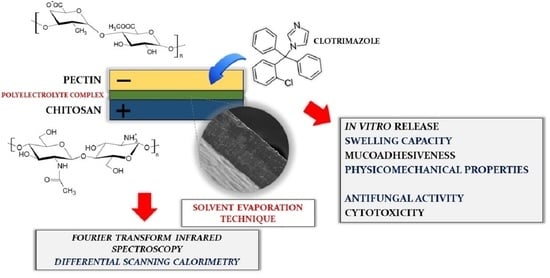
Multilayer Films Based on Chitosan/Pectin Polyelectrolyte Complexes as Novel Platforms for Buccal Administration of Clotrimazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Films
2.3. Evaluation of Films Thickness and Weight Variations
2.4. pH Analysis
2.5. Moisture Content
2.6. High-Performance Liquid Chromatography (HPLC) Analysis of CLO
2.7. Determination of Drug Content
2.8. Physicomechanical Analysis
2.9. Scanning Electron Microscopy (SEM)
2.10. Differential Scanning Calorimetry (DSC) Analysis
2.11. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR–FTIR)
2.12. Zeta Potential Measurement
2.13. In Vitro Release Study
2.14. Swelling and Disintegration Study
2.15. Erosion Test
2.16. Determination of Mucoadhesiveness
2.17. Antifungal Activity Test
2.18. Cytotoxicity Tests
2.18.1. Cells
2.18.2. Preparation of Samples
2.18.3. MTT Assay
2.18.4. [3H]–Thymidine Incorporation (DNA Biosynthesis Assay)
2.18.5. ROS Production Rate
2.18.6. Real-Time-GloTM Annexin V Assay
2.19. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Films with Regard to Thickness, Weight Uniformity, Microenvironmental pH, Moisture, and Drug Content
3.2. Physicomechanical Evaluation of Films
3.3. Morphology of Films
3.4. Thermal Behavior
3.5. FTIR Analysis
3.6. Zeta Potential
3.7. In Vitro Release of CLO
3.8. Swelling Performance
3.9. Susceptibility of the Films to Erosion
3.10. Mucoadhesive Properties
3.11. Antifungal Activity
3.12. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapall, V.R.M.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Potaś, J.; Szymańska, E.; Winnicka, K. Challenges in developing of chitosan–based polyelectrolyte complexes as a platform for mucosal and skin drug delivery. Eur. Polym. J. 2020, 140, 110020. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef] [Green Version]
- Szymańska, E.; Winnicka, K. Stability of chitosan–a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Quindós, G.; Gil–Alonso, S.; Marcos–Arias, C.; Sevillano, E.; Mateo, E.; Jauregizar, N.; Eraso, E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e172–e180. [Google Scholar] [CrossRef] [PubMed]
- Balata, G.; Mahdi, M.; Bakera, R.A. Improvement of solubility and dissolution properties of clotrimazole by solid dispersions and inclusion complexes. Indian J. Pharm. Sci. 2011, 73, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drugbank.ca. 2021. Available online: https://go.drugbank.com/drugs/DB00257 (accessed on 4 July 2021).
- Qiu, W.; Ren, B.; Dai, H.; Zhang, L.; Zhang, Q.; Zhou, X.; Li, Y. Clotrimazole and econazole inhibit Streptococcus mutans biofilm and virulence in vitro. Arch. Oral Biol. 2017, 73, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y.; Li, W.; Gao, P.; Xiang, D.; Ren, X.; Liu, D. Mucoadhesive buccal film containing ornidazole and dexamethasone for oral ulcers: In vitro and in vivo studies. Pharm. Dev. Technol. 2019, 24, 118–126. [Google Scholar] [CrossRef]
- Alves, T.F.R.; Rios, A.C.; da Silva Pontes, K.; Portella, D.L.; Aranha, N.; Severino, P.; Souto, E.B.; Gonsalves, J.K.M.; de Souza Nunes, R.; Chaud, M.V. Bilayer mucoadhesive buccal film for mucosal ulcers treatment: Development, characterization, and single study case. Pharmaceutics 2020, 12, 657. [Google Scholar] [CrossRef]
- Mady, O.Y.; Donia, A.M.; Al–Madboly, L.A. Miconazole–urea in a buccal film as a new trend for treatment of resistant mouth fungal white patches. Front. Microbiol. 2018, 9, 837. [Google Scholar] [CrossRef] [Green Version]
- Rogawski, M.A.; Heller, A.H. Diazepam buccal film for the treatment of acute seizures. Epilepsy Behav. 2019, 101 Pt B, 106537. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.B.; Shah, J.; Jacob, S.; Al–Dhubiab, B.E.; Patel, V.; Sreeharsha, N.; Shinu, P. Development of mucoadhesive buccal film for rizatriptan: In vitro and in vivo evaluation. Pharmaceutics 2021, 13, 728. [Google Scholar] [CrossRef]
- Giordani, B.; Abruzzo, A.; Prata, C.; Nicoletta, F.P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Ondansetron buccal administration for paediatric use: A comparison between films and wafers. Int. J. Pharm. 2020, 580, 119228. [Google Scholar] [CrossRef]
