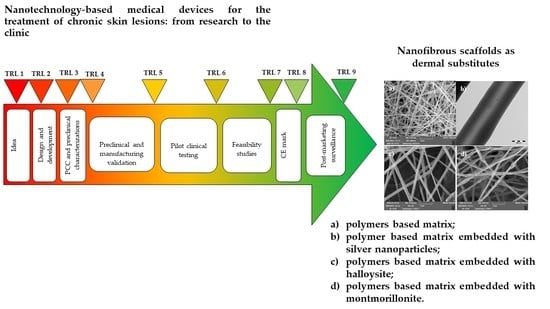
Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic
Abstract
:1. Introduction
2. Medical Devices: Definition and Classification
- −
- diagnosis, prevention, monitoring, treatment, or alleviation of disease,
- −
- diagnosis, monitoring, treatment, alleviation of or compensation for an injury,
- −
- investigation, replacement, modification, or support of the anatomy or of a physiological process,
- −
- supporting or sustaining life,
- −
- control of conception,
- −
- disinfection of MDs,
- −
- providing information by means of in vitro examination of specimens derived from the human body.
- −
- class I: less critical (low risk) devices, such as most of the non-active and non-invasive ones (two subclasses can be identified within class I: sterile class Is—those supplied in a sterile state—and class Im—those that perform a measurement function);
- −
- class IIa: medium risk devices, such as some non-active devices (invasive and non-invasive) and active devices that interact with the body in a non-dangerous way;
- −
- class IIb: medium/high risk devices, such as some non-active devices (invasive species) and active devices that interact with the body in a dangerous way;
- −
- class III: high-risk devices, such as most of those implantable, those containing drugs or animal derivatives and some MDs that act on the functions of vital organs.
3. Medical Devices for Wound Healing
3.1. Current Commercially Available Skin Substitutes in Wound Healing
3.2. Nanotechnology-Based Medical Devices for Wound Healing
4. Quality by Design (QbD) Approach
5. Definition of the Quality Attributes
5.1. Chemical and Solid State Assessement
5.2. Stability
5.3. Particle Size
5.4. Mechanical Properties
5.5. Surface Properties
5.6. Release of Nanomaterials from Medical Devices
5.7. Apyrogenicity and Sterility
6. Preclinical Evaluation of Medical Devices
6.1. Biocompatibility Testing
6.2. Alternative In Vitro Tests for Irritation and Sensitization
6.3. Wound Healing Test
6.4. Animal Wound Models
7. Clinical Evaluation of Medical Devices
8. CE Marking and Product Life Cycle
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Villen, F.; Faccendini, A.; Aguzzi, C.; Cerezo, P.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Ruggeri, M.; Ferrari, F.; Sandri, G.; et al. Montmorillonite-norfloxacin nanocomposite intended for healing of infected wounds. Int. J. Nanomed. 2019, 14, 5051–5060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadi, K.; Ganchi, P. Chronic wounds. Clin. Plast. Surg. 2005, 32, 209–222. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J.; Järbrink, K.; Ni, G.; Sönnergren, H.; et al. Prevalence and incidence of chronic wounds and related complications: A protocol for a systematic review. Syst. Rev. 2016, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Syst. Rev. 2017, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Ray, J.A.; Valentine, W.J.; Secnik, K.; Oglesby, A.K.; Cordony, A.; Gordois, A.; Davey, P.; Palmer, A.J. Review of the cost of diabetes complications in Australia, Canada, France, Germany, Italy and Spain. Curr. Med. Res. Opin. 2005, 21, 1617–1629. [Google Scholar] [CrossRef]
- Nussbaum, SR.; Carter, MJ.; Fife, CE.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health. 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashtikar, M.; Wacker, M.G. Nanopharmaceuticals for wound healing—Lost in translation? Adv. Drug Deliv. Rev. 2018, 129, 194–218. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Medical Devices. 2019. Available online: https://www.who.int/medical_devices/en/ (accessed on 21 March 2020).
- Global Medical Devices Market Report 2019–2022—A $521+ Billion Opportunity Analysis, Research and Markets. Available online: https://www.globenewswire.com/news-release/2019/09/19/1918062/0/en/Global-Medical-Devices-Market-Report-2019-2022-A-521-Billion-Opportunity-Analysis.html (accessed on 13 August 2020).
- European Commission. MEDDEV 2.4/1 Rev.9. Classification of Medical Devices. Published June 2010. Available online: http://ec.europa.eu/DocsRoom/documents/10337/attachments/1/translations (accessed on 24 August 2020).
- Directive 93/42/EEC. 1993. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31993L0042 (accessed on 24 August 2020).
- Aronson, J.K.; Heneghan, C.; Ferner, R.E. Medical Devices: Definition, Classification, and Regulatory Implications. Drug Saf. 2020, 43, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.J.; Jones, T. Medical device classification: Focus on vascular access. Br. J. Nurs. 2018, 27, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Badylak, S.F. The Use of Biologic Scaffolds in the Treatment of Chronic Nonhealing Wounds. Adv. Wound Care 2015, 4, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, L.E.; Gerecht, S. Engineered Biopolymeric Scaffolds for Chronic Wound Healing. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, D.; Sullivan, N.; Margolis, D.; Schoelles, K. Skin Substitutes for Treating Chronic Wounds; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012. Available online: https://effectivehealthcare.ahrq.gov/products/skin-substitutes/protocol (accessed on 24 August 2020).
