IPEC-J2 rMdr1a, a New Cell Line with Functional Expression of Rat P-glycoprotein Encoded by Rat Mdr1a for Drug Screening Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Transfection and Cell Culture
2.3. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction
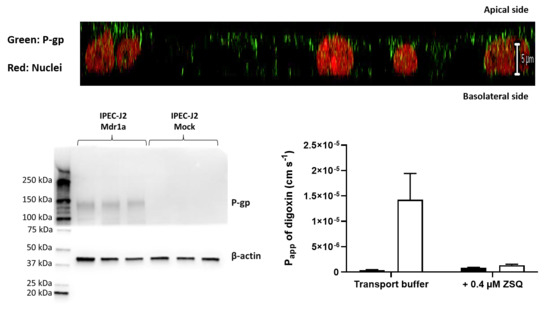
2.4. Immunocytochemistry
2.5. Western Blotting
2.6. Transepithelial Electrical Resistance
2.7. Transport Experiments
2.8. Data Treatment
3. Results
3.1. IPEC-J2 Cells Transfected with Rat Mdr1a Exhibited Marked P-glycoprotein Functionality and High Electrical Resistances
3.2. IPEC-J2 rMdr1a Cells Expressed High Levels of Rat Mdr1a and Exhibited Polarized Expression of P-gp in the Apical Membrane
3.3. Bidirectional Transport of Selected Model Compounds across Monolayers of IPEC-J2 rMdr1a Cells Was Not Significantly Different from Bidirectional Transport across Monolayers of IPEC-J2 MDR1 Cells Overexpressing Human P-gp
4. Discussion
4.1. IPEC-J2 rMdr1a Cells Express High Levels of Functional Rat P-glycoprotein
4.2. Expression of rMdr1a Encoding for Rat P-glycoprotein Was Higher than Selected Endogenous Efflux Transporters in IPEC-J2 rMdr1a Cells
4.3. Bidirectional Transport of Selected Model Drugs Was Not Different between IPEC-J2 rMdr1a Cells Overexpressing Rat P-gp and IPEC-J2 MDR1 Cells Overexpressing Human P-gp
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.J.; Chin, J.E.; Ueda, K.; Clark, D.P.; Pastan, I.; Gottesman, M.M.; Roninson, I.B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 1986, 47, 381–389. [Google Scholar] [CrossRef]
- Hamada, H.; Tsuruo, T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J. Biol. Chem. 1988, 263, 1454–1458. [Google Scholar] [PubMed]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.I.; Lothstein, L.; Horwitz, S.B. Differential overexpression of three mdr gene family members in multidrug-resistant J774.2 mouse cells. Evidence that distinct P-glycoprotein precursors are encoded by unique mdr genes. J. Biol. Chem. 1989, 264, 12053–12062. [Google Scholar] [PubMed]
- Van der Bliek, A.M.; Kooiman, P.M.; Schneider, C.; Borst, P. Sequence of mdr3 cDNA encoding a human P-glycoprotein. Gene 1988, 71, 401–411. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Smit, J.J.M.; van Tellingen, M.; Beijnen, J.H.; Wagenaar, E.; Van Deemter, L.; Mol, C.A.A.M.; Van der Valk, M.A.; Robanus-Maandag, E.C.; Te Riele, H.P.J.; et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 1994, 77, 491–502. [Google Scholar] [CrossRef]
- Jette, L.; Pouliot, J.F.; Murphy, G.F.; Beliveau, R. Isoform I (mdr3) is the major form of P-glycoprotein expressed in mouse brain capillaries. Evidence for cross-reactivity of antibody C219 with an unrelated protein. Biochem. J. 1995, 305, 761–766. [Google Scholar] [CrossRef]
- Baltes, S.; Gastens, A.M.; Fedrowitz, M.; Potschka, H.; Kaever, V.; Loscher, W. Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology 2007, 52, 333–346. [Google Scholar] [CrossRef]
- Summerfield, S.G.; Zhang, Y.; Liu, H. Examining the Uptake of Central Nervous System Drugs and Candidates across the Blood-Brain Barrier. J. Pharmacol. Exp. Ther. 2016, 358, 294–305. [Google Scholar] [CrossRef]
- Gartzke, D.; Fricker, G. Establishment of optimized MDCK cell lines for reliable efflux transport studies. J. Pharm. Sci. 2014, 103, 1298–1304. [Google Scholar] [CrossRef]
- Goh, L.B.; Spears, K.J.; Yao, D.; Ayrton, A.; Morgan, P.; Roland Wolf, C.R.; Friedberg, T. Endogenous drug transporters in in vitro and in vivo models for the prediction of drug disposition in man. Biochem. Pharmacol. 2002, 64, 1569–1578. [Google Scholar] [CrossRef]