- Gajdošová, M.; Vetchý, D.; Muselík, J.; Gajdziok, J.; Juřica, J.; Vetchá, M.; Hauptman, K.; Jekl, V. Bilayer mucoadhesive buccal films with prolonged release of ciclopirox olamine for the treatment of oral candidiasis: In vitro development, ex vivo permeation testing, pharmacokinetic and efficacy study in rabbits. Int. J. Pharm. 2021, 592, 120086. [Google Scholar] [CrossRef] [PubMed]
- Kononova, S.V.; Kruchinina, E.V.; Petrova, V.A.; Baklagina, Y.G.; Klechkovskaya, V.V.; Orekhov, A.S.; Vlasova, E.N.; Popova, E.N.; Gubanova, G.N.; Skorik, Y.A. Pervaporation membranes of a simplex type with polyelectrolyte layers of chitosan and sodium hyaluronate. Carbohydr. Polym. 2019, 209, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Barroso, G.N.; Guaresti, O.; Perez, L.; Ruiz–Rubio, L.; Gabilondo, N.; Vilas, J. Self–healable hyaluronic acid/chitosan polyelectrolyte complex hydrogels and multilayers. Eur. Polym. J. 2019, 120, 109268. [Google Scholar] [CrossRef]
- Choi, D.; Hong, J. Layer–by–layer assembly of multilayer films for controlled drug release. Arch. Pharm. Res. 2014, 37, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Das, B.P.; Tsianou, M. From polyelectrolyte complexes to polyelectrolyte multilayers: Electrostatic assembly, nanostructure, dynamics, and functional properties. Adv. Colloid Interface Sci. 2017, 244, 71–89. [Google Scholar] [CrossRef]
- Petrila, L.-M.; Bucatariu, F.; Mihai, M.; Teodosiu, C. Polyelectrolyte multilayers: An overview on fabrication, properties, and biomedical and environmental applications. Materials 2021, 14, 4152. [Google Scholar] [CrossRef]
- Montenegro–Nicolini, M.; Reyes, P.E.; Jara, M.O.; Vuddanda, P.R.; Neira–Carrillo, A.; Butto, N.; Velaga, S.; Morales, J.O. The effect of inkjet printing over polymeric films as potential buccal biologics delivery systems. AAPS PharmSciTech. 2018, 19, 3376–3387. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D.; Andreadis, D.A.; Boetker, J.; Rantanen, J.; Fatouros, D.G. Unidirectional drug release from 3D printed mucoadhesive buccal films using FDM technology: In vitro and ex vivo evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef]
- Petrova, V.A.; Chernyakov, D.D.; Poshina, D.N.; Gofman, I.V.; Romanov, D.P.; Mishanin, A.I.; Golovkin, A.S.; Skorik, Y.A. Electrospun bilayer chitosan/hyaluronan material and its compatibility with mesenchymal stem cells. Materials 2019, 12, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimling, B.; Karolewicz, B.; Nawrot, U.; Włodarczyk, K.; Górniak, A. Physicochemical and antifungal properties of clotrimazole in combination with high–molecular weight chitosan as a multifunctional excipient. Mar. Drugs 2020, 18, 591. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, F.; Zerrouk, N.; Chemtob, C.; Mura, P. Influence of chitosan and its glutamate and hydrochloride salts on naproxen dissolution rate and permeation across Caco–2 cells. Int. J. Pharm. 2004, 271, 257–267. [Google Scholar] [CrossRef]
- Shete, A.S.; Yadav, A.V.; Murthy, S.M. Chitosan and chitosan chlorhydrate based various approaches for enhancement of dissolution rate of carvedilol. DARU J. Pharm. Sci. 2012, 20, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.R.; Naresh, L.; Dhuldhoya, N.C.; Merchant, S.U. An overview on pectins. Times Food Process. J. 2006, 23, 44–51. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan–present status and application. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Domján, A.; Bajdik, J.; Pintye–Hódi, K. Understanding of the plasticizing effects of glycerol and PEG 400 on chitosan films using solid–state NMR spectroscopy. Macromolecules 2009, 42, 4667–4673. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Löbenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Tech. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Mishra, R.; Soni, K.; Mehta, T. Mucoadhesive vaginal film of fluconazole using cross–linked chitosan and pectin. J. Therm. Anal. Calorim. 2017, 130, 1683–1695. [Google Scholar] [CrossRef]
