Lutein-Loaded, Biotin-Decorated Polymeric Nanoparticles Enhance Lutein Uptake in Retinal Cells
Abstract
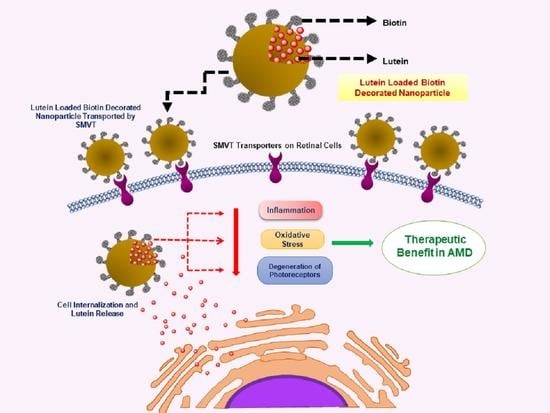
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Lutein-Loaded Polymeric Nanoparticles
2.2.2. Determination of Size, Polydispersity Index, and Zeta Potential
2.2.3. Lutein Quantification Using HPLC
2.2.4. Determination of Lutein Encapsulation Efficiency (%EE) and Drug Loading (%DL)
2.2.5. Differential Scanning Calorimetry (DSC)
2.2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.7. In-Vitro Release Studies
2.2.8. Cell Culture Studies
Cell Culture
FITC Labelling
In Vitro Cellular Uptake Studies Using Flow Cytometry (Fluorescence-Activated Cell Sorting (FACS))
In Vitro Cellular Uptake Studies Using Confocal Laser Scanning Microscopy
In Vitro Cell Viability Studies (MTT Assay)
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Determination of Particle Size, PDI, and ZP
3.2. Determination of Encapsulation Efficiency (%EE) and Drug Loading (%DL)
3.3. DSC
3.4. FTIR
3.5. In-Vitro Release Studies
3.6. In-Vitro Cellular Uptake Studies
3.6.1. FACS Analysis
3.6.2. Confocal Microscopy
3.7. In-Vitro Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaudana, R.; Jwala, J.; Boddu, S.H.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2008, 26, 1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharm. 2018, 107, 1564–1582. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Recent developments in ocular drug delivery. J. Drug Target. 2015, 23, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.; Vitale, S.; Agrón, E.; Domalpally, A.; Antoszyk, A.N.; Elman, M.J.; Clemons, T.E.; Chew, E.Y.; Age-Related Eye Disease Study 2 Research Group. AREDS2 Research Group Visual Acuity Outcomes after Anti–Vascular Endothelial Growth Factor Treatment for Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2020, 4, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yan, S.-F.; Huang, Y.-M.; Lu, X.-R.; Qian, F.; Pang, H.-L.; Xu, X.-R.; Zou, Z.; Dong, P.-C.; Xiao, X.; et al. Effect of Lutein and Zeaxanthin on Macular Pigment and Visual Function in Patients with Early Age-related Macular Degeneration. Ophthalmology 2012, 119, 2290–2297. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.; Stiles, W.; Statkute, L.; Pulido, J.; Frankowski, J.; Rudy, D.; Pei, K.; Tsipursky, M.; Nyland, J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: The Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optom. J. Am. Optom. Assoc. 2004, 75, 216–229. [Google Scholar] [CrossRef]
- De Jong, P.T. Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef]
- Hu, D.; Lin, C.; Liu, L.; Li, S.; Zhao, Y. Preparation, characterization, and in vitro release investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluids. J. Food Eng. 2012, 109, 545–552. [Google Scholar] [CrossRef]
- Li, S.-Y.; Fu, Z.; Ma, H.; Jang, W.-C.; So, K.-F.; Wong, D.; Lo, A.C.Y. Effect of Lutein on Retinal Neurons and Oxidative Stress in a Model of Acute Retinal Ischemia/Reperfusion. Investig. Opthalmol. Vis. Sci. 2009, 50, 836–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschos, M.M.; Dettoraki, M.; Tsatsos, M.; Kitsos, G.; Kalogeropoulos, C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis. 2017, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Allison, G.S.; Draper, C.S.; Soekamto, C.; Gong, X.M.; Rubin, L.P. Lutein Protects the Retinal Pigment Epithelium Against Hypoxic and Oxidative Stress: In Vitro Studies. FASEB J. 2016, 30, 2–5. [Google Scholar]
- Gong, X.; Draper, C.S.; Allison, G.S.; Marisiddaiah, R.; Rubin, L.P. Effects of the Macular Carotenoid Lutein in Human Retinal Pigment Epithelial Cells. Antioxidants 2017, 6, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozawa, Y.; Sasaki, M.; Takahashi, N.; Kamoshita, M.; Miyake, S.; Tsubota, K. Neuroprotective Effects of Lutein in the Retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Lutein. 2020. Available online: https://www.drugbank.ca/drugs/DB00137 (accessed on 31 May 2020).
