3D Printed Drug Delivery Systems Based on Natural Products
Abstract
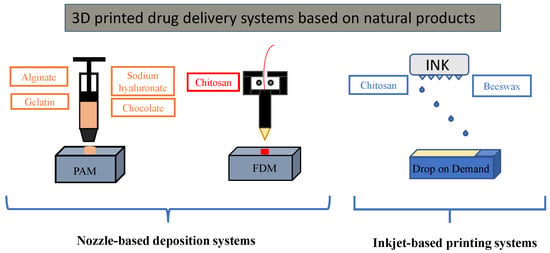
:1. Introduction
2. Nozzle-Based Deposition Systems
2.1. Pressure-Assisted Microsyringe (PAM)
2.1.1. PAM Technique Employing Polysaccharides
2.1.2. PAM Technique Employing Proteins
2.1.3. PAM Technique Employing Mixture of Polysaccharides and Proteins
2.1.4. PAM Technique Employing Lipids
2.2. Fused Deposition Modeling (FDM)
HME Filaments Containing Natural Products as APIs
3. Inkjet-Based Printing Systems
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Khatri, P.; Shah, M.K.; Vora, N. Formulation strategies for solid oral dosage form using 3D printing technology: A mini-review. J. Drug Deliv. Sci. Technol. 2018, 46, 148–155. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bashyal, S.; Keum, T.; Noh, G.; Seo, J.E.; Bastola, R.; Choi, J.; Sohn, D.H.; Lee, S. Complex formulations, simple techniques: Can 3D printing technology be the Midas touch in pharmaceutical industry? Asian J. Pharm. Sci. 2019, 14, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Gault, R.S. A comparison of rapid prototyping technologies. Int. J. Mach. Tools Manuf. 1998, 38, 1257–1287. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Kim, E.H.; Choi, H.H.; Jung, Y.G. Fabrication of a ceramic core for an impeller blade using a 3D printing technique and inorganic binder. J. Manuf. Process. 2020, 53, 43–47. [Google Scholar] [CrossRef]
- Markstedt, K.; Håkansson, K.; Toriz, G.; Gatenholm, P. Materials from trees assembled by 3D printing—Wood tissue beyond nature limits. Appl. Mater. Today 2019, 15, 280–285. [Google Scholar] [CrossRef]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.R.P.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. The digital pharmacies era: How 3D printing technology using fused deposition modeling can become a reality. Pharmaceutics 2019, 11, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Lv, J.; Yang, W.; Pi, X.; Lin, W.; Lin, Z.; Zhang, W.; Pang, J.; Zeng, Y.; Lv, Z.; et al. Preparation and application of subdivided tablets using 3D printing for precise hospital dispensing. Eur. J. Pharm. Sci. 2020, 149, 105293. [Google Scholar] [CrossRef] [PubMed]
- Van Riet-Nales, D.A.; Doeve, M.E.; Nicia, A.E.; Teerenstra, S.; Notenboom, K.; Hekster, Y.A.; Van Den Bemt, B.J.F. The accuracy, precision and sustainability of different techniques for tablet subdivision: Breaking by hand and the use of tablet splitters or a kitchen knife. Int. J. Pharm. 2014, 466, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursan, I.D.; Chiu, L.; Pierce, A. Three-dimensional drug printing: A structured review. J. Am. Pharm. Assoc. 2013, 53, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.M.; Borland, S.W.; Giordano, R.A.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Solid free-form fabrication of drug delivery devices. J. Control. Release 1996, 40, 77–87. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of bioprinter technologies. Addit. Manuf. 2017, 13, 179–200. [Google Scholar] [CrossRef] [Green Version]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Linares, V.; Casas, M.; Caraballo, I. Printfills: 3D printed systems combining Fused Deposition Modeling and Injection Volume Filling. Application to colon-specific drug delivery. Eur. J. Pharm. Biopharm. 2018, 134, 138–143. [Google Scholar] [CrossRef]
- Piyush; Kumar, R.; Kumar, R. 3D printing of food materials: A state of art review and future applications. Mater. Today Proc. 2000, 2–6. [Google Scholar] [CrossRef]
- Galbis, J.A.; García-Martín, M.D.G.; de Paz, M.V.; Galbis, E. Synthetic Polymers from Sugar-Based Monomers. Chem. Rev. 2016, 116, 1600–1636. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, M.; Ghaffari, A.; Yazdanpanah, Y.; Luo, D.K.M. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Whyte, D.J.; Rajkhowa, R.; Allardyce, B.; Kouzani, A.Z. A review on the challenges of 3D printing of organic powders. Bioprinting 2019, 16, e00057. [Google Scholar] [CrossRef]