- Martins, J.G.; de Oliveira, A.C.; Garcia, P.S.; Kipper, M.J.; Martins, A.F. Durable pectin/chitosan membranes with self–assembling, water resistance and enhanced mechanical properties. Carbohydr. Polym. 2018, 188, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jain, S.; Muthu, M.S.; Tiwari, S.; Tilak, R. Preparation and evaluation of buccal bioadhesive films containing clotrimazole. AAPS Pharm. Sci. Tech. 2008, 9, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Sosnowska, K.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Poly(amidoamine) dendrimers increase antifungal activity of clotrimazole. Biol. Pharm. Bull. 2011, 34, 1129–1133. [Google Scholar] [CrossRef] [Green Version]
- Timur, S.S.; Yüksel, S.; Akca, G.; Şenel, S. Localized drug delivery with mono and bilayered mucoadhesive films and wafers for oral mucosal infections. Int. J. Pharm. 2019, 559, 102–112. [Google Scholar] [CrossRef]
- Almeida, N.; de Lima, R.; Alves, T.; Rebelo, M.A.; Severino, P.; Chaud, M.V. A novel dosage form for buccal administration of bupropion. Braz. J. Pharm. Sci. 2015, 51, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Sakloetsakun, D.; Preechagoon, D.; Bernkop–Schnürch, A.; Pongjanyakul, T. Chitosan–gum arabic polyelectrolyte complex films: Physicochemical, mechanical and mucoadhesive properties. Pharm. Dev. Technol. 2016, 21, 590–599. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al–Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef]
- Centkowska, K.; Ławrecka, E.; Sznitowska, M. Technology of orodispersible polymer films with micronized loratadine–influence of different drug loadings on film properties. Pharmaceutics 2020, 12, 250. [Google Scholar] [CrossRef] [Green Version]
- Senthilkumar, K.; Vijaya, C. Formulation development of mouth dissolving film of etoricoxib for pain management. Adv. Pharm. 2015, 2015, 702963. [Google Scholar] [CrossRef]
- Nafee, N.A.; Boraie, M.A.; Ismail, F.A.; Mortada, L.M. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta Pharm. 2003, 53, 199–212. [Google Scholar]
- Potaś, J.; Szymańska, E.; Basa, A.; Hafner, A.; Winnicka, K. Tragacanth gum/chitosan polyelectrolyte complexes–based hydrogels enriched with xanthan gum as promising materials for buccal application. Materials 2021, 14, 86. [Google Scholar] [CrossRef]
- United States Pharmacopoeia and National Formulary (USP 41–NF 36). United States Pharmacopoeial Convention; The United States Pharmacopeia: Rockville, MD, USA, 2016. [Google Scholar]
- Yüksel, N.; Dinç, E.; Onur, F.; Baykara, T. Influence of swelling degree on release of nicardipine hydrochloride from acrylic microspheres prepared by solvent evaporation method. Pharm. Dev. Technol. 1998, 3, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Wróblewska, M.; Trofimiuk, M.; Basa, A.; Winnicka, K. Alginate oligosaccharides affect mechanical properyties and antifungal activity of alginate buccal films with posaconazole. Mar. Drugs 2019, 17, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, J.H. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI Document M27–A3; Clinical & Laboratory Standards Institute: Wayne, IL, USA, 2008. [Google Scholar]
- Wróblewska, M.; Szymańska, E.; Szekalska, M.; Winnicka, K. Different types of gel carriers as metronidazole delivery systems to the oral mucosa. Polymers 2020, 12, 680. [Google Scholar] [CrossRef] [Green Version]
- Chronopoulou, L.; Nocca, G.; Castagnola, M.; Paludetti, G.; Ortaggi, G.; Sciubba, F.; Bevilacqua, M.; Lupi, A.; Gambarini, G.; Palocci, C. Chitosan based nanoparticles functionalized with peptidomimetic derivatives for oral drug delivery. New Biotechnol. 2016, 33, 23–31. [Google Scholar] [CrossRef]
- Priprem, A.; Damrongrungruang, T.; Limsitthichaikoon, S.; Khampaenjiraroch, B.; Nukulkit, C.; Thapphasaraphong, S.; Limphirat, W. Topical niosome gel containing an anthocyanin complex: A potential oral wound healing in rats. AAPS Pharm. Sci. Tech. 2018, 19, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Use of International Standard ISO 10993–1, “Biological Evaluation of Medical Devices–Part 1: Evaluation and Testing within a Risk Management Process”, Guidance for Industry and Food and Drug Administration Staff; Center for Devices and Radiological Health: Rockville, MD, USA, 2020.