- Kemp, P.D. Problems and Pitfalls in Tissue-Engineered Therapy. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Chapter 65; pp. 871–875. [Google Scholar]
- Zaulyanov, L.; Kirsner, R.S. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin. Interv. Aging 2007, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isaacs, C.; Paquette, D.; Downing, G.; Kouttab, N.; Butmarc, J.; Badiavas, E.; Hardin-Young, J. Wounding of bioengineered skin: Cellular and molecular aspects after injury. J. Investig. Dermatol. 2002, 119, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Hart, C.E.; Loewen-Rodriguez, A.; Lessem, J. Dermagraft: Use in the Treatment of Chronic Wounds. Adv. Wound Care 2012, 1, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Marston, W.A.; Hanft, J.; Norwood, P.; Pollak, R.; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: Results of a prospective randomized trial. Diabetes Care 2003, 26, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Naughton, G.; Mansbridge, J.; Gentzkow, G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artif. Organs 1997, 21, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Landsman, A.S.; Cook, J.; Cook, E.; Landsman, A.R.; Garrett, P.; Yoon, J.; Kirkwood, A.; Desman, E. A retrospective clinical study of 188 consecutive patients to examine the effectiveness of a biologically active cryopreserved human skin allograft (TheraSkin®) on the treatment of diabetic foot ulcers and venous leg ulcers. Foot Ankle Spec. 2011, 4, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Mostow, E.N.; Haraway, G.D.; Dalsing, M.; Hodde, J.P.; King, D.; OASIS Venus Ulcer Study Group. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: A randomized clinical trial. J. Vasc. Surg. 2005, 41, 837–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Ronfard, V. Biochemical and biomechanical characterization of porcine small intestinal submucosa (SIS): A mini review. Int. J. Burns Trauma 2013, 3, 173–179. [Google Scholar] [PubMed]
- Cullen, B.; Smith, R.; McCulloch, E.; Silcock, D.; Morrison, L. Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Wound Repair Regen. 2002, 10, 16–25. [Google Scholar] [CrossRef]
- Lantis, J.C.; Paredes, J.A. Topical Wound Care Treatment and Indications for Their Use. In The Diabetic Foot; Veves, A., Giurini, J., Guzman, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 281–304. [Google Scholar]
- Simman, R.; Mari, W.; Younes, S.; Wilson, M. Use of Hyaluronic Acid-Based Biological Bilaminar Matrix in Wound Bed Preparation: A Case Series. Eplasty 2018, 18, e10. [Google Scholar]
- Myers, S.R.; Partha, V.N.; Soranzo, C.; Price, R.D.; Navsaria, H.A. Hyalomatrix: A temporary epidermal barrier, hyaluronan delivery, and neodermis induction system for keratinocyte stem cell therapy. Tissue Eng. 2007, 13, 2733–2741. [Google Scholar] [CrossRef]
- Maus, E.A. Successful treatment of two refractory venous stasis ulcers treated with a novel poly-N-acetyl glucosamine-derived membrane. BMJ Case Rep. 2012, 2012, bcr0320126091. [Google Scholar] [CrossRef]
- Scherer, S.S.; Pietramaggiori, G.; Matthews, J.; Perry, S.; Assmann, A.; Carothers, A.; Demcheva, M.; Muise-Helmericks, R.C.; Seth, A.; Vournakis, J.N.; et al. Poly-N-acetyl glucosamine nanofibers: A new bioactive material to enhance diabetic wound healing by cell migration and angiogenesis. Ann. Surg. 2009, 250, 322–330. [Google Scholar] [CrossRef]
- Carter, M.J.; Waycaster, C.; Schaum, K.; Gilligan, A.M. Cost-effectiveness of three adjunct cellular/tissue-derived products used in the management of chronic venous leg ulcers. Value Health 2014, 17, 801–813. [Google Scholar] [CrossRef] [Green Version]
- Rennert, R.C.; Rodrigues, M.; Wong, V.W.; Duscher, D.; Hu, M.; Maan, Z.; Longaker, M.T. Biological therapies for the treatment of cutaneous wounds: Phase III and launched therapies. Expert Opin. Biol. Ther. 2013, 13, 1523–1541. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic Acid and Chitosan-Based Nanosystems: A New Dressing Generation for Wound Care. Expert Opin. Drug Deliv. 2019, 16, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Korrapati, P.S.; Karthikeyan, K.; Satish, A.; Krishnaswamy, V.R.; Venugopal, J.R.; Ramakrishna, S. Recent advancements in nanotechnological strategies in selection, design and delivery of biomolecules for skin regeneration. Mater. Sci. Eng. C 2016, 67, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Mordorski, B.; Rosen, J.; Friedman, A. Nanotechnology as an innovative approach for accelerating wound healing in diabetes. Diabetes Manag. 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef]
- O’Brien, J.F. Biomaterials & scaffolds for tissue engineering. Mat. Today 2011, 14, 88–94. [Google Scholar]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef]
- Gaspar, A.; Moldovan, L.; Constantin, D.; Stanciuc, A.M.; Sarbu Boeti, P.M.; Efrimescu, I.C. Collagen-based scaffolds for skin tissue engineering. J. Med. Life 2011, 4, 172–177. [Google Scholar]
- Powell, H.M.; Supp, D.M.; Boyce, S.T. Influence of electrospun collagen on wound contraction of engineered skin substitutes. Biomaterials 2008, 29, 834–843. [Google Scholar] [CrossRef]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.-M.; Park, Y.J.; Hong, S.D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.-M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Rujitanaroj, P.-O.; Pimpha, N.; Supaphol, P. Wound dressing materials with antibacterial activity from electrospun gelatin fiber mats containing silver nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Gu, S.Y.; Wang, Z.M.; Ren, J.; Zhang, C.Y. Electrospinning of gelatin and gelatin/poly(L-lactide) blend and its characteristics for wound dressing. Mater. Sci. Eng. C 2009, 29, 1822–1828. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Icaro Cornaglia, I.; Boselli, C.; Luxbacher, T.; et al. Chitosan/glycosaminoglycan Scaffolds for Skin Reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandri, G.; Faccendini, A.; Longo, M.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Prina-Mello, A.; Aguzzi, C.; Viseras, C.; et al. Halloysite- and Montmorillonite-Loaded Scaffolds as Enhancers of Chronic Wound Healing. Pharmaceutics 2020, 12, 179. [Google Scholar] [CrossRef] [Green Version]
- Faccendini, A.; Ruggeri, M.; Rossi, S.; Bonferoni, M.C.; Aguzzi, C.; Grisoli, P.; Viseras, C.; Sandri, G.; Ferrari, F. Norfloxacin loaded electrospun scaffolds: Montmorillonite nanocomposite vs. free drug. Pharmaceutics 2020, 12, 325. [Google Scholar] [CrossRef] [Green Version]
