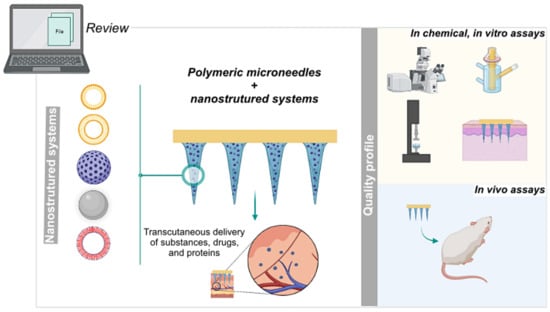
Dissolving Microneedles Developed in Association with Nanosystems: A Scoping Review on the Quality Parameters of These Emerging Systems for Drug or Protein Transdermal Delivery
Abstract
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Information Sources and Search Strategy
2.3. Selection of Sources of Evidence
2.4. Eligibility Criteria
2.5. Data Items and Data Extraction
2.6. Synthesis of the Results
3. Results
3.1. Selection and General Characteristics of Sources of Evidence
3.2. Characteristics of Devices
3.3. Methods Employed in Quality Control
3.3.1. Microscopy and Complementary Physical and Physicochemical Characterization (FTIR, TGA, DSC, and XRD)
3.3.2. Drug or Protein Content
3.3.3. Mechanical Properties, In Vitro Assays
| Technique | Equipment | Applied Force (N)/Weight (g) | Compression Rate (mm/s) | Result Expression | Ref. |
|---|---|---|---|---|---|
| Dynamic force | CT3 texture | NI | NI | Compressive force-displacement curve | [66] |
| NI | 1.00 | Compressive force-displacement curve | [77,78] | ||
| Displacement-force test station (Model 921A) | NI | 1.10 | Compressive force-displacement curve | [72] | |
| 10 N | 0.008 | Failure force | [60,79] | ||
| DTS delaminator | 10 N | 0.05 | Compressive force-displacement curve Failure force | [32] | |
| Force displacement tester (model 925) | NI | 1.25 | Compressive force-displacement curve Failure force | [42] | |
| Hounsfield universal mechanical testing machine | 10 N | 0.017 | Compressive force-displacement curve | [23] | |
| Mechanical testing system (5943 MicroTester) | NI | 0.50 | Compressive force-displacement curve Failure force | [47,74,80] | |
| 10 N | 0.10 | Compressive force-displacement curve | [81] | ||
| NI | 0.10 | Compressive force-displacement curve Failure force | [82] | ||
| 50 N | 0.50 | Compressive force-displacement curve | [83] | ||
| Side-shaking test stand (HCS-500) and a thrust meter (HF-50) | 2, 4, 8, 12, 16, and 20 N | NI | Observations of the MN deformations with a digital camera | [84] | |
| Tensile load frame | NI | 0.01 | Compressive force-displacement curve | [85] | |
| Tensile machine (Instron) | NI | 0.017 | Failure force | [86] | |
| Tensile testing machine (MTS 30G) | 10 N | 0.10 | Compressive force-displacement curve Failure force | [20,61] | |
| Texture analyzer (TA-XT2, Stable microsystems) | 45 N | 0.05 | Percentage height reduction (digital microscopy) | [19] | |
| 45 N, held for 30 s | NI | Percentage height reduction (digital microscopy) | [57,73,75] | ||
| 32 N, held for 30 s | 1.19 | Percentage height reduction (stereomicroscopy, digital microscopy) | [22,38,39,44,55,56,69,87] | ||
| 32 N, held for 30 s | 0.50 | Percentage height reduction (stereomicroscopy) | [41,51,70] | ||
| NI | 0.10 | Compressive force-displacement curve Failure force Stereomicroscopy | [76] | ||
| 0.049 N | 0.50 | Failure force | [62] | ||
| 40 N | 0.01 | Failure force MN morphology (Scanning electronic microscopy) | [53] | ||
| Texture analyzer (XT plus, Stable microsystems) | NI | 1.00 | Compressive force-displacement curve Failure force | [52] | |
| Universal testing machine (MARK-10) | NI | 1.00 | Compressive force-displacement curve | [68] | |
| Atomic force microscopy | 1.0 mN, 10 mm SiO2 sphere probe | 500 nm/s | The moduli of the needles were calculated from the force-displacement curves | [46] | |
| NI | NI | NI | Compressive force-displacement curve | [40] | |
| Static force | Standard weight | 50, 100, 200, and 500 g, held for 1 min | NA | Optical images of the MN deformation | [81] |
| Standard weight | 500 g, held for 5 min | NA | Optical images of the MN deformation | [47,74,80] | |
