles. MS-II (1:0.75) had the highest profiles, followed by MS-I (1:0.75) and EZ-RM, represented by a first time-point between one and two hours (
Figure 6). Several studies report that the increase in the pharmacokinetic profile of poorly soluble drugs was due to different pharmaceutical systems that improve their solubility and dissolution rates [
5,
25].
Figure 6 also shows the second time-point of EZ-RM at six hours, whereas in MS-II (1:0.75) and MS-I (1:0.75) this second point occurred at eight hours. These multiple peaks are characteristic of the enterohepatic circulation of EZ formulations [
1,
12], and may be due to the EZ excreted into the bile after undergoing extensive glucuronidation to phenolic glucuronide in the intestine. The enhanced residence time via enterohepatic circulation increases its cholesterol-lowering activity [
1].
The mean pharmacokinetic parameters (C
max, T
max and AUC
0–24h) for the three groups are summarized in
Table 2. The data revealed significant improvements in the rate and extent of drug absorption from MS-II (1:0.75) and MS-I (1:0.75) compared to EZ-RM. The C
max of MS-II (1:0.75) was 156.02% and 173.86% compared to MS-I (1:0.75) and EZ-RM, respectively. MS-II (1:0.75) and MS-I (1:0.75) exhibited an earlier T
max (1.5 h) than EZ-RM (T
max 2 h). Previous studies with micellar systems showed increased plasma concentrations in the early stages [
5].
The relative bioavailability of EZ in MS-II (1:0.75) was about 142.99% compared to EZ-RM, indicating rapid absorption and higher bioavailability of EZ from these micellar systems. The statistically significant (p < 0.001) higher bioavailability of EZ from MS-II (1:0.75) highlights the role of the fast dissolution profile in enhancing drug absorption. The slight improvement (114.24%) in relative bioavailability observed between MS-II (1:0.75) and MS-I (1:0.75) could be attributed to the smaller difference in early dissolution times between these systems. However, the mean Cmax of MS-II (1:0.75) showed significant increases (p < 0.001) of 173.86% and 156.02% compared to EZ-RM and MS-I (1:0.75), respectively.
The interactions of MS-II (1:0.75) with biological fluids may vary due to differences in the preparation process of both types of micellar systems, which could account for the variation in C
max values between MS-II (1:0.75) and MS-I (1:0.75). Both EZ formulations have the same proportions of Kolliphor
® RH40; this amount of surfactant may produce a similar increase in permeability and inhibit the outflow of P-gp [
13]. The differences between MS-II (1:0.75) and MS-I (1:0.75) have been attributed to a higher proportion of amorphous EZ in PXRD and DSC studies, which improved its dissolution.
An increase in the pharmacokinetic parameters of MS-II (1:0.75) could enhance cholesterol-lowering activity. Studies on the efficacy of MS-II (1:0.75) are important to evaluate the effect of this micellar system on total cholesterol and lipid parameters (TC, TG, LDL and HDL) compared to EZ-RM.
3.8. Biochemical Parameters
For this study, the rats in the HFD, EZ-RM and MS-II groups were fed a high-fat diet (HFD) to induce hyperlipidemia, while the animals in the control group were fed a standard diet. After two and four weeks of treatment, the EZ-RM and MS-II (1:0.75) groups exhibited significant changes in TC, TG, LDL, HDL, ALT and AST. All these values are shown in
Figure 7.
After two weeks of treatment, the hyperlipidemia induced in the animals by the HFD produced an increase of 53.37% in TC, 19.53% in TG and 119.30% in LDL levels, and a reduction of 17.80% in HDL levels compared to the animals in the control group fed a standard diet (
Figure 7A–D). AST and ALT levels also increased significantly (
p < 0.05) by 69.82% and 82.88%, respectively, in comparison with the control group (
Figure 7E,F). Previous work indicates that these high levels of TC and lipids are related to liver steatosis and inflammation, and are indicative of non-alcoholic fatty liver disease [
26].