- Malgarim Cordenonsi, L.; Faccendini, A.; Rossi, S.; Bonferoni, M.C.; Malavasi, L.; Raffin, R.; Scherman Schapoval, E.E.; Del Fante, C.; Vigani, B.; Miele, D.; et al. Platelet lysate loaded electrospun scaffolds: Effect of nanofiber types on wound healing. Eur. J. Pharm. Biopharm. 2019, 142, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.H.; Boyce, S.T. Engineered Human Skin Fabricated Using Electrospun collagen-PCL Blends: Morphogenesis and Mechanical Properties. Tissue Eng. Part A 2009, 15, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun Poly (lactic Acid-Co-Glycolic Acid) Scaffolds for Skin Tissue Engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackwood, K.A.; McKean, R.; Canton, I.O.; Freeman, C.; Franklin, K.L.; Cole, D.; Brook, I.; Farthing, P.; Rimmer, S.; Haycock, J.W.; et al. Development of Biodegradable Electrospun Scaffolds for Dermal Replacement. Biomaterials 2008, 29, 3091–3104. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.G.; Zhu, X.L.; Yang, Y.; Li, X.H.; Jin, Y. Evaluation of electrospun fibrous scaffolds of poly(DL-lactide) and poly(ethylene glycol) for skin tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 869–1876. [Google Scholar] [CrossRef]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Caramella, C.; Ferrari, F. Electrospinning Technologies in Wound Dressing Applications. In Therapeutic Dressings and Wound Healing Applications; Boateng, J., Ed.; Chichester: West Sussex, UK, 2020; Chapter 14; pp. 315–336. [Google Scholar]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 03400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.; Ma, H.; Wu, Z.; Zeng, H.; Li, Z.; Wang, Y.; Liu, G.; Xu, B.; Lin, Y.; Zhang, P.; et al. Enhancement of skin wound healing with decellularized scaffolds loaded with hyaluronic acid and epidermal growth factor. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 440–448. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, T.; Zhi, W.; Wei, L.; Weng, J.; Zhang, C.; Li, X. Promotion of Skin Regeneration in Diabetic Rats by Electrospun Core-Sheath Fibers Loaded With Basic Fibroblast Growth Factor. Biomaterials 2011, 32, 4243–4254. [Google Scholar] [CrossRef]
- Vijayan, A.; Sabareeswaran, A.; Kumar, G.S.V. PEG grafted chitosan scaffold for dual growth factor delivery for enhanced wound healing. Sci. Rep. 2019, 9, 19165. [Google Scholar] [CrossRef] [Green Version]
- Madhavan, R.V.; Rosemary, M.J.; Nandkumar, M.A.; Krishnan, K.V.; Krishnan, L.K. Silver nanoparticle impregnated poly (ε-caprolactone) scaffolds: Optimization of antimicrobial and noncytotoxic concentrations. Tissue Eng. Part A 2011, 17, 439–449. [Google Scholar] [CrossRef]
- Madhumathi, K.; Sudheesh Kumar, P.T.; Abhilash, S.; Sreeja, V.; Tamura, H.; Manzoor, K.; Nair, S.V.; Jayakumar, R. Development of Novel Chitin/Nanosilver Composite Scaffolds for Wound Dressing Applications. J. Mater. Sci. Mater. Med. 2010, 21, 807–813. [Google Scholar] [CrossRef]
- Loan Khanh, L.; Thanh Truc, N.; Tan Dat, N.; Phuong Nghi, N.T.; van Toi, V.; Thu Hoai, N.T.; Ngoc Quyen, T.; Thanh Loan, T.T.; Thi Hiepa, N. Gelatin-stabilized composites of silver nanoparticles and curcumin: Characterization, antibacterial and antioxidant study. Sci. Technol. Adv. Mater. 2019, 20, 276–290. [Google Scholar] [CrossRef] [Green Version]
- Mary, G.; Bajpai, S.K.; Chand, N. Copper (II) Ions and Copper Nanoparticles-Loaded Chemically Modified Cotton Cellulose Fibers with Fair Antibacterial Properties. J. Appl. Polym. 2009, 113, 757–766. [Google Scholar] [CrossRef]
- Ullah, S.; Zainol, I.; Idrus, R.H. Incorporation of zinc oxide nanoparticles into chitosan-collagen 3D porous scaffolds: Effect on morphology, mechanical properties and cytocompatibility of 3D porous scaffolds. Int. J. Biol. Macromol. 2017, 104, 1020–1029. [Google Scholar] [CrossRef]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds. RSC Adv. 2014, 93, 51528–51536. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.; Mishra, A.; Sharma, D.; Singh, K. Antiviral and Antimicrobial Potentiality of Nano Drugs. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S., Ranjan, S., Dasgupta, N., Kumar, R., Thomas, S., Eds.; Elsevier: Amsterdam, Netherlands, 2019; Chapter 13; pp. 343–356. [Google Scholar]
- Fornaguera, C.; García-Celma, M. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacker, M.G.; Proykova, A.; Mendes, G.; Santos, L. Dealing with nanosafety around the globe—Regulation vs. innovation. Int. J. Pharm. 2016, 509, 95–106. [Google Scholar] [CrossRef]
- Ragelle, H.; Danhier, F.; Préat, V.; Langer, R.; Anderson, D.G. Nanoparticle-based drug delivery systems: A commercial and regulatory outlook as the field matures. Expert Opin. Drug Deliv. 2017, 14, 851–864. [Google Scholar] [CrossRef]
- CDRH INNOVATION INITIATIVE February 2011 Center for Devices and Radiological Health, U.S. Food and Drug Administration. Available online: https://www.fda.gov/about-fda/cdrh-innovation/medical-device-innovation-initiative-white-paper (accessed on 26 August 2020).
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Wacker, M.G.; Janas, C.; Ferreira, F.S.; Pires Vieira, F. Manufacturing and Safety Guidelines for Manufactured Functionalized Nanomaterials in Pharmaceutics. In Biomedical Applications of Functionalized Nanomaterials; Sarmento, B., das Neves, J., Eds.; Elsevier: Amsterdam, Netherlands, 2018; Chapter 21; pp. 623–651. [Google Scholar]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control Release 2011, 150, 128–141. [Google Scholar] [CrossRef]
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential Applications of Nanocellulose-Containing Materials in the Biomedical Field. Materials 2017, 10, 977. [Google Scholar] [CrossRef] [Green Version]
- Muthu, M.S.; Feng, S.S. Pharmaceutical stability aspects of nanomedicines. Nanomedicine 2009, 4, 857–860. [Google Scholar] [CrossRef] [PubMed]
- ICH Q1A (R2). Stability Testing Guidelines: Stability Testing of New Drug Substances and Products. ICH Step 5. CPMP/ICH/2736/99. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products (accessed on 24 August 2020).
- ICH Q1C. Stability Testing for New Dosage Forms. ICH Step 5. CPMP/ICH/280/95. Available online: https://www.ema.europa.eu/en/ich-q1c-stability-testing-requirements-new-dosage-forms (accessed on 24 August 2020).
- ICH Q5C. Stability Testing of Biotechnological/Biological Products. ICH Step 4. CPMP/ICH/138/95. Available online: https://www.ema.europa.eu/en/ich-q5c-stability-testing-biotechnologicalbiological-products (accessed on 24 August 2020).
- ICH Q1B. Photostability Testing of New Drug Substances and Products. ICH Step 5. CPMP/ICH/279/95. Available online: https://www.ema.europa.eu/en/ich-q1b-photostability-testing-new-active-substances-medicinal-products (accessed on 24 August 2020).
- Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. In Natural Polymer Drug Delivery Systems; Bhatia, S. (Ed.) Springer International Publishing: Cham, Switzerland, 2016; Chapter 2; pp. 33–93. [Google Scholar]
- Akbari, B.; Pirhadi Tavandashti, M.; Zandrahimi, M. Particle size characterization of nanoparticles—A practical approach. Iran. J. Mater. Sci. Eng. 2011, 8, 48–56. [Google Scholar]
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices. 2015. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwjjufKR-bXrAhXEKewKHT46BEgQFjAAegQIBRAB&url=https%3A%2F%2Fec.europa.eu%2Fhealth%2Fscientific_committees%2Femerging%2Fdocs%2Fscenihr_o_045.pdf&usg=AOvVaw1Z8Vcg8DewoqXssCNlDPav (accessed on 25 August 2020).
- Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Drug Products, Including Biological Products, That Contain Nanomaterials, Guidance for Industry. 2017. Available online: https://www.fda.gov/files/drugs/published/Drug-Products--Including-Biological-Products--that-Contain-Nanomaterials---Guidance-for-Industry.pdf (accessed on 27 March 2020).