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H. Ocular Drug Delivery Barriers—Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, D.F.; Lim, J.I. Topical Drug Delivery for Posterior Segment Disease, Retin. Available online: https://retinatoday.com/pdfs/0510RT_Feature_Lim_Mosh.pdf (accessed on 24 July 2020).
- Cholkar, K.; Ray, A.; Agrahari, V.; Pal, D.; Mitra, A.K. Transporters and receptors in the anterior segment of the eye. In Ocular Transporters and Receptors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 115–168. [Google Scholar]
- Patel, A.; Gokulgandhi, M.; Khurana, V.; Mitra, A.K. 5-Transporters and Receptors in the Posterior Segment of the Eye; Woodhead Publishing: Cambridge, UK, 2013; pp. 169–205. [Google Scholar]
- Dey, S.; Mitra, A.K. Transporters and receptors in ocular drug delivery: Opportunities and challenges. Expert Opin. Drug Deliv. 2005, 2, 201–204. [Google Scholar] [CrossRef]
- Boddu, S.H.; Nesamony, J. Utility of transporter/receptor(s) in drug delivery to the eye. World J. Pharmacol. 2013, 2, 1–17. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Vadlapatla, R.K.; Pal, D.; Mitra, A.K. Functional and Molecular Aspects of Biotin Uptake via SMVT in Human Corneal Epithelial (HCEC) and Retinal Pigment Epithelial (D407) Cells. AAPS J. 2012, 14, 832–842. [Google Scholar] [CrossRef] [Green Version]
- Singh, Y.; Viswanadham, K.D.R.; Jajoriya, A.K.; Meher, J.G.; Raval, K.; Jaiswal, S.; Dewangan, J.; Bora, H.K.; Rath, S.K.; Lal, J.; et al. Click Biotinylation of PLGA Template for Biotin Receptor Oriented Delivery of Doxorubicin Hydrochloride in 4T1 Cell-Induced Breast Cancer. Mol. Pharm. 2017, 14, 2749–2765. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Rouhani, H.; Sepehri, N.; Varshochian, R.; Ghahremani, M.H.; Amini, M.; Gharghabi, M.; Ostad, S.N.; Atyabi, F.; Baharian, A.; et al. Biotin decorated PLGA nanoparticles containing SN-38 designed for cancer therapy. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Meng, X.; Su, J.; Ma, H.; Wang, W.; Fang, L.; Zheng, H.; Qin, Y.; Chen, T. Biotin-Modified Polylactic-co-Glycolic Acid Nanoparticles with Improved Antiproliferative Activity of 15,16-Dihydrotanshinone I in Human Cervical Cancer Cells. J. Agric. Food Chem. 2018, 66, 9219–9230. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Wood, T.; Chen, C.-C.; Corry, K.; Snyder, J.M.; Juul, S.E.; Parikh, P.; Nance, E. Curcumin-loaded polymeric nanoparticles for neuroprotection in neonatal rats with hypoxic-ischemic encephalopathy. Nano Res. 2018, 11, 5670–5688. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 8, 271–299. [Google Scholar] [CrossRef]
- Lim, C.; Kim, D.-W.; Sim, T.; Hoang, N.H.; Lee, J.W.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Preparation and characterization of a lutein loading nanoemulsion system for ophthalmic eye drops. J. Drug Deliv. Sci. Technol. 2016, 36, 168–174. [Google Scholar] [CrossRef]