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A flexible-dose dispenser for immediate and extended release 3D printed tablets. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik e.V 2015, 96, 380–387. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- El Aita, I.; Breitkreutz, J.; Quodbach, J. On-demand manufacturing of immediate release levetiracetam tablets using pressure-assisted microsyringe printing. Eur. J. Pharm. Biopharm. 2019, 134, 29–36. [Google Scholar] [CrossRef]
- Sadia, M.; So, A.; Arafat, B.; Isreb, A.; Ahmed, W. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]
- Deng, N.; Sun, J.; Li, Y.; Chen, L.; Chen, C.; Wu, Y.; Wang, Z.; Li, L. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone. Arch. Oral Biol. 2019, 102, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chang, T.W.; Peng, T.K. Three-dimensional plotted alginate fibers embedded with diclofenac and bone cells coated with chitosan for bone regeneration during inflammation. J. Biomed. Mater. Res. Part A 2018, 106, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.F.; Olhero, S.M.; Torres, P.M.C.; Abrantes, J.C.C.; Fateixa, S.; Nogueira, H.I.S.; Ribeiro, I.A.C.; Bettencourt, A.; Sousa, A.; Granja, P.L.; et al. Novel sintering-free scaffolds obtained by additive manufacturing for concurrent bone regeneration and drug delivery: Proof of concept. Mater. Sci. Eng. C 2019, 94, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Zhang, X.; Zhao, X.; Ma, J.; Pu, X.; Wang, Y.; Ran, F.; Wang, Y.; Leng, F.; et al. Snakegourd root/Astragalus polysaccharide hydrogel preparation and application in 3D printing. Int. J. Biol. Macromol. 2019, 121, 309–316. [Google Scholar] [CrossRef]
- Etxabide, A.; Long, J.; Guerrero, P.; de la Caba, K.; Seyfoddin, A. 3D printed lactose-crosslinked gelatin scaffolds as a drug delivery system for dexamethasone. Eur. Polym. J. 2019, 114, 90–97. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Luo, Y.; Ma, J. Layer-by-layer coated porous 3D printed hydroxyapatite composite scaffolds for controlled drug delivery. Colloids Surf. B Biointerfaces 2019, 179, 121–127. [Google Scholar] [CrossRef]
- Karavasili, C.; Gkaragkounis, A.; Moschakis, T.; Ritzoulis, C.; Fatouros, D.G. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur. J. Pharm. Sci. 2020, 147, 105291. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Cook, W.G.; Fenton, M.E. Handbook of Pharmaceutical Excipients; American Pharmacists Association: Washington, DC, USA, 2012; ISBN 9780857110275 (UK). [Google Scholar]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Tian, L.; Singh, A.; Singh, A.V. Synthesis and characterization of pectin-chitosan conjugate for biomedical application. Int. J. Biol. Macromol. 2020, 153, 533–538. [Google Scholar] [CrossRef]
- Salman, S.; Tang, E.K.Y.; Cheung, L.C.; Nguyen, M.N.; Sommerfield, D.; Slevin, L.; Lim, L.Y.; von Ungern Sternberg, B.S. A novel, palatable paediatric oral formulation of midazolam: Pharmacokinetics, tolerability, efficacy and safety. Anaesthesia 2018, 73, 1469–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martir, J.; Flanagan, T.; Mann, J.; Fotaki, N. Recommended strategies for the oral administration of paediatric medicines with food and drinks in the context of their biopharmaceutical properties: A review. J. Pharm. Pharmacol. 2017, 69, 384–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, L.C.; Nguyen, M.; Tang, E.; von Ungern Sternberg, B.S.; Salman, S.; Tuleu, C.; Mohamed Ahmed, A.H.A.; Soto, J.; Lim, L.Y. Taste evaluation of a novel midazolam tablet for pediatric patients: In vitro drug dissolution, in vivo animal taste aversion and clinical taste perception profiles. Int. J. Pharm. 2018, 535, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Synaridou, M.S.; Morichovitou, A.K.; Markopoulou, C.K. Innovative Pediatric Formulations: Ibuprofen in Chocolate-Coated Honey Core. J. Pharm. Innov. 2019. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D. European Journal of Pharmaceutics and Biopharmaceutics Unidirectional drug release from 3D printed mucoadhesive buccal fi lms using FDM technology: In vitro and ex vivo evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Chatzitaki, A.; Karavasili, C.; Katsamenis, O.L.; Tzetzis, D.; Mystiridou, E.; Bouropoulos, N.; Fatouros, D.G. Controlled Release of 5-Fluorouracil from Alginate Beads Encapsulated in 3D Printed pH-Responsive Solid Dosage Forms. AAPS PharmSciTech 2018, 19, 3362–3375. [Google Scholar] [CrossRef] [Green Version]