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control. Release 2019, 299, 31–43. [Google Scholar] [CrossRef]
- Blundell, E.L.C.J.; Mayne, L.J.; Billinge, E.R.; Platt, M. Emergence of tunable resistive pulse sensing as a biosensor. Anal. Methods 2015, 7, 7055. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.; Hamid, Z.; Cheong, K. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser. 2018, 1082, 012080. [Google Scholar] [CrossRef]
- ISO 6892-1. Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature; ICS: Waltham, MA, USA, 2019. [Google Scholar]
- ISO 178. Plastics—Determination of Flexural Properties; ICS: Waltham, MA, USA, 2019. [Google Scholar]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. Methods. Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef]
- Buksek, H.; Luxbacher, T.; Petrini, I. Zeta Potential Determination of Polymeric Materials Using Two Differently Designed Measuring Cells of an Electrokinetic Analyzer. Acta Chim. Slov. 2010, 57, 700–706. [Google Scholar] [PubMed]
- Madhukumar, R.; Asha, S.; Lakshmeesha Rao, B.; Sarojini, B.; Byrappa, K.; Wang, Y.; Sangappa, Y. Optical properties of γ-irradiated Bombyx mori silk fibroin films. Radiat. Eff. Defects Solids 2015, 906–915. [Google Scholar] [CrossRef]
- Bremer-Hoffmann, S.; Halamoda-Kenzaoui, B.; Borgos, S.V. Identification of regulatory needs for nanomedicines. J. Interdiscip. Nanomed. 2018, 3, 4–15. [Google Scholar] [CrossRef]
- European pharmacopoeia 5.0 2.6.14. Bacterial endotoxins. Available online: https://gmpua.com/Validation/Method/LAL/EUPHARMACOPOEIA.pdf (accessed on 3 August 2020).
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the correlation between in vitro and ex immunotoxicity tests for nanomedicines. J. Control. Release 2013, 172, 456–466. [Google Scholar] [CrossRef] [Green Version]
- ISO 10993-1. Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process; ICS: Waltham, MA, USA, 2018. [Google Scholar]
- ISO 10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ICS: Waltham, MA, USA, 2009. [Google Scholar]
- ISO 10993-10. Biological Evaluation of Medical Devices—Part 10: Biological Evaluation of Medical Devices Tests for Irritation and Skin Sensitization; ICS: Waltham, MA, USA, 2010. [Google Scholar]
- Frankild, S.; Vølund, A.; Wahlberg, J.E.; Andersen, K.E. Comparison of the Sensitivities of the Buehler Test and the Guinea Pig Maximization Test for Predictive Testing of Contact Allergy. Acta Derm. Venereol. 2000, 80, 256–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.K.; Nusair, T.L.; Fletcher, E.R.; Ritz, H.L. Review of the Buehler Guinea Pig Skin Sensitization Test and Its Use in a Risk Assessment Process for Human Skin Sensitization. Toxicology 1990, 61, 91–107. [Google Scholar] [CrossRef]
- Gerberick, G.F.; Ryan, C.A.; Dearman, R.J.; Kimber, I. Local lymph node assay (LLNA) for detection of sensitization capacity of chemicals. Methods 2007, 41, 54–60. [Google Scholar] [CrossRef]
- Appendix D Overview of the GHS Classification Scheme in Hazard Classification. Available online: https://www.nap.edu/read/18872/chapter/20 (accessed on 27 March 2020).
- Bosshard, E. Review on skin and mucous-membrane irritation tests and their application. Food Chem. Toxicol. 1985, 23, 149–154. [Google Scholar] [CrossRef]
- Botham, P.A.; Basketter, D.A.; Maurer, T.; Mueller, D.; Potokar, M.; Bontinck, W.J. Skin sensitization—A critical review of predictive test methods in animals and man. Food Chem. Toxicol. 1991, 29, 275–286. [Google Scholar] [CrossRef]
- Myers, D.; Goldberg, A.; Poth, A.; Wolf, M.; Carraway, J.; McKim, J.; Coleman, K.; Hutchinson, R.; Brown, R.; Krug, H.; et al. From in vivo to in vitro: The medical device testing paradigm shift. ALTEX-Altern. Anim. Exp. 2017, 34, 479–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method. In Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Alépée, N.; Grandidier, M.H. The EpiSkin™ Human Epidermis Model for In Vitro Skin Corrosion of Test Chemicals. In Alternatives for Dermal Toxicity Testing; Eskes, C., van Vliet, E., Maibach, H.I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 107–125. [Google Scholar]
- Kandárová, H.; Hayden, P.; Klausner, M.; Kubilus, J.; Sheasgreen, J. An in vitro skin irritation test (SIT) using the EpiDerm reconstructed human epidermal (RHE) model. J. Vis. Exp. 2009, 29, e1366. [Google Scholar] [CrossRef] [Green Version]
- De Brugerolle de Fraissinette, A.; Picarles, V.; Chibout, S.; Kolopp, M.; Medina, J.; Burtin, P.; Ebelin, M.E.; Osborne, S.; Mayer, F.K.; Spake, A.; et al. Predictivity of an in vitro model for acute and chronic skin irritation (SkinEthic) applied to the testing of topical vehicles. Cell Biol. Toxicol. 1999, 15, 121–135. [Google Scholar] [CrossRef]
- Kandárová, H.; Liebsch, M.; Schmidt, E.; Genschow, E.; Traue, D.; Spielmann, H.; Meyer, K.; Steinhoff, C.; Tornier, C.; De Wever, B.; et al. Assessment of the skin irritation potential of chemicals by using the SkinEthic reconstructed human epidermal model and the common skin irritation protocol evaluated in the ECVAM skin irritation validation study. Altern. Lab. Anim. 2006, 34, 393–406. [Google Scholar] [CrossRef]
- Kojima, H.; Katoh, M.; Shinoda, S.; Hagiwara, S.; Suzuki, T.; Izumi, R.; Yamaguchi, Y.; Nakamura, M.; Kasahawa, T.; Shibai, A. A catch-up validation study of an in vitro skin irritation test method using reconstructed human epidermis LabCyte EPI-MODEL24. J. Appl. Toxicol. 2014, 34, 766–774. [Google Scholar] [CrossRef]
- Lee, M.; Hwang, J.H.; Lim, K.M. Alternatives to In Vivo Draize Rabbit Eye and Skin Irritation Tests with a Focus on 3D Reconstructed Human Cornea-Like Epithelium and Epidermis Models. Toxicol. Res. 2017, 33, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faller, C.; Bracher, M.; Dami, N.; Roguet, R. Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics. Toxicol. In Vitro 2002, 16, 557–572. [Google Scholar] [CrossRef]
- Faller, C.; Bracher, M. Reconstructed skin kits: Reproducibility of cutaneous irritancy testing. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 74–91. [Google Scholar] [CrossRef]