- Tan, T.B.; Yussof, N.S.; Abas, F.; Mirhosseini, H.; Nehdi, I.A.; Tan, C.P. Stability evaluation of lutein nanodispersions prepared via solvent displacement method: The effect of emulsifiers with different stabilizing mechanisms. Food Chem. 2016, 205, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Muhoza, B.; Zhang, Y.; Xia, S.; Cai, J.; Zhang, X.; Su, J. Improved stability and controlled release of lutein-loaded micelles based on glycosylated casein via Maillard reaction. J. Funct. Foods 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int. J. Pharm. 2011, 420, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci. Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Silva, J.; Geiss, J.M.T.; Oliveira, S.M.; Brum, E.D.S.; Sagae, S.C.; Becker, D.; Leimann, F.V.; Ineu, R.P.; Guerra, G.P.; Gonçalves, O.H. Nanoencapsulation of lutein and its effect on mice’s declarative memory. Mater. Sci. Eng. C 2017, 76, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Brum, A.A.S.; Dos Santos, P.P.; Da Silva, M.M.; Paese, K.; Guterres, S.S.; Costa, T.M.; Pohlmann, A.R.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O. Lutein-loaded lipid-core nanocapsules: Physicochemical characterization and stability evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 477–484. [Google Scholar] [CrossRef]
- Jwala, J.; Boddu, S.H.; Shah, S.; Sirimulla, S.; Pal, D.; Mitra, A.K. Ocular Sustained Release Nanoparticles Containing Stereoisomeric Dipeptide Prodrugs of Acyclovir. J. Ocul. Pharmacol. Ther. 2011, 27, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Boddu, S.H.; Vaishya, R.; Jwala, J.; Vadlapudi, A.; Pal, D.; Mitra, A.K. Preparation and Characterization of Folate Conjugated Nanoparticles of Doxorubicin using Plga-Peg-Fol Polymer. Med. Chem. 2012, 2, 068–075. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Sun, M.; Liu, X.; Fan, A.; Wang, Z.; Zhao, Y. Interplay of stimuli-responsiveness, drug loading and release for a surface-engineered dendrimer delivery system. Int. J. Pharm. 2014, 462, 103–107. [Google Scholar] [CrossRef]
- Chaganti, L.K.; Venkatakrishnan, N.; Bose, K. An efficient method for FITC labelling of proteins using tandem affinity purification. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Michlewska, S.; Kubczak, M.; Maroto-Díaz, M.; Del Olmo, N.S.; Ortega, P.; Shcharbin, D.; Gómez, R.; De La Mata, F.J.; Ionov, M.; Bryszewska, M. Synthesis and Characterization of FITC Labelled Ruthenium Dendrimer as a Prospective Anticancer Drug. Biomolecules 2019, 9, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damgé, C.; Maincent, P.; Ubrich, N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J. Control. Release 2007, 117, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Cholkar, K.; Khurana, V.; Shah, A.; Agrahari, V.; Bisht, R.; Pal, D.; Mitra, A.K. Topical Formulation of Self-Assembled Antiviral Prodrug Nanomicelles for Targeted Retinal Delivery. Mol. Pharm. 2017, 14, 2056–2069. [Google Scholar] [CrossRef] [PubMed]
- Javidfar, S.; Pilehvar-Soltanahmadi, Y.; Farajzadeh, R.; Lotfi-Attari, J.; Shafiei-Irannejad, V.; Hashemi, M.; Zarghami, N. The inhibitory effects of nano-encapsulated metformin on growth and hTERT expression in breast cancer cells. J. Drug Deliv. Sci. Technol. 2018, 43, 19–26. [Google Scholar] [CrossRef]
- Firouzi-Amandi, A.; Dadashpour, M.; Nouri, M.; Zarghami, N.; Serati-Nouri, H.; Jafari-Gharabaghlou, D.; Karzar, B.H.; Mellatyar, H.; Aghebati-Maleki, L.; Babaloo, Z.; et al. Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: Possible application in tissue regeneration. Biomed. Pharm. 2018, 105, 773–780. [Google Scholar] [CrossRef]
- USFDA, Inactive Ingredient Search for Approved Drug Products, (n.d.). Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed on 28 July 2019).