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic colorimetric proliferation assays: MTT, WST, and Resazurin. In BT–Cell Viability Assays: Methods and Protocols; Gilbert, D.F., Friedrich, O., Eds.; Springer: New York, NY, USA, 2017; pp. 1–17. [Google Scholar]
- Griffiths, M.; Sundaram, H. Drug design and testing: Profiling of antiproliferative agents for cancer therapy using a cell–based methyl–[3H]–thymidine incorporation assay. In BT–Cancer Cell Culture: Methods and Protocols; Cree, I.A., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 451–465. [Google Scholar]
- Kuan, Y.H.; Huang, F.M.; Li, Y.C.; Chang, Y.C. Proinflammatory activation of macrophages by bisphenol A–glycidyl–methacrylate involved NFκB activation via PI3K/Akt pathway. Food Chem. Toxicol. 2012, 50, 4003–4009. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Szarmach, I.; Żendzian–Piotrowska, M.; Maciejczyk, M. The effect of N–acetylcysteine on respiratory enzymes, ADP/ATP ratio, glutathione metabolism, and nitrosative stress in the salivary gland mitochondria of insulin resistant rats. Nutrients 2020, 12, 458. [Google Scholar] [CrossRef] [Green Version]
- Maciel, V.B.V.; Yoshida, C.M.P.; Franco, T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Norcino, L.B.; de Oliveira, J.E.; Moreira, F.K.V.; Marconcini, J.M.; Mattoso, L.H.C. Rheological and thermos–mechanical evaluation of bio–based chitosan/pectin blends with tunable ionic cross–linking. Int. J. Biol. Macromol. 2018, 118, 1817–1823. [Google Scholar] [CrossRef]
- Tejada, G.; Barrera, M.G.; Piccirilli, G.N.; Sortino, M.; Frattini, A.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Development and evaluation of buccal films based on chitosan for the potential treatment of oral candidiasis. AAPS Pharm. Sci. Tech. 2017, 18, 936–946. [Google Scholar] [CrossRef]
- Pires, A.L.R.; de Azevedo Motta, L.; Dias, A.M.A.; de Sousa, H.C.; Moraes, A.M.; Braga, M.E.M. Towards wound dressings with improved properties: Effects of poly(dimethylsiloxane) on chitosan–alginate films loaded with thymol and beta–carotene. Mater. Sci. Eng. C 2018, 93, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Tejada, G.; Piccirilli, G.N.; Sortino, M.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Formulation and in vitro efficacy of antifungal mucoadhesive polymeric matrices for the delivery of miconazole nitrate. Mater. Sci. Eng. C 2017, 79, 140–150. [Google Scholar] [CrossRef]
- Petrova, V.A.; Orekhov, A.S.; Chernyakov, D.D.; Baklagina, Y.G.; Romanov, D.P.; Kononova, S.V.; Volod’ko, A.V.; Ermak, I.M.; Klechkovskaya, V.V.; Skorik, Y.A. Preparation and analysis of multilayer composites based on polyelectrolyte complexes. Crystallogr. Rep. 2016, 61, 945–953. [Google Scholar] [CrossRef]
- Karolewicz, B.; Gajda, M.; Owczarek, M.; Pluta, J.; Górniak, A. Physicochemical characterization and dissolution studies of solid dispersions of clotrimazole with Pluronic F127. TroP. J. Pharm. Res. 2014, 13, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Garcia Ferreira, P.; Guimarães de Souza Lima, C.; Noronha, L.L.; de Moraes, M.C.; Silva, F.; Lifsitch Viçosa, A.; Omena Futuro, D.; Francisco Ferreira, V. Development of a method for the quantification of clotrimazole and itraconazole and study of their stability in a new microemulsion for the treatment of sporotrichosis. Molecules 2019, 24, 2333. [Google Scholar] [CrossRef] [Green Version]