- Kuteykin-Teplyakov, K.; Luna-Tortos, C.; Ambroziak, K.; Loscher, W. Differences in the expression of endogenous efflux transporters in MDR1-transfected versus wildtype cell lines affect P-glycoprotein mediated drug transport. Br. J. Pharmacol. 2010, 160, 1453–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saaby, L.; Helms, H.C.; Brodin, B. IPEC-J2 MDR1, a Novel High-Resistance Cell Line with Functional Expression of Human P-glycoprotein (ABCB1) for Drug Screening Studies. Mol. Pharm. 2016, 13, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Perriere, N.; Demeuse, P.; Garcia, E.; Regina, A.; Debray, M.; Andreux, J.P.; Couvreur, P.; Scherrmann, J.M.; Temsamani, J.; Couraud, P.O.; et al. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood-brain barrier-specific properties. J. Neurochem. 2005, 93, 279–289. [Google Scholar] [CrossRef]
- Brouwer, K.L.; Keppler, D.; Hoffmaster, K.A.; Bow, D.A.; Cheng, Y.; Lai, Y.; Palm, J.E.; Stieger, B.; Evers, R.; International Transporter Consortium. In Vitro methods to support transporter evaluation in drug discovery and development. Clin. Pharmacol. Ther. 2013, 94, 95–112. [Google Scholar] [CrossRef] [Green Version]
- Garberg, P.; Ball, M.; Borg, N.; Cecchelli, R.; Fenart, L.; Hurst, R.D.; Lindmark, T.; Mabondzo, A.; Nilsson, J.E.; Raub, T.J.; et al. In Vitro models for the blood-brain barrier. Toxicol. in Vitro. 2005, 19, 299–334. [Google Scholar] [CrossRef]
- Hellinger, E.; Veszelka, S.; Toth, A.E.; Walter, F.; Kittel, A.; Bakk, M.L.; Tihanyi, K.; Háda, V.; Nakagawa, S.; Duy, T.D.H.; et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur. J. Pharm. Biopharm. 2012, 82, 340–351. [Google Scholar] [CrossRef]
- Gartzke, D.; Delzer, J.; Laplanche, L.; Uchida, Y.; Hoshi, Y.; Tachikawa, M.; Terasaki, T.; Sydor, J.; Fricker, G. Genomic Knockout of Endogenous Canine P-Glycoprotein in Wild-Type, Human P-Glycoprotein and Human BCRP Transfected MDCKII Cell Lines by Zinc Finger Nucleases. Pharm. Res. 2015, 32, 2060–2071. [Google Scholar] [CrossRef]
- Horio, M.; Chin, K.V.; Currier, S.J.; Goldenberg, S.; Williams, C.; Pastan, I.; Gottesman, M.M.; Handler, J. Transepithelial transport of drugs by the multidrug transporter in cultured Madin-Darby canine kidney cell epithelia. J. Biol. Chem. 1989, 264, 14880–14884. [Google Scholar]
- Stephens, R.H.; O’Neill, C.A.; Bennett, J.; Humphrey, M.; Henry, B.; Rowland, M.; Warhurst, G. Resolution of P-glycoprotein and non-P-glycoprotein effects on drug permeability using intestinal tissues from mdr1a (-/-) mice. Br. J. Pharmacol. 2002, 135, 2038–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helms, H.C.; Hersom, M.; Kuhlmann, L.B.; Badolo, L.; Nielsen, C.U.; Brodin, B. An Electrically Tight In Vitro Blood-Brain Barrier Model Displays Net Brain-to-Blood Efflux of Substrates for the ABC Transporters, P-gp, Bcrp and Mrp-1. AAPS J. 2014, 16, 1046–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.S.; Taylor, R.; Ruan, Y.; Donovan, J.L.; Markowitz, J.S.; Lindsay De Vane, C. Olanzapine penetration into brain is greater in transgenic Abcb1a P-glycoprotein-deficient mice than FVB1 (wild-type) animals. Neuropsychopharmacology 2004, 29, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, A.; Hammerle, S.; Suda, K.; Rothen-Rutishauser, B.; Gunthert, M.; Kramer, S.D.; Wunderli-Allenspach, H. Cell cultures as tools in biopharmacy. Eur. J. Pharm. Sci. 2000, 11 (Suppl. S2), S51–S60. [Google Scholar] [CrossRef]
- Matsson, P.; Englund, G.; Ahlin, G.; Bergstrom, C.A.; Norinder, U.; Artursson, P. A global drug inhibition pattern for the human ATP-binding cassette transporter breast cancer resistance protein (ABCG2). J. Pharmacol. Exp. Ther. 2007, 323, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Matsson, P.; Pedersen, J.M.; Norinder, U.; Bergstrom, C.A.; Artursson, P. Identification of novel specific and general inhibitors of the three major human ATP-binding cassette transporters P-gp, BCRP and MRP2 among registered drugs. Pharm. Res. 2009, 26, 1816–1831. [Google Scholar] [CrossRef]