After two weeks of treatment, the animals in the EZ-RM group showed a slight improvement (
p > 0.05) in TC, TG, LDL and HDL values compared to the HFD group. However, the animals treated with the MS-II (1:0.75) formulation presented a significant decrease (
p < 0.05) in TC (24.53%), TG (25.71%) and LDL (30.44%) levels (
Figure 7A–C) in comparison with the HFD group. The HDL values after two weeks of treatment with MS-II (1:0.75) were similar to those of the HFD group (
Figure 7D). The decrease in LDL levels with the administration of MS-II (1:0.75) could improve the recovery time of the liver cells.
Transaminase levels showed no differences for AST values compared to the HFD group after two weeks of EZ-RM treatment, and only a slight decrease in AST was seen in the MS-II (1:0.75) group compared to the HFD group (
Figure 7E). However, after two weeks of treatment the ALT values presented significant increases (
p < 0.05) of 59.25% for the EZ-RM group and 48.14% for the MS-II (1:0.75) group (
Figure 7F). The high values of ALT in the EZ treatment groups (EZ-RM and MS-II (1:0.75)) may be due to increased liver metabolism due to fat accumulation in the hepatocytes [
27].
At four weeks the animals fed the high-fat diet (HFD group) presented similar values of TC, LDL, HDL, AST and ALT compared to the levels at two weeks, and only the TG parameters showed an increase of 21.60% (
Figure 7B).
After four weeks of EZ-RM treatment, TC and TG levels showed a significant decrease (
p < 0.05) of 16.80% and 34.59%, respectively (
Figure 7A,B), and an increase (
p < 0.05) in HDL levels of about 20.10% compared to the HFD group (
Figure 7D). LDL values showed a slight decrease, with no significant differences with the HFD group (
Figure 7C). These LDL and TC levels were significantly different from the control group. At this dose of ezetimibe (3 mg/kg/day), more prolonged treatment may be necessary to achieve lipid levels similar to those seen in the control group [
28].
The serum analysis of the animals at the end of the treatment (four weeks) with MS-II (1:0.75) showed improvements of 32.53% for TC, 40.04% for TG, 44.95% for LDL and 23.00% for HDL compared to the HFD group (
Figure 7A–D). These values presented no significant differences (
p > 0.05) with the animals fed the standard diet (control group). However, the TC and LDL levels of the MS-II (1:0.75) group were significantly (
p < 0.05) different from the EZ-RM group.
The higher plasma concentrations of EZ with MS-II (1:0.75) explain the greater TC and lipid reduction achieved with this treatment, due to the enhanced solubility of ezetimibe in this formulation. The decrease in the accumulation of TC, TG and LDL in the liver plays an important role in reducing hepatic steatosis [
26].
The AST levels at four weeks of treatment showed a significant decrease (
p > 0.05) of 17.83% with the EZ-RM treatment, and 33.89% with MS-II (1:0.75) compared to the HFD group (
Figure 7E), significantly different (
p > 0.05) from the control group. However, the AST values in the MS-II (1:0.75) group showed no significant differences (
p < 0.05) with the control group. The EZ-RM and MS-II (1:0.75) groups presented a decrease of 4.71% and 13.89% in ALT values, respectively, compared to the HFD group (
Figure 7F).
The lower ALT and AST levels in the EZ-RM and MS-II (1:0.75) groups could be due to a decrease in the hepatic cholesterol metabolism after four weeks of treatment [
29]. The ALT levels of animals treated with the MS-II (1:0.75) formulation were not significantly different (
p < 0.05) to those of the control group. This treatment had an advantage over EZ-RM due to the significant decrease in TC and lipid parameters, and its lower AST and ALT levels could imply fewer adverse effects on muscles and liver than in the EZ-RM and HFD groups [
30].
These results demonstrate the importance of ALT and AST analysis to evaluate the metabolism of liver cells in patients with antihyperlipidemic treatment. Elevated transaminase levels at two weeks indicate higher liver metabolism, whereas decreases in ALT and AST values at four weeks of treatment could indicate lower metabolic activity due to a decrease in fat accumulation in hepatocytes.