- Tejada, G.; Lamas, M.C.; Svetaz, L.; Salomón, C.J.; Alvarez, V.A.; Leonardi, D. Effect of drug incorporation technique and polymer combination on the performance of biopolymeric antifungal buccal films. Int. J. Pharm. 2018, 548, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Darwesh, B.; Aldawsari, H.M.; Badr–Eldin, S.M. Optimized chitosan/anion polyelectrolyte complex based inserts for vaginal delivery of fluconazole: In vitro/in vivo evaluation. Pharmaceutics 2018, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Sonje, A.G.; Mahajan, H.S. Nasal inserts containing ondansetron hydrochloride based on chitosan–gellan gum polyelectrolyte complex: In vitro–in vivo studies. Mater. Sci. Eng. C 2016, 64, 329–335. [Google Scholar] [CrossRef]
- Mittal, G.N.; Kaur, G. In situ gelling ophthalmic drug delivery system: Formulation and evaluation. J. Appl. Polym. Sci. 2014, 131, 39788. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural–based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Siswanta, D.; Farida, F.; Zunaim, D.; Aprilita, N.H. Adsorption of HA (humic acid) using sulfuric acid–crosslinked chitosan/pectin polyelectrolyte complex film. J. Phys. Conf. Ser. 2019, 1156, 012003. [Google Scholar] [CrossRef]
- Kelemen, H.; Noszal, B.; Orgovan, B. Analysis of inclusion complexes of clotrimazole and differently substituted α, β and γ cyclodextrins by NMR spectroscopy. Rev. Chim. 2020, 71, 497–506. [Google Scholar] [CrossRef]
- Peschka, M.; Roberts, P.H.; Knepper, T.P. Analyis, fate studies and monitoring of the antifungal agent clotrimazole in the aquatic environment. Anal. Bioanal. Chem. 2007, 389, 959–968. [Google Scholar] [CrossRef]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Cruciani, F.; Vitali, B.; Luppi, B. Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohydr. Polym. 2012, 87, 581–588. [Google Scholar] [CrossRef]
- Šešlija, S.; Nešić, A.; Ružić, J.; Kalagasidis Krušić, M.; Veličković, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible blend films of pectin and poly(ethylene glycol): Preparation and physico–chemical evaluation. Food Hydrocoll. 2018, 77, 494–501. [Google Scholar] [CrossRef]
- Pilicheva, B.; Uzunova, Y.; Bodurov, I.; Viraneva, A.; Exner, G.; Sotirov, S.; Yovcheva, T.; Marudova, M. Layer–by–layer self–assembly films for buccal drug delivery: The effecr of polymer cross–linking. J. Drug Deliv. Sci. Technol. 2020, 59, 101897. [Google Scholar] [CrossRef]
- Ang, L.F.; Por, L.Y.; Yam, M.F. Study on different molecular weights of chitosan as an immobilization matrix for a glucose biosensor. PLoS ONE 2013, 8, e70597. [Google Scholar] [CrossRef] [Green Version]
- Perioli, L.; Pagano, C. Preformulation studies of mucoadhesive tablets for carbamazepine sublingual administration. Colloids Surf. B 2013, 102, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, M.; Olejniczak–Rabinek, M.; Bartkowiak, A.; Snela, A.; Prochaska, K.; Lulek, J. The wettability and swelling of selected mucoadhesive polymers in simulated saliva and vaginal fluids. Colloids Surf. B 2017, 156, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ren, B.; Tong, Y.; Dai, H.; Zhang, L. Synergistic combinations of antifungals and anti–virulence agents to fight against Candida albicans. Virulence 2015, 6, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starkov, A.A. Measurement of mitochondrial ROS production. Methods Mol. Biol. 2010, 648, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Formulation | CLO Content (%) | |