- Fojo, A.T.; Ueda, K.; Slamon, D.J.; Poplack, D.G.; Gottesman, M.M.; Pastan, I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Bertelsen, K.M.; Greenblatt, D.J.; von Moltke, L.L. Apparent active transport of MDMA is not mediated by P-glycoprotein: A comparison with MDCK and Caco-2 monolayers. Biopharm. Drug Dispos. 2006, 27, 219–227. [Google Scholar] [CrossRef]
- De Souza, J.; Benet, L.Z.; Huang, Y.; Storpirtis, S. Comparison of bidirectional lamivudine and zidovudine transport using MDCK, MDCK-MDR1, and Caco-2 cell monolayers. J. Pharm. Sci. 2009, 98, 4413–4419. [Google Scholar] [CrossRef]
- Booth-Genthe, C.L.; Louie, S.W.; Carlini, E.J.; Li, B.; Leake, B.F.; Eisenhandler, R.; Hochman, J.H.; Mei, Q.; Kim, R.B.; Rushmore, T.H.; et al. Development and characterization of LLC-PK1 cells containing Sprague-Dawley rat Abcb1a (Mdr1a): Comparison of rat P-glycoprotein transport to human and mouse. J. Pharmacol. Toxicol. Methods 2006, 54, 78–89. [Google Scholar] [CrossRef]
- Takeuchi, T.; Yoshitomi, S.; Higuchi, T.; Ikemoto, K.; Niwa, S.; Ebihara, T.; Katoh, M.; Yokoi, T.; Asahi, S. Establishment and characterization of the transformants stably-expressing MDR1 derived from various animal species in LLC-PK1. Pharm. Res. 2006, 23, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Dickens, D.; Radisch, S.; Chiduza, G.N.; Giannoudis, A.; Cross, M.J.; Malik, H.; Schaeffeler, E.; Sison-Young, R.L.; Wilkinson, E.L.; Goldring, C.E.; et al. Cellular Uptake of the Atypical Antipsychotic Clozapine Is a Carrier-Mediated Process. Mol. Pharm. 2018, 15, 3557–3572. [Google Scholar] [CrossRef] [PubMed]





| Protein Name | Target Gene Name | Forward Primer | Reverse Primer | Product Size (bp) |
|---|---|---|---|---|
| pβ-actin | Atcb | AGGCCAACCGTGAGAAGATG | CATGACAATGCCAGTGGTGC | 122 |
| pBCRP | Abcg2 | ATCCTGGGCCTGGTTATAGG | GAGACGCTGCTGAAACACTG | 170 |
| pClaudin1 | CLDN5 | TATGACCCCATGACCCCAGT | GGGCCTTGGTGTTGGGTAA | 150 |
| pClaudin5 | CLDN1 | CCCATGTCGCAGAAGTACGA | GGCCGAATACTTGACAGGGA | 150 |
| pGAPDH | GAPDH | GTCCACTGGTGTCTTCACGA | TCTCATGGTTCACGCCCATC | 125 |
| pMDR1 | Abcb1 | GCCTCGTATCTTGCTTCTGG | TCAAGTCTGCGTTCTGGATG | 146 |
| pMrp-1 | Abcc1 | AACTTTCTGGCTGGTAGCCC | AGCACGAGGGCGAAGTAAAT | 135 |
| pMrp-2 | Abcc2 | GATGCTCACGTGGGAAGACA | GTGCCATTTCCCACAACCAC | 140 |
| pMrp-3 | Abcc3 | CGTGGCGAGGTGGAGTTC | CATGGATGATTTGCCAGCCC | 141 |
| pZO-1 | TJP1 | CTGAGGGAATTGGGCAGGAA | CCAAAGGACTCAGCAGGGTT | 102 |
| rMdr1a | Abcb1a | TTAGATACGGCCGCGAGAAC | TTTCTCCCACGAGTGTGTCG | 117 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saaby, L.; Trasborg, J.; Rasmussen, M.A.; Holst, B.; Brodin, B. IPEC-J2 rMdr1a, a New Cell Line with Functional Expression of Rat P-glycoprotein Encoded by Rat Mdr1a for Drug Screening Purposes. Pharmaceutics 2020, 12, 673. https://doi.org/10.3390/pharmaceutics12070673
Saaby L, Trasborg J, Rasmussen MA, Holst B, Brodin B. IPEC-J2 rMdr1a, a New Cell Line with Functional Expression of Rat P-glycoprotein Encoded by Rat Mdr1a for Drug Screening Purposes. Pharmaceutics. 2020; 12(7):673. https://doi.org/10.3390/pharmaceutics12070673
Chicago/Turabian StyleSaaby, Lasse, Josefine Trasborg, Mikkel A. Rasmussen, Bjørn Holst, and Birger Brodin. 2020. "IPEC-J2 rMdr1a, a New Cell Line with Functional Expression of Rat P-glycoprotein Encoded by Rat Mdr1a for Drug Screening Purposes" Pharmaceutics 12, no. 7: 673. https://doi.org/10.3390/pharmaceutics12070673
APA StyleSaaby, L., Trasborg, J., Rasmussen, M. A., Holst, B., & Brodin, B. (2020). IPEC-J2 rMdr1a, a New Cell Line with Functional Expression of Rat P-glycoprotein Encoded by Rat Mdr1a for Drug Screening Purposes. Pharmaceutics, 12(7), 673. https://doi.org/10.3390/pharmaceutics12070673