- Pinho, L.A.G.; Souza, S.G.; Marreto, R.N.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Dissolution enhancement in cocoa extract, combining hydrophilic polymers through hot-melt extrusion. Pharmaceutics 2018, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Kang, Q.; Liu, N.; Zhang, Q.; Zhang, Y.; Li, H.; Zhao, B.; Chen, Y.; Lan, Y.; Ma, Q.; et al. Enhanced dissolution rate and oral bioavailability of Ginkgo biloba extract by preparing solid dispersion via hot-melt extrusion. Fitoterapia 2015, 102, 189–197. [Google Scholar] [CrossRef]
- Jiang, Y.; Piao, J.; Cho, H.-J.; Kang, W.-S.; Kim, H.-Y. Improvement in antiproliferative activity of Angelica gigas Nakai by solid dispersion formation via hot-melt extrusion and induction of cell cycle arrest and apoptosis in HeLa cells. Biosci. Biotechnol. Biochem. 2015, 79, 1635–1643. [Google Scholar] [CrossRef]
- Chuah, A.M.; Jacob, B.; Jie, Z.; Ramesh, S.; Mandal, S.; Puthan, J.K.; Deshpande, P.; Vaidyanathan, V.V.; Gelling, R.W.; Patel, G.; et al. Enhanced bioavailability and bioefficacy of an amorphous solid dispersion of curcumin. Food Chem. 2014, 156, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease risk: An evidence based approach. Food Funct. 2017, 8, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummins, G.; Desmulliez, M.P.Y. Inkjet printing of conductive materials: A review. Circuit World 2012, 38, 193–213. [Google Scholar] [CrossRef]
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int. J. Pharm. 2019, 555, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Klammert, U.; Ewald, A.; Barralet, J.E.; Gbureck, U. Simultaneous immobilization of bioactives during 3D powder printing of bioceramic drug-release matrices. Adv. Funct. Mater. 2010, 20, 1585–1591. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Pravin, S.; Sudhir, A. Integration of 3D printing with dosage forms: A new perspective for modern healthcare. Biomed. Pharmacother. 2018, 107, 146–154. [Google Scholar] [CrossRef]
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Stanton, M.M.; Samitier, J.; Sánchez, S. Bioprinting of 3D hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef] [Green Version]
- Compaan, A.M.; Christensen, K.; Huang, Y. Inkjet Bioprinting of 3D Silk Fibroin Cellular Constructs Using Sacrificial Alginate. ACS Biomater. Sci. Eng. 2017, 3, 1519–1526. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2017, 45, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.B.H.; Seo, D.Y.; Kim, J.S.; Lee, H.; Lee, O.; Ajiteru, M.T.; Sultan, O.J.; Lee, S.H.; Lee, C.H.P. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020, 232, 119679. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, E.; Sandler, N.; El Aita, I.; Breitkreutz, J.; Quodbach, J.; Zidan, A.; Alayoubi, A.; Coburn, J.; Asfari, S.; Ghammraoui, B.; et al. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2019, 554, 110–123. [Google Scholar]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D printing in pharmaceutical and medical applications. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [Green Version]







| Natural Product | Type of Printer Used | Scaffolds Dimensions | Printing Temperature | Printing Speed | Reference |
|---|---|---|---|---|---|
| Chitosan | 3D bioprinter (Youni Technology Co., Ltd., organization 2500 X, Shenzhen, China | 13 × 6 × 4 mm | 4 °C | 4 mm/min | [31] |
| Chitosan and alginate | 3D plotting system (SR2000D, Ganbow Technology, Banqiao, Taiwan) | 10 × 10 × 2 mm | Room temperature | 35 mm/s | [32] |
| Chitosan | Robotic deposition device (3-D Inks, Stillwater, OK, USA) | 3 × 3 × 3 mm | Room temperature | 10 mm/s | [33] |
| Chitosan and pectin | BioBot1 3D printer (Allevi, Philadelphia, PA, USA) | 23.50 × 23.50 × 1.00 mm | Room temperature | 6 mm/s | [34] |