|---|---|---|
| PC Layer | CS Layer | |
| F1 | 50.0 | 50.0 |
| F2 1 | 33.3 | 66.7 |
| F3 1 | 0.0 | 100.0 |
| Formulations | Thickness (µm) | Weight Uniformity (mg) | Moisture Content (%) | CLO Content (%) |
|---|---|---|---|---|
| F1 | 127.0 ± 14.8 | 30.8 ± 4.2 | 5.0 ± 1.1 | 86.1 ± 6.6 |
| F2 | 147.0 ± 11.8 | 28.2 ± 2.1 | 7.9 ± 2.0 | 105.2 ± 8.1 |
| F3 | 128.9 ± 6.7 | 23.5 ± 1.8 | 4.9 ± 1.1 | 84.6 ± 6.4 |
| PF | 132.5 ± 16.0 | 23.9 ± 5.1 | 7.0 ± 3.6 | not applicable |
| Formulation | Young’s modulus (kPa) | Tear Resistance (N) | Tensile Strength (N/mm2) | Elongation at Break (%) | Folding Endurance |
|---|---|---|---|---|---|
| F1 | 35.9 ± 1.4 | 34.1 ± 4.2 | 14.3 ± 2.0 | 6.2 ± 1.3 | >300 |
| F2 | 33.7 ± 7.2 | 25.6 ± 9.8 | 7.0 ± 1.3 | 3.0 ± 1.5 | >150 |
| F3 | 26.1 ± 8.9 | 24.9 ± 1.0 | 9.5 ± 1.9 | 6.9 ± 1.8 | >300 |
| PF | 35.3 ± 7.1 | 34.9 ± 7.1 | 11.5 ± 2.7 | 5.5 ± 1.1 | >300 |
| CLO Suspension | Zeta Potential (mV) | |
|---|---|---|
| T0 | T24h | |
| CLO in water | −43.2 ± 1.4 | −21.1 ± 2.1 |
| CLO in SS 1 pH 4.8 | −4.1 ± 0.6 | −2.7 ± 0.6 |
| CLO in SS 1 pH 6.8 | −11.2 ± 0.6 | −14.2 ± 1.1 |
| Formulation | Erosion (%) | |||
|---|---|---|---|---|
| 15 Min | 60 Min | |||
| pH 4.8 | pH 6.8 | pH 4.8 | pH 6.8 | |
| F1 | 39.06 ± 19.24 | 56.06 ± 9.00 | 50.99 ± 8.59 | 48.4 ± 1.83 |
| F2 | 24.1 ± 16.16 | 50.01 ± 0.43 | 34.09 ± 5.38 | 57.53 ± 0.04 |
| F3 | 26.79 ± 5.40 | 50.03 ± 12.52 | 35.54 ± 3.94 | 51.34 ± 6.77 |
| PF | 26.12 ± 6.94 | 59.10 ± 1.87 | 43.27 ± 10.03 | 50.91 ± 9.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potaś, J.; Szymańska, E.; Wróblewska, M.; Kurowska, I.; Maciejczyk, M.; Basa, A.; Wolska, E.; Wilczewska, A.Z.; Winnicka, K. Multilayer Films Based on Chitosan/Pectin Polyelectrolyte Complexes as Novel Platforms for Buccal Administration of Clotrimazole. Pharmaceutics 2021, 13, 1588. https://doi.org/10.3390/pharmaceutics13101588
Potaś J, Szymańska E, Wróblewska M, Kurowska I, Maciejczyk M, Basa A, Wolska E, Wilczewska AZ, Winnicka K. Multilayer Films Based on Chitosan/Pectin Polyelectrolyte Complexes as Novel Platforms for Buccal Administration of Clotrimazole. Pharmaceutics. 2021; 13(10):1588. https://doi.org/10.3390/pharmaceutics13101588
Chicago/Turabian StylePotaś, Joanna, Emilia Szymańska, Magdalena Wróblewska, Izabela Kurowska, Mateusz Maciejczyk, Anna Basa, Eliza Wolska, Agnieszka Zofia Wilczewska, and Katarzyna Winnicka. 2021. "Multilayer Films Based on Chitosan/Pectin Polyelectrolyte Complexes as Novel Platforms for Buccal Administration of Clotrimazole" Pharmaceutics 13, no. 10: 1588. https://doi.org/10.3390/pharmaceutics13101588
APA StylePotaś, J., Szymańska, E., Wróblewska, M., Kurowska, I., Maciejczyk, M., Basa, A., Wolska, E., Wilczewska, A. Z., & Winnicka, K. (2021). Multilayer Films Based on Chitosan/Pectin Polyelectrolyte Complexes as Novel Platforms for Buccal Administration of Clotrimazole. Pharmaceutics, 13(10), 1588. https://doi.org/10.3390/pharmaceutics13101588