- Casas, J.W.; Lewerenz, G.M.; Rankin, E.A.; Willoughby, J.A.; Blakeman, L.C.; McKim, J.M.; Coleman, K.P. In vitro human skin irritation test for evaluation of medical device extracts. Toxicol. In Vitro 2013, 27, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guidelines for the Testing of Chemicals. In Chemico Skin Sensitisation: Assays Addressing the Adverse Outcome Pathway Key Event on Covalent Binding to Proteins; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Wong, C.L.; Lam, A.L.; Smith, M.T.; Ghassabian, S. Evaluation of a High-Throughput Peptide Reactivity Format Assay for Assessment of the Skin Sensitization Potential of Chemicals. Front. Pharmacol. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerberick, G.F.; Vassallo, J.D.; Bailey, R.E.; Chaney, J.C.; Morrall, S.W.; Lepoittevin, J.-P. Development of a peptide reactivity assay for screening contact allergens. Toxicol. Sci. 2004, 81, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Natsch, A. The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers—Functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol. Sci. 2010, 113, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Natsch, A.; Bauch, C.; Foertsch, L.; Gerberick, F.; Norman, K.; Hilberer, A.; Inglis, H.; Landsiedel, R.; Onken, S.; Reuter, H.; et al. The intra-and inter-laboratory reproducibility and predictivity of the KeratinoSens assay to predict skin sensitisers in vitro: Results of a ring-study in five laboratories. Toxicol. In Vitro 2011, 25, 733–744. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Ashikaga, T.; Miyazawa, M.; Kosaka, N.; Ito, Y.; Yoneyama, K.; Sono, S.; Itagaki, H.; Toyoda, H.; Suzuki, H. The relationship between CD86/CD54 expression and THP-1 cell viability in an in vitro skin sensitization test—human cell line activation test (h-CLAT). Cell Biol. Toxicol. 2009, 25, 109–126. [Google Scholar] [CrossRef]
- Stamm, A.; Reimers, K.; Strauß, S.; Vogt, P.; Scheper, T.; Pepelanova, I. In vitro wound healing assays—State of the art. BioNanoMat 2016, 17, 79–87. [Google Scholar] [CrossRef]
- Zordan, M.D.; Mill, C.P.; Riese, J.; Leary, J.F. A high throughput, interactive imaging, bright-field wound healing assay. Cytom. Part A 2011, 79, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, R.; Geng, H.; Hwang, Y.; Mishra, P.; Skloss, W.L.; Sprague, E.A.; Saikumar, P.; Venkatacha. A novel wounding device suitable for quantitative biochemical analysis of wound healing and regeneration of cultured epithelium. Wound Repair Regen. 2010, 18, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Wang, Y.-L.; Ren, F.; Lele, T.P. Stamp wound assay for studying coupled cell migration and cell debris clearance. Letter 2010, 26, 16672–16676. [Google Scholar] [CrossRef] [PubMed]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szulcek, R.; Bogaard, H.J.; van Nieuw Amerongen, G.P. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. J. Vis. Exp. 2014, 28. [Google Scholar] [CrossRef] [Green Version]
- Jonkman, J.E.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef] [Green Version]
- García-Villén, F.; Faccendini, A.; Miele, D.; Ruggeri, M.; Sánchez-Espejo, R.; Borrego-Sánchez, A.; Cerezo, P.; Rossi, S.; Viseras, C.; Sandri, G. Wound Healing Activity of Nanoclay/Spring Water Hydrogels. Pharmaceutics 2020, 12, 467. [Google Scholar] [CrossRef]
- Ansell, D.M.; Holden, K.A.; Hardman, M.J. Animal models of wound repair: Are they cutting it? Exp. Dermatol. 2012, 21, 581–585. [Google Scholar] [CrossRef]
- Dusinska, M.; Tulinska, J.; El Yamani, N.; Kuricova, M.; Liskova, A.; Rollerova, E.; Rundén-Pran, E.; Smolkova, B. Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials: New strategies for toxicity testing? Food Chem. Toxicol. 2017, 109, 797–811. [Google Scholar] [CrossRef]
- Dorsett-Martin, W.A. Rat models of skin wound healing: A review. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef]
- Ito, M.; Cotsarelis, G. Is the Hair Follicle Necessary for Normal Wound Healing? J. Investig. Dermatol. 2008, 128, 1059–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sami, D.G.; Heiba, H.H.; Abdellatif, A. Wound Healing Models; A Systematic Review of Animal and Non-Animal Models. Wound Med. 2018, 24, 8–17. [Google Scholar] [CrossRef]
- FDA Wound Healing Clinical Focus Group. Guidance for Industry: Chronic Cutaneous Ulcer and Burn Wounds-Developing Products for Treatment. Available online: https://www.fda.gov/media/71278/download (accessed on 27 March 2020).
- Van Kilsdonk, J.W.; van den Bogaard, E.H.; Jansen, P.A.; Bos, C.; Bergers, M.; Schalkwijk, J. An in vitro wound healing model for evaluation of dermal substitutes. Wound Repair Regen. 2013, 21, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.; Mishra, A.K.; Ali, S.S.; Ahmad, A.; Abuzinadah, M.F.; Khan, N.A. In vivo models for assesment of wound healing potential: A systematic review. Wound Med. 2018, 20, 43–53. [Google Scholar] [CrossRef]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen. 2015, 23, 874–877. [Google Scholar] [CrossRef] [Green Version]
- Lanning, D.A.; Nwomeh, B.C.; Montante, S.J.; Yager, D.R.; Diegelmann, R.F.; Haynes, J.H. TGF-β1 alters the healing of cutaneous fetal excisional wounds. J. Pediatr. Surg. 1999, 34, 695–700. [Google Scholar] [CrossRef]
- Shailajan, S.; Menon, S.; Pednekar, S.; Singh, A. Wound healing efficacy of Jatyadi Taila: In vivo evaluation in rat using excision wound model. J. Ethnopharmacol. 2011, 138, 99–104. [Google Scholar] [CrossRef]
- Ansell, D.M.; Campbell, L.; Thomason, H.A.; Brass, A.; Hardman, M.J. A statistical analysis of murine incisional and excisional acute wound models. Wound Repair Regen. 2014, 22, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Mogford, J.E.; Tawil, B.; Jia, S.; Mustoe, T.A. Fibrin sealant combined with fibroblasts and platelet-derived growth factor enhance wound healing in excisional wounds. Wound Repair Regen. 2009, 17, 405–410. [Google Scholar] [CrossRef]
- Qian, L.W.; Fourcaudot, A.B.; Leung, K.P. Silver Sulfadiazine Retards Wound Healing and Increases Hypertrophic Scarring in a Rabbit Ear Excisional Wound Model. J. Burn Care Res. 2017, 38, e418–e422. [Google Scholar] [CrossRef]
- Ravishankar, K.; Kiranmayi, G.V.N.; Prasad, Y.R.; Devi, L. Wound healing activity in rabbits and antimicrobial activity of Hibiscus hirtus ethanolic extract. Braz. J Pharm. Sci. 2018, 54, e17075. [Google Scholar] [CrossRef] [Green Version]
