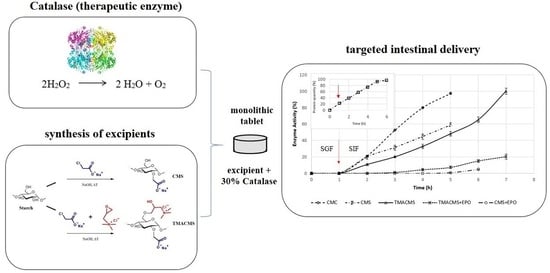
Design of Catalase Monolithic Tablets for Intestinal Targeted Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Starch Derivatives
2.2.2. Characterization of Polymeric Powders
2.2.3. Degree of Substitution (DS)
2.2.4. Fourier-Transform Infrared (FTIR)
2.2.5. X-ray Diffraction
2.2.6. Preparation of Tablets and Their Characterization in the Dry Phase
2.2.7. Determination of the Fluid Uptake and Erosion
2.2.8. In Vitro Dissolution Tests
2.2.9. Catalase Activity
2.2.10. Protein Dosage
3. Results and Discussion
3.1. Characterization of Excipients
3.2. Formulation Studies
3.3. Gastroprotection
3.4. Fluid Uptake, Swelling, and Erosion
3.5. In Vitro Dissolution Assays
3.6. Commercial Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbr. | Expansion |
| CAT | Catalase |
| CD | Crohn’s disease |
| CMC | Sodium CarboxyMethylCellulose |
| CMS | CarboxyMethylStarch |
| DS | Degree of substitution |
| DR | Delayed Release |
| FTIR | Fourier-transform infrared |
| GTMAC | Glycidyltrimethylammonium |
| IBD | Inflammatory bowel disease |
| MIF | Migration inhibitory factor |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| SMCA | Sodium monochloroacetate |
| TGA | Thermogravimetric analyses |
| TMACMS | TriMethylAmmoniumCarboxyMethylStarch |
| ROI | Reactive oxygen intermediates |
| UC | Ulcerative colitis |
References
- Strus, M.; Gosiewski, T.; Fyderek, K.; Wedrychowicz, A.; Kowalska-Duplaga, K.; Kochan, P.; Adamski, P.; Heczko, P. A role of hydrogen peroxide producing commensal bacteria present in colon of adolescents with inflammatory bowel disease in perpetuation of the inflammatory process. J. Physiol. Pharm. 2009, 60, 49–54. [Google Scholar]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prokopidis, K.; Cervo, M.M.; Gandham, A.; Scott, D. Impact of Protein Intake in Older Adults with Sarcopenia and Obesity: A Gut Microbiota Perspective. Nutrients 2020, 12, 2285. [Google Scholar] [CrossRef] [PubMed]
- Basu Thakur, P.; Long, A.R.; Nelson, B.J.; Kumar, R.; Rosenberg, A.F.; Gray, M.J. Complex Responses to Hydrogen Peroxide and Hypochlorous Acid by the Probiotic Bacterium Lactobacillus reuteri. mSystems 2019, 4, e00453-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera-Chávez, F.; Lopez, C.A.; Bäumler, A.J. Oxygen as a driver of gut dysbiosis. Free Radical. Biol. Med. 2017, 105, 93–101. [Google Scholar] [CrossRef]
- Pérez, S.; Taléns-Visconti, R.; Rius-Pérez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003, 201, 28–36. [Google Scholar] [CrossRef]
- Schreck, R.; Baeuerle, P.A. Assessing oxygen radicals as mediators in activation of inducible eukaryotic transcription factor NF-kappa B. Methods Enzymol. 1994, 234, 151–163. [Google Scholar]
- Cao, W.-G.; Morin, M.; Metz, C.; Maheux, R.; Akoum, A. Stimulation of Macrophage Migration Inhibitory Factor Expression in Endometrial Stromal Cells by Interleukin 1, beta Involving the Nuclear Transcription Factor NFκB1. Biol. Reprod. 2005, 73, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Pricolo, V.E.; Zhang, L.; Behar, J.; Biancani, P.; Kirber, M.T. Gq-linked NK2 receptors mediate neurally induced contraction of human sigmoid circular smooth muscle. Gastroenterology 2000, 119, 51–61. [Google Scholar] [CrossRef]
- Chappell, A.E.; Bunz, M.; Smoll, E.; Dong, H.; Lytle, C.; Barrett, K.E.; McCole, D.F. Hydrogen peroxide inhibits Ca2+-dependent chloride secretion across colonic epithelial cells via distinct kinase signaling pathways and ion transport proteins. FASEB J. 2008, 22, 2023–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everse, J. Heme Proteins. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 532–538. [Google Scholar]