| Snakegourd root and Astragalus root | Melt-extrusion 3D printer | Square, circle and rectangle shapes | Room temperature | 50 mm/s | [35] |
| Gelatin | BioBots 3D printer (Allevi, Philadelphia, PA, USA) | 22.20 × 11.20 × 0.80 | 27 °C | 4 mm/s | [36] |
| Sodium hyaluronate and chitosan | 3D bio-printing (Regenovo Bio-printer system, Hangzhou Regenovo biotechnology Co., Ltd., Hangzhou, China) | Cylindrical shape (Diameter = 10 mm, Height = 5 mm) | 37 °C | 8 mm/s | [37] |
| Chocolate | 3D Food Printer (Model 3C10A) | Different shapes ranging from 59.1 × 33.1 × 3.0 mm to 61.8 × 84.1 × 6.0 mm | 45 °C | 5 mm/s | [38] |
| Natural Product | Type of Printer Used | Scaffolds Dimensions | Printing Temperature | Printing Speed | Reference |
|---|---|---|---|---|---|
| Chitosan and alginate | Makerbot Replicator® 2X FDM printer (Makerbot Inc., New York, NY, USA) | 20 × 20 × 0.2 mm EC backing layer: 20 × 20 × 0.1 mm | 200 °C (PVA) 190 °C (EC) | 10 mm/s | [47] |
| Chitosan | MakerBot Replicator® 2X 3D printer (MakerBot Inc., New York, NY, USA) | Cylindrical shape (Diameter = 15 mm, Height = 4.2 mm) | 182 °C (Eudragit®) 215 °C (PLA) | 20 mm/s | [48] |
| Natural Product | Type of Printer Used | Dimensions of the Scaffolds | Printing Temperature | Reference |
|---|---|---|---|---|
| Beeswax | Inkjet printer (PiXDRO LP50, Meyer Burger Technology Ltd., Gwatt (Thun), Switzerland ) | Cylindrical shape: 0.20, 0.41, 0.61, 1.22 and 1.83 mm diameter and 3.22 mm height | 90 °C | [60] |
| Chitosan | Multicolor 3D powder printing system (spectrum Z510, Z Corporation, Burlington, MA, USA) | ---- | ---- | [57] |
| Natural Product | 3D Printing Technique | Advantages | Printability | References |
|---|---|---|---|---|
| Beeswax | inkjet | Solvent free ink/Controlled release for hydrophobic drugs | Suitable surface tension and droplet formation at 90 °C | [60] |
| Chitosan | PAM | Ability to control the drug release Protection of the bone cells imbibed in the scaffold/Increase of the amount of drug loaded in the scaffold | Adequate rheological properties of hydrogels with low viscosity and fast gelling reaction with negatively charged polymers and polyanions | [31,32,35,37] |
| FDM | Mucoadhesive properties | [47] | ||
| Inkjet | Fast gelling reaction/Retention of drug in microporous implants | [57] | ||
| Chocolate | PAM | Taste masking | No nozzle clogging and correct strand extruded at 45 °C | [38] |
| Gelatin | PAM | Shape control of structure/Controlled drug release | Extrudable hydrogels with variable viscosity depending on the printing temperature | [36] |
| Viscosity change at 37 °C which increase cohesiveness with HAP | [37] | |||
| Sodium hyaluronate | PAM | Adjustment of the viscosity of the slurry for 3D printing/Extrusion at body temperature | ---- | [37] |
| Coadyuvant of the controlled release | ||||
| Snakegourd root and Astragalus root | PAM | Ability to form hydrogels/hypoglycemic activity | ---- | [35] |
| Alginate | PAM | Very mild gelling condition biologically safe for cells | Ease gelation with low percentage and mild conditions | [32] |
| Control of the drug release | ||||
| Physically crosslinked Chitosan/Pectin | PAM | Avoidance of chemical crosslinkers typically toxic/entrapment of the drug in the hydrogel matrix | Extrudable gel structure after cooling up at ambient temperature | [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-de-Leyva, Á.; Linares, V.; Casas, M.; Caraballo, I. 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics 2020, 12, 620. https://doi.org/10.3390/pharmaceutics12070620
Aguilar-de-Leyva Á, Linares V, Casas M, Caraballo I. 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics. 2020; 12(7):620. https://doi.org/10.3390/pharmaceutics12070620
Chicago/Turabian StyleAguilar-de-Leyva, Ángela, Vicente Linares, Marta Casas, and Isidoro Caraballo. 2020. "3D Printed Drug Delivery Systems Based on Natural Products" Pharmaceutics 12, no. 7: 620. https://doi.org/10.3390/pharmaceutics12070620
APA StyleAguilar-de-Leyva, Á., Linares, V., Casas, M., & Caraballo, I. (2020). 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics, 12(7), 620. https://doi.org/10.3390/pharmaceutics12070620