- Mehdinezhad, B.; Rezaei, A.; Mohajeri, D.; Safarmashaei, S. Comparison of in-vivo wound healing activity of Verbascum thapsus flower extract with zinc oxide on experimental wound model in rabbits. Adv. Environ. Biol. 2011, 5, 1501–1509. [Google Scholar] [CrossRef]
- Singer, A.J.; McClain, S.A. Development of a porcine excisional wound model. Acad. Emerg. Med. 2003, 10, 1029–1033. [Google Scholar] [CrossRef]
- Wang, J.F.; Olson, M.E.; Reno, C.R.; Wright, J.B.; Hart, D.A. The pig as a model for excisional skin wound healing: Characterization of the molecular and cellular biology, and bacteriology of the healing process. Comp. Med. 2001, 51, 341–348. [Google Scholar]
- Beitz, A.J.; Newman, A.; Shepard, M.; Ruggles, T.; Eikmeier, L. A new rodent model of hind limb penetrating wound injury characterized by continuous primary and secondary hyperalgesia. J. Pain 2004, 5, 26–37. [Google Scholar] [CrossRef]
- Beitz, A.J.; Newman, A.; Kahn, A.R.; Ruggles, T.; Eikmeier, L. A polymeric membrane dressing with antinociceptive properties: Analysis with a rodent model of stab wound secondary hyperalgesia. J. Pain 2004, 5, 38–47. [Google Scholar] [CrossRef]
- Ziv-Polat, O.; Topaz, M.; Brosh, T.; Margel, S. Enhancement of incisional wound healing by thrombin conjugated iron oxide nanoparticles. Biomaterials 2010, 31, 741–747. [Google Scholar] [CrossRef]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef] [Green Version]
- Pereira, T.; dos Santos, D.; Lima-Ribeiro, M.H.M.; Pontes-Filho, D.; Teles, N.; Mdos, A.; Carneiro-Leão, A.; Tdos, M.; Correia, S. Development of animal model for studying deep second-degree thermal burns. BioMed Res. Int. 2012, 2012. [Google Scholar] [CrossRef]
- Calum, H.; Høiby, N.; Moser, C. Burn mouse models. Methods Mol. Biol. 2014, 1149, 793–802. [Google Scholar] [CrossRef]
- Friedrich, E.E.; Niknam-Bienia, S.; Xie, P.; Jia, S.X.; Hong, S.J.; Mustoe, T.A.; Galiano, R.D. Thermal injury model in the rabbit ear with quantifiable burn progression and hypertrophic scar. Wound Repair Regen. 2017, 25, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.-Y.; Wang, W.-L.; Fu, Y.-T.; Lin, S.-C.; Lei, Y.-C.; Liao, J.-H.; Yao, C.-H. The pig as an experimental model for mid-dermal burns research. Burns 2014, 40, 1679–1688. [Google Scholar] [CrossRef]
- Singer, A.J.; McClain, S.A.; Taira, B.R.; Romanov, A.; Rooney, J.; Zimmerman, T. Validation of a porcine comb burn model. Am. J. Emerg. Med. 2009, 27, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P. Burns as a model of SIRS. Front. Biosci. (Landmark Ed.) 2009, 14, 4962–4967. [Google Scholar] [CrossRef] [PubMed]
- Campelo, A.P.; Campelo, M.W.; Britto, G.A.; Ayala, A.P.; Guimaraes, S.B.; Vasconcelos, P.R. An optimized animal model for partial and total skin thickness burns studies. Acta Cir. Bras. 2011, 26, 38–42. [Google Scholar] [CrossRef]
- Zelt, R.G.; Daniel, R.K.; Ballard, P.A.; Brissette, Y.; Heroux, P. High-voltage electrical injury: Chronic wound evolution. Plast. Reconstr. Surg. 1988, 82, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Aguzzi, C.; Rossi, S.; Bonferoni, M.C.; Bruni, G.; Boselli, C.; Icaro Cornaglia, A.; Riva, F.; Viseras, C.; Caramella, C.M.; et al. Halloysite and chitosan oligosaccharide nanocomposite for wound healing. Acta Biomater. 2017, 57, 216–224. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Dellera, E.; Invernizzi, A.; Boselli, C.; Icaro Cornaglia, A.; Del Fante, C.; Perotti, C.; Vigani, B.; et al. Association of Alpha Tocopherol and Ag Sulfadiazine Chitosan Oleate Nanocarriers in Bioactive Dressings Supporting Platelet Lysate Application to Skin Wounds. Mar. Drugs 2018, 16, 56. [Google Scholar] [CrossRef] [Green Version]
- Branski, L.K.; Mittermayr, R.; Herndon, D.N.; Norbury, W.B.; Masters, O.E.; Hofmann, M.; Traber, D.L.; Redl, H.; Jeschke, M.G. A porcine model of full-thickness burn, excision and skin autografting. Burns 2008, 34, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ge, J.; Tredget, E.E.; Wu, Y. The Mouse Excisional Wound Splinting Model, Including Applications for Stem Cell Transplantation. Nat. Protoc. 2013, 8, 302–309. [Google Scholar] [CrossRef]
- Yao, Z.; Huang, Y.; Luo, G.; Wu, J.; He, W. A biological membrane-based novel excisional wound-splinting model in mice (With video). Burns Trauma 2014, 2, 196–200. [Google Scholar] [PubMed] [Green Version]
- Galiano, R.D.; Michaels, J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and Reproducible Murine Model of Excisional Wound Healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Michaels, J.; Churgin, S.S.; Blechman, K.M.; Greives, M.R.; Aarabi, S.; Galiano, R.D.; Gurtner, G.C. Db/Db Mice Exhibit Severe Wound-Healing Impairments Compared With Other Murine Diabetic Strains in a Silicone-Splinted Excisional Wound Model. Wound Repair Regen. 2007, 15, 665–670. [Google Scholar] [CrossRef]
- McLennan, S.V.; Bonner, J.; Milne, S.; Lo, L.; Charlton, A.; Kurup, S.; Jia, J.; Yue, D.K.; Twigg, S.M. The Anti-Inflammatory Agent Propolis Improves Wound Healing in a Rodent Model of Experimental Diabetes. Wound Repair Regen. 2008, 16, 706–713. [Google Scholar] [CrossRef]
- Gu, X.Y.; Shen, S.E.; Huang, C.F.; Liu, Y.N.; Chen, Y.C.; Luo, L.; Zeng, Y.; Wang, A.P. Effect of activated autologous monocytes/macrophages on wound healing in a rodent model of experimental diabetes. Diabetes Res. Clin. Pract. 2013, 102, 53–59. [Google Scholar] [CrossRef]
- Mendes, J.J.; Leandro, C.I.; Bonaparte, D.P.; Pinto, A.L. A Rat Model of Diabetic Wound Infection for the Evaluation of Topical Antimicrobial Therapies. Comp. Med. 2012, 62, 37–48. [Google Scholar]