- Gagnière, J.; Bonnet, M. Chapter 15—Molecular Mechanism Underlying the Actions of Antioxidant Molecules in Digestive Disorders. In Gastrointestinal Tissu; Gracia-Sancho, J., Salvadó, J., Eds.; Elsevier: London, UK; Academic Press: London, UK, 2017; pp. 197–216. [Google Scholar]
- Iborra, M.; Moret, I.; Rausell, F.; Bastida, G.; Aguas, M.; Cerrillo, E.; Nos, P.; Beltrán, B. Role of oxidative stress and antioxidant enzymes in Crohn’s disease. Biochem. Soc. Trans. 2011, 39, 1102–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trang, T.; Chan, J.; Graham, D.Y. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency in the 21(st) century. World J. Gastroenterol. 2014, 20, 11467–11485. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, I.P.; Gourmel, C.; Berkovska, O.; Burger, M.; Leroux, J.-C. A microparticulate based formulation to protect therapeutic enzymes from proteolytic digestion: Phenylalanine ammonia lyase as case study. Sci. Rep. 2020, 10, 3651. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Leroux, J.C. Improving the stability and activity of oral therapeutic enzymes-recent advances and perspectives. Pharm. Res. 2014, 31, 1099–1105. [Google Scholar] [CrossRef]
- Lapuhs, P.; Fuhrmann, G. Engineering Strategies for Oral Therapeutic Enzymes to Enhance Their Stability and Activity. Adv. Exp. Med. Biol. 2019, 1148, 151–172. [Google Scholar]
- Chung, B.Y.; Choi, S.M.; Roh, T.H.; Lim, D.S.; Ahn, M.Y.; Kim, Y.J.; Kim, H.S.; Lee, B.M. Risk assessment of phthalates in pharmaceuticals. J. Toxicol. Environ. Health Part A 2019, 82, 351–360. [Google Scholar] [CrossRef]
- Kang, J.-H.; Hwang, J.-Y.; Seo, J.-W.; Kim, H.-S.; Shin, U.S. Small intestine- and colon-specific smart oral drug delivery system with controlled release characteristic. Mater. Sci. Eng. C 2018, 91, 247–254. [Google Scholar] [CrossRef]
- Calinescu, C.; Mondovi, B.; Federico, R.; Ispas-Szabo, P.; Mateescu, M.A. Carboxymethyl starch: Chitosan monolithic matrices containing diamine oxidase and catalase for intestinal delivery. Int. J. Pharm. 2012, 428, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Calinescu, C.; Mulhbacher, J.; Nadeau, E.; Fairbrother, J.M.; Mateescu, M.A. Carboxymethyl high amylose starch (CM-HAS) as excipient for Escherichia coli oral formulations. Eur. J. Pharm. Biopharm. 2005, 60, 53–60. [Google Scholar] [CrossRef]
- Calinescu, C.; Nadeau, E.; Mulhbacher, J.; Fairbrother, J.M.; Mateescu, M.A. Carboxymethyl high amylose starch for F4 fimbriae gastro-resistant oral formulation. Int. J. Pharm. 2007, 343, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sakeer, K.; Ispas-Szabo, P.; Benyerbah, N.; Mateescu, M.A. Ampholytic starch excipients for high loaded drug formulations: Mechanistic insights. Int. J. Pharm. 2018, 535, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Benyerbah, N.; Ispas-Szabo, P.; Sakeer, K.; Chapdelaine, D.; Mateescu, M.A. Ampholytic and Polyelectrolytic Starch as Matrices for Controlled Drug Delivery. Pharmaceutics 2019, 11, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric Systems for Controlled Drug Release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef]
- Stojanović, Ž.; Jeremić, K.; Jovanović, S.; Lechner, M.D. A Comparison of Some Methods for the Determination of the Degree of Substitution of Carboxymethyl Starch. Starch Stärke 2005, 57, 79–83. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–524. [Google Scholar] [CrossRef]
- Labelle, M.A.; Ispas-Szabo, P.; Masseau, I.; Chorfi, Y.; Mateescu, M.A. In vivo evaluation of targeted delivery of biological agents using barium sulfate. Int. J. Pharm. 2019, 572, 118801. [Google Scholar] [CrossRef]