- Bolla, P.K.; Kalhapure, R.S.; Rodriguez, V.A.; Ramos, D.V.; Dahl, A.; Renukuntla, J. Preparation of solid lipid nanoparticles of furosemide-silver complex and evaluation of antibacterial activity. J. Drug Deliv. Sci. Technol. 2019, 49, 6–13. [Google Scholar] [CrossRef]
- Bolla, P.K.; Meraz, C.A.; Rodriguez, V.A.; Deaguero, I.G.; Singh, M.; Yellepeddi, V.K.; Renukuntla, J. Clotrimazole Loaded Ufosomes for Topical Delivery: Formulation Development and In-Vitro Studies. Molecules 2019, 24, 3139. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, V.A.; Bolla, P.K.; Kalhapure, R.S.; Boddu, S.H.; Neupane, R.; Franco, J.; Renukuntla, J. Preparation and Characterization of Furosemide-Silver Complex Loaded Chitosan Nanoparticles. Processes 2019, 7, 206. [Google Scholar] [CrossRef] [Green Version]
- Morissette, S.L. High-throughput crystallization: Polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliv. Rev. 2004, 56, 275–300. [Google Scholar] [CrossRef]
- Musumeci, T.; Serapide, M.; Pellitteri, R.; Dalpiaz, A.; Ferraro, L.; Magro, R.D.; Bonaccorso, A.; Carbone, C.; Veiga, F.; Sancini, G.; et al. Oxcarbazepine free or loaded PLGA nanoparticles as effective intranasal approach to control epileptic seizures in rodents. Eur. J. Pharm. Biopharm. 2018, 133, 309–320. [Google Scholar] [CrossRef]
- Bragagni, M.; Gil-Alegre, M.E.; Mura, P.; Cirri, M.; Ghelardini, C.; Mannelli, L.D.C. Improving the therapeutic efficacy of prilocaine by PLGA microparticles: Preparation, characterization and in vivo evaluation. Int. J. Pharm. 2018, 547, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoub, M.M.; Elantouny, N.G.; Elnahas, H.; Ghazy, F.E.-D.S. Injectable PLGA Adefovir microspheres; the way for long term therapy of chronic hepatitis-B. Eur. J. Pharm. Sci. 2018, 118, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, J.; Deemer, E.M.; Narayan, M. Chitosan Nanoparticles Rescue Rotenone-Mediated Cell Death. Materials 2019, 12, 1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| S.no | Particle Type | Size (nm) | PDI | ZP (mV) | EE (%) |
|---|---|---|---|---|---|
| 1 | Lutein PLGA | 196.4 ± 20.04 | 0.087 ± 0.016 | −11.12 ± 2.12 | 56.05 ± 7.28 |
| 2 | Lutein PLGA–PEG–biotin | 208.0 ± 3.38 | 0.206 ± 0.016 | −27.2 ± 2.04 | 74.56 ± 10.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-Loaded, Biotin-Decorated Polymeric Nanoparticles Enhance Lutein Uptake in Retinal Cells. Pharmaceutics 2020, 12, 798. https://doi.org/10.3390/pharmaceutics12090798
Bolla PK, Gote V, Singh M, Patel M, Clark BA, Renukuntla J. Lutein-Loaded, Biotin-Decorated Polymeric Nanoparticles Enhance Lutein Uptake in Retinal Cells. Pharmaceutics. 2020; 12(9):798. https://doi.org/10.3390/pharmaceutics12090798
Chicago/Turabian StyleBolla, Pradeep Kumar, Vrinda Gote, Mahima Singh, Manan Patel, Bradley A. Clark, and Jwala Renukuntla. 2020. "Lutein-Loaded, Biotin-Decorated Polymeric Nanoparticles Enhance Lutein Uptake in Retinal Cells" Pharmaceutics 12, no. 9: 798. https://doi.org/10.3390/pharmaceutics12090798
APA StyleBolla, P. K., Gote, V., Singh, M., Patel, M., Clark, B. A., & Renukuntla, J. (2020). Lutein-Loaded, Biotin-Decorated Polymeric Nanoparticles Enhance Lutein Uptake in Retinal Cells. Pharmaceutics, 12(9), 798. https://doi.org/10.3390/pharmaceutics12090798