- O’Loughlin, A.; Kulkarni, M.; Creane, M.; Vaughan, E.E.; Mooney, E.; Shaw, G.; Murphy, M.; Dockery, P.; Pandit, A.; O’Brien, T. Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes 2013, 62, 2588–2594. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, L.; Cai, X.; Wu, S.; Andersen, N.D.; Martin, M.; Malek, J.; Guthrie, P.; Veves, A.; Logerfo, F.W. Gene Expression of Pro-Inflammatory Cytokines and Neuropeptides in Diabetic Wound Healing. J. Surg. Res. 2011, 167, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Pradhan Nabzdyk, L.; Kuchibhotla, S.; Guthrie, P.; Chun, M.; Auster, M.E.; Nabzdyk, C.; Deso, S.; Andersen, N.; Gnardellis, C.; LoGerfo, F.W.; et al. Expression of neuropeptides and cytokines in a rabbit model of diabetic neuroischemic wound healing. J. Vasc. Surg. 2013, 58, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Velander, P.; Theopold, C.; Hirsch, T.; Bleiziffer, O.; Zuhaili, B.; Fossum, M.; Hoeller, D.; Gheerardyn, R.; Chen, M.; Visovatti, S.; et al. Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia. Wound Repair Regen. 2008, 16, 288–293. [Google Scholar] [CrossRef]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine Models of Cutaneous Wound Healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef]
- Adhirajan, N.; Shanmugasundaram, N.; Shanmuganathan, S.; Babu, M. Collagen-based Wound Dressing for Doxycycline Delivery: In-Vivo Evaluation in an Infected Excisional Wound Model in Rats. J. Pharm. Pharmacol. 2009, 61, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.V.; Demir, M.; Onem, G.; Goksin, I.; Baltalarli, A.; Topkara, V.K.; Kaleli, I. Topical versus systemic vancomycin for deep sternal wound infection caused by methicillin-resistant Staphylococcus aureus in a rodent experimental model. Tex. Heart Inst. J. 2006, 33, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Don Chae, J.; Kim, D.G.; Hong, S.H.; Lee, W.M.; Ki, M. Comparison of the Efficacies of Silver-Containing Dressing Materials for Treating a Full-Thickness Rodent Wound Infected by Methicillin-Resistant Staphylococcus Aureus. Korean J. Lab. Med. 2010, 30, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Seth, A.K.; Geringer, M.R.; Hong, S.J.; Leung, K.P.; Galiano, R.D.; Mustoe, T.A. Comparative analysis of single-species and polybacterial wound biofilms using a quantitative, in vivo, rabbit ear model. PLoS ONE 2012, 7, e42897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratford, A.F.; Zoutman, D.E.; Davidson, J.S.D. Effect of Lidocaine and Epinephrine on Staphylococcus Aureus in a Guinea Pig Model of Surgical Wound Infection. Plast. Reconstr. Surg. 2002, 110, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Svedman, P.; Ljungh, A.; Rausing, A.; Banck, G.; Sandén, G.; Miedzobrodzki, J.; Wadström, T. Staphylococcal Wound Infection in the Pig: Part I. Course. Ann. Plast. Surg. 1989, 23, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Nakamura, S.; Nambu, M.; Sasaki, K.; Yanagibayashi, S.; Azuma, R.; Kiyosawa, T. New model of radiation-induced skin ulcer in rats. J. Plast. Surg. Hand Surg. 2011, 45, 258–262. [Google Scholar] [CrossRef]
- Roessner, E.D.; Thier, S.; Hohenberger, P.; Schwarz, M.; Pott, P.; Dinter, D.; Smith, M. Acellular Dermal Matrix Seeded with Autologous Fibroblasts Improves Wound Breaking Strength in a Rodent Soft Tissue Damage Model in Neoadjuvant Settings. J. Biomater. Appl. 2009, 25, 413–427. [Google Scholar] [CrossRef]
- Fujita, K.; Nishimoto, S.; Fujiwara, T.; Sotsuka, Y.; Tonooka, M.; Kawai, K.; Kakibuchi, M. A new rabbit model of impaired wound healing in an X-ray-irradiated field. PLoS ONE 2017, 12, e0184534. [Google Scholar] [CrossRef] [Green Version]
- Bernatchez, S.F.; Parks, P.J.; Grussing, D.M.; Matalas, S.L.; Nelson, G.S. Histological characterization of a delayed wound healing model in pig. Wound Repair Regen. 1998, 6, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Udupa, A.L.; Udupa, S.L. Effect of Ixora coccinea flowers on dead space wound healing in rats. Fitoterapia 1999, 70, 233–236. [Google Scholar] [CrossRef]
- Deshmukh, P.T.; Fernandes, J.; Atul, A.; Toppoa, E. Wound healing activity of Calotropis gigantea root bark in rats. J. Ethnopharmacol. 2009, 125, 178–181. [Google Scholar] [CrossRef]
- Shivananda Nayak, B.; Isitor, G.; Davis, E.M.; Pillai, G.K. The Evidence Based Wound Healing Activity of Lawsonia Inermis Linn. Phytother. Res. 2007, 21, 827–831. [Google Scholar] [CrossRef]
- Suh, H.; Lee, A.-Y.; Park, E.J.; Hong, J.P. Negative Pressure Wound Therapy on Closed Surgical Wounds with Dead Space. Ann. Plast. Surg. 2016, 76, 717–722. [Google Scholar] [CrossRef] [Green Version]
- European Commission. MEDDEV 2.7/1, Revision 4. Clinical Evaluation: A Guide for Manufacturers and Notified Bodies under Directives 93/42/EEC and 90/385/EEC. 2016. Available online: http://ec.europa.eu/DocsRoom/documents/17522/attachments/1/translations/ (accessed on 24 August 2020).
- Zenner, H.P.; Božić, M. Clinical Evaluation of Medical Devices in Europe. In Personalized Medicine in Healthcare Systems, Legal, Medical and Economic Implications; Bodiroga-Vukobrat, N., Rukavina, D., Pavelic, K., Sander, G.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 21–32. [Google Scholar] [CrossRef]
- ISO 14155. Clinical Investigation of Medical Devices for Human Subjects—Good Clinical Practice; ICS: Waltham, MA, USA, 2011. [Google Scholar]
- European Commission. MEDDEV 2.7/3, Revision 3. Clinical Investigations: Serious Adverse Reporting under Directives 90/385/EEC and 93/42/EC. 2015. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjtkoL6n7TrAhUR2qQKHbucB3IQFjAAegQIAhAB&url=https%3A%2F%2Fec.europa.eu%2Fdocsroom%2Fdocuments%2F16477%2Fattachments%2F1%2Ftranslations%2Fen%2Frenditions%2Fnative&usg=AOvVaw1ZVXsWXv48Ye-Dcg-hMqPH (accessed on 25 August 2020).
- European Commission. MEDDEV 2.7/2, Revision 2. Guidelines for Competent Authorities for Making a Validation/Assessment of a Clinical Investigation Application under Directives 90/385/EEC and 93/42/EC. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwi6x7be97XrAhUR2aQKHSozD1AQFjACegQIAhAB&url=https%3A%2F%2Fec.europa.eu%2Fdocsroom%2Fdocuments%2F17522%2Fattachments%2F1%2Ftranslations%2Fen%2Frenditions%2Fnative&usg=AOvVaw1L6oboIQ-Y__oC1BjIiwhG (accessed on 24 August 2020).
- European Commission. MEDDEV 2.7/4. Guidelines on Clinical Investigations: A Guide for Manufacturers and Notified Bodies. 2010. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwicsKqDLXrAhUwNOwKHctMDukQFjAAegQIBRAB&url=https%3A%2F%2Fec.europa.eu%2Fdocsroom%2Fdocuments%2F10336%2Fattachments%2F1%2Ftranslations%2Fen%2Frenditions%2Fnative&usg=AOvVaw1jGES6UQzHEID0BVIhuL4Z (accessed on 24 August 2020).