- Sakeer, K.; Scorza, T.; Romero, H.; Ispas-Szabo, P.; Mateescu, M.A. Starch materials as biocompatible supports and procedure for fast separation of macrophages. Carbohydr. Polym. 2017, 163, 108–117. [Google Scholar] [CrossRef]
- Ispas-Szabo, P.; De Koninck, P.; Calinescu, C.; Mateescu, M.A. Carboxymethyl Starch Excipients for Drug Chronodelivery. AAPS PharmSciTech 2017, 18, 1673–1682. [Google Scholar] [CrossRef]
- Mulhbacher, J.; Ispas-Szabo, P.; Lenaerts, V.; Mateescu, M.A. Cross-linked high amylose starch derivatives as matrices for controlled release of high drug loadings. J. Control. Release 2001, 76, 51–58. [Google Scholar] [CrossRef]
- Labelle, M.-A.; Ispas-Szabo, P.; Mateescu, M.A. Structure-Functions Relationship of Modified Starches for Pharmaceutical and Biomedical Applications. Starch Stärke 2020, 72, 2000002. [Google Scholar] [CrossRef]
- Ispas-Szabo, P.; Ravenelle, F.; Hassan, I.; Preda, M.; Mateescu, M.A. Structure–properties relationship in cross-linked high-amylose starch for use in controlled drug release. Carbohydr. Res. 2000, 323, 163–175. [Google Scholar] [CrossRef]
- Mateescu, M.A.; Ispas-Szabo, P.; Assaad, E. Starch and derivatives as pharmaceutical excipients: From nature to pharmacy. In Controlled Drug Delivery the Role of Self-Assembling Multi-Task Excipients, 1st ed.; Woodhead Publishing: Oxford, UK; Elsevier: Oxford, UK, 2015; pp. 21–84. [Google Scholar]
- Moustafine, R.I.; Bukhovets, A.V.; Sitenkov, A.Y.; Kemenova, V.A.; Rombaut, P.; Van den Mooter, G. Eudragit E PO as a Complementary Material for Designing Oral Drug Delivery Systems with Controlled Release Properties: Comparative Evaluation of New Interpolyelectrolyte Complexes with Countercharged Eudragit L100 Copolymers. Mol. Pharm. 2013, 10, 2630–2641. [Google Scholar] [CrossRef]
- Xie, T.; Gao, W.; Taylor, L.S. Impact of Eudragit EPO and hydroxypropyl methylcellulose on drug release rate, supersaturation, precipitation outcome and redissolution rate of indomethacin amorphous solid dispersions. Int. J. Pharm. 2017, 531, 313–323. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. Rapid kinetic microassay for catalase activity. J. Biomol. Tech. 2007, 18, 185–187. [Google Scholar]










| Formulation | CAT Load (% w/w) | Table Weight (mg) | Dimensions of Dry Tables | Hardness (N) | Time of Complete Disintegration in SIF | ||
|---|---|---|---|---|---|---|---|
| Dimeter (mm) | Thickness (mm) | ||||||
| 1 | CMC + CAT | 30 | 300 | 9.65 ± 0.11 | 3.17 ± 0.07 | 103.8 ± 0.5 | 3–4 h |
| 2 | CMS + CAT | 30 | 300 | 9.52 ± 0.12 | 2.91 ± 0.05 | >150 | 3–4 h |
| 3 | TMACMS + CAT | 30 | 300 | 9.63 ± 0.19 | 2.84 ± 0.08 | >150 | >5 h |
| 4 | CMC + CAT | 30 | 500 | 13.21 ± 0.15 | 3.06 ± 0.06 | 99.6 ± 0.03 | 3–4 h |
| 5 | CMC + CAT | 30 | 500 | 12.92 ± 0.17 | 2.63 ± 0.07 | >150 | 3–4 h |
| 6 | TMACMS + CAT | 30 | 500 | 12.93 ±0.15 | 2.67 ± 0.05 | >150 | >5 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alothman, M.; Ispas-Szabo, P.; Mateescu, M.A. Design of Catalase Monolithic Tablets for Intestinal Targeted Delivery. Pharmaceutics 2021, 13, 69. https://doi.org/10.3390/pharmaceutics13010069
Alothman M, Ispas-Szabo P, Mateescu MA. Design of Catalase Monolithic Tablets for Intestinal Targeted Delivery. Pharmaceutics. 2021; 13(1):69. https://doi.org/10.3390/pharmaceutics13010069
Chicago/Turabian StyleAlothman, Mirna, Pompilia Ispas-Szabo, and Mircea Alexandru Mateescu. 2021. "Design of Catalase Monolithic Tablets for Intestinal Targeted Delivery" Pharmaceutics 13, no. 1: 69. https://doi.org/10.3390/pharmaceutics13010069
APA StyleAlothman, M., Ispas-Szabo, P., & Mateescu, M. A. (2021). Design of Catalase Monolithic Tablets for Intestinal Targeted Delivery. Pharmaceutics, 13(1), 69. https://doi.org/10.3390/pharmaceutics13010069