- Fiedler, B.A.; David, Y. Reframing Product Life Cycle for Medical Devices. In Managing Medical Devices within a Regulatory Framework; Fiedler, B.A., Ed.; Elsevier: Amsterdam, Netherlands, 2017; pp. 3–16. [Google Scholar]







| Class | Risk | Examples |
|---|---|---|
| I | Low | Examination gloves, colostomy bags or oxygen masks |
| IIa | Low/medium | Hearing aids, urinary and peripheral vascular catheters |
| IIb | Medium/high | Ventilators and intensive care monitoring equipment |
| III | High | Implants, balloon catheters, pacemakers |
| Intended use | Clinical treatment of skin chronic wounds |
| Device description | Nanotechnology-based medical device |
| Expected efficacy | Tissue reparation with minimal scarring |
| Quality | Control of the inter-individual response |
| Contraindications | No serious adverse effect |
| Preclinical testing | Cell adhesion and proliferation onto the systems |
| Clinical testing | Therapeutic efficacy |
| QA | Description | Methods | References | |
|---|---|---|---|---|
| Physico-chemical chacterization | Chemical and solid-state assessment | Information on chemical characterization of materials and surfaces, allowable limits for leachable substances, degradation products, tolerable intake for extractable substances, ethylene oxide sterilization residuals, physical form and crystal or amorphous form, | UV-vis, HPLC, GC/LC- MS, XRD, NMR; XPS | ISO 10993-7, 12, 13, 14, 15, 17, 18, 19 |
| Stability | Information on chemical, physical stability (tendency to aggregation, growth of crystals, variation of solubility and dissolution), chemical–physical stability related to the characteristics of the solid state such as morphology, as well as the adsorption of components of the formulation, assessment of the photostability and identification of storage conditions | FTIR, XRD, UV-vis, HPLC, GC/LC- MS | ICH Q1A (R2), Q1C (R2) and Q5C guidelines | |
| Particle size/morphology | Information on the primary and secondary particle size and system morphology | SEM, TEM, XRD, DLS, AFM, NTA, TRPS | ISO 10993-1 | |
| Mechanical properties | Investigations on the biomechanical of the bioengineered matrix, and the properties that change when cells spread onto the system during its degradation. | Compressive, tensile and flexural tests | ISO 6892-1, ISO 178:2019 | |
| Surface properties | Information on zeta potential of the medical device | LDV-PALS, Electrokinetic analyzer for solid surface analysis | ISO 10993-18 | |
| Release of nanomaterials | Evaluation of the nanomaterial release from a medical device | Currently, a robust methodology, especially for the measurements of low level release of nanomaterials, is lacking. | ISO 10993-18 | |
| Sterility and apirogenicity | Application of different methods of sterilization to investigate which is the most suitable method to ensure the SAL (sterility assurance level), and evaluation of apirogenicity | Steam and dry heat sterilization, ethylene oxide sterilization, LAL test | European and United State Ph. | |
| Biological characterizations | Biocompatibility | Evaluation of cell damage, the effect on cell growth, and specific aspects of cellular metabolism altered following direct or indirect exposure with medical devices. | MTT assay, NRU cytotoxicity test, colony formation cytotoxicity test and XTT cytotoxicity test | ISO 10993-5 |
| Skin irritation | Evaluation of local inflammatory response to single, repeated and continuous applications of the test material, without an immune mechanism | Draize rabbit skin | ISO 10993-10 | |
| Skin sensitization | Evaluation of the medical device potential to cause a sensitizing effect or an allergenic reaction | Buehler test, guinea pig maximization, LLNA assay | ISO 10993-10 | |
| Medical Devices | Biological Effect | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contact | Contact Duration | C | S | I/IR | ST | S/ST | G | I | H | CT | CG | R/DT | |
| Surface MD | Skin | Limited | X | X | X | ||||||||
| Prolonged | X | X | X | ||||||||||
| Permanent | X | X | X | ||||||||||
| Mucosal Membranes | Limited | X | X | X | |||||||||
| Prolonged | X | X | X | ||||||||||
| Permanent | X | X | X | X | X | X | |||||||
| Breached/ compromised surface | Limited | X | X | X | |||||||||
| Prolonged | X | X | X | ||||||||||
| Permanent | X | X | X | X | X | X | |||||||
| Ext. communicating MD | Blood path indirect | Limited | X | X | X | X | X | ||||||
| Prolonged | X | X | X | X | X | ||||||||
| Permanent | X | X | X | X | X | X | X | X | X | ||||
| Tissue, bone and dentin | Limited | X | X | X | |||||||||
| Prolonged | X | X | X | X | X | X | X | X | |||||
| Permanent | X | X | X | X | X | X | X | X | X | X | |||
| Circulating blood | Limited | X | X | X | X | X | |||||||
| Prolonged | X | X | X | X | X | X | X | X | X | ||||
| Permanent | X | X | X | X | X | X | X | X | X | X | X | ||
| Implant MD | Bone, tissue | Limited | X | X | X | ||||||||
| Prolonged | X | X | X | X | X | X | X | X | |||||
| Permanent | X | X | X | X | X | X | X | X | X | X | |||
| Blood | Limited | X | X | X | X | X | X | X | X | ||||
| Prolonged | X | X | X | X | X | X | X | X | X | ||||
| Permanent | X | X | X | X | X | X | X | X | X | X | X | ||
| Model Injury | Animal | Method |
|---|---|---|
| Excisional wound | - Rodent [144,145,146,147] - Rabbit [148,149,150,151] - Pig [152,153] | Full-thickness circular excision |
| Incisional wound | - Rodent [143,154,155,156] | Linear/longitudinal incision |
| Burn wound | - Rodent [157,158,159] - Rabbit [160] - Pig [161,162] | Contact of skin with a heated metal [163,164], electricity [165], and heated water [163] |
| Burn/excisional wound | - Rodent [52,166,167] - Pig [168] | Contact of the skin with a hot device followed by full-thickness lesion |
| Excisional wound splinting | - Rodent [169,170,171] | Full-thickness excisional wounds followed by application of a splinting ring tightly around the wound, to inhibit wound skin contraction |
| Diabetic wound | - Rodent [172,173,174,175] - Rabbit [176,177,178] - Pig [179,180] | Transgenic db/db mice or induction of diabetes (Alloxan, Streptozotocin) followed by full thickness excisional wound |
| Infected Model | - Rodent [181,182,183] - Rabbit [184] - Pig [185,186] | Full-thickness wound followed by inoculation of bacteria (P. aeruginosa, S. aureus, S. hyicus) |
| Radiation-induced ulcer | - Rodent [187,188] - Rabbit [189] - Pig [190] | Radiation exposure followed by full-thickness excisional lesion |
| Dead space wound | - Rodent [191,192,193] - Pig [194] | Subcutaneous implantation of polypropylene tubes [109,110] below the skin |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggeri, M.; Bianchi, E.; Rossi, S.; Vigani, B.; Bonferoni, M.C.; Caramella, C.; Sandri, G.; Ferrari, F. Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic. Pharmaceutics 2020, 12, 815. https://doi.org/10.3390/pharmaceutics12090815
Ruggeri M, Bianchi E, Rossi S, Vigani B, Bonferoni MC, Caramella C, Sandri G, Ferrari F. Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic. Pharmaceutics. 2020; 12(9):815. https://doi.org/10.3390/pharmaceutics12090815
Chicago/Turabian StyleRuggeri, Marco, Eleonora Bianchi, Silvia Rossi, Barbara Vigani, Maria Cristina Bonferoni, Carla Caramella, Giuseppina Sandri, and Franca Ferrari. 2020. "Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic" Pharmaceutics 12, no. 9: 815. https://doi.org/10.3390/pharmaceutics12090815
APA StyleRuggeri, M., Bianchi, E., Rossi, S., Vigani, B., Bonferoni, M. C., Caramella, C., Sandri, G., & Ferrari, F. (2020). Nanotechnology-Based Medical Devices for the Treatment of Chronic Skin Lesions: From Research to the Clinic. Pharmaceutics, 12(9), 815. https://doi.org/10.3390/pharmaceutics12090815