| Standard weight | 100, 200, 500, and 1000 g, held for 5 min | NA | Optical images of the MN deformation and images by confocal microscopy | [88] |
| Model Category | Matrix | Insertion Force | Method of Mensuration | Observations | Ref. |
|---|---|---|---|---|---|
| Skin model | Chicken skin | Manual force | Histological analysis | - | [23] |
| Human skin | Manual force, held for 30 s | CLSM | Skin from abdominal plastic surgeries | [53] | |
| Minipig skin | 20.0 N | Histological analysis | Applicator: not informed | [18] | |
| Mouse skin | 20.0 N | Staining with trypan blue | Equipment: Mechanical testing system (5943 MicroTester) | [83] | |
| Manual force, held for 5 min | Staining with trypan blue Histological analysis OCT | - | [35] | ||
| NI | Staining with trypan blue Histological analysis | - | [89] | ||
| NI, held for 3 min | Staining with trypan blue Histological analysis | - | [33] | ||
| Mouse skin (Balb-c) | NI | Histological analysis | - | [85] | |
| NI | Force-displacement curve | Equipment: Texture analyzer, insertion speed 0.10 mm/s The skin was placed on Styrofoam block support | [76] | ||
| NI | Histological analysis | - | [36] | ||
| Porcine skin | 1.0, 2.0, and 4.0 N | Staining with trypan blue | Insertion speed 0.5 mm/s The skin was placed on sheet of dental wax support topped with parafilm, and this set was fixed on a wooden block for support | [84] | |
| 1.5 N | Staining with trypan blue Histological analysis Fluoresce microscopy | Homemade electric applicator | [71] | ||
| 8.0, 11.0, and 16.0 N | OCT | Spring-loaded applicator The skin was placed on sheet of dental wax support | [19] | ||
| 10.0 N | Digital microscopy Staining with Shandon™ Blue tissue marker dye Histological analysis | Spring-loaded applicator | [62] | ||
| 10.0 to 50.0 N | OCT | Equipment: TA-XT2 Texture Analyser, insertion speed 0.50 mm/s | [44] | ||
| 11.0 N | Histological analysis | Custom-made spring-loaded applicator; Insertion test in association with the permeation test (Franz-type diffusion cell) | [49] | ||
| 32.0 N, held for 30 s | OCT | Equipment: TA-XT2 Texture Analyser, insertion speed 1.19 mm/s or 0.50 mm/s | [22,38,39,41,51,56,70] | ||
| Manual force (~1.5 N) | Fluorescence stereomicroscopy | - | [60,72,79] | ||
| Manual force | OCT | The skin was placed on sheet of dental wax support | [57,73,75] | ||
| Manual force | Stereomicroscopy Histological analysis | Evaluation of single, double, and triple insertion | [45] | ||
| Manual force, held for 5 min | Staining with trypan blue Histological analysis | - | |||
| NI | Staining with trypan blue Histological analysis | - | [67] | ||
| NI | Digital images of skin MN prepared with brilliant blue dye >> penetration efficacy | - | [64] | ||
| NI | Staining with trypan blue | Equipment: CT3 texture, insertion speed 20.0 mm/s | [77,78] | ||
| NI | SEM Fluorescence microscopy | - | [50] | ||
| Rat skin | Manual force (~5 N) | Staining with trypan blue | - | [88] | |
| NI, held for 1 min | Staining with trypan blue Histological analysis CLSM OCT | - | [43] | ||
| Rat skin (Sprague–Dawley) | NI | CLSM | - | [47] | |
| NI | Histological analysis CLSM | - | [74,80,82] | ||
| NI | Staining with trypan blue Histological analysis | - | [90] | ||
| NI, MN held for 3 min | Histological analysis Staining with trypan blue | - | [34,68,91] | ||
| 15 N, held for 1 min | Staining with trypan blue Histological analysis CLSM | - | [92] | ||
| Origin not described | 0.08 N | Fracture force | Equipment: CT3 texture, insertion speed 0.50 mm/s Additional analyses of bioadhesion and post-wetting bioadhesion | [54] | |
| Artificial skin model | Agarose disc (3% w/v) | Manual force, held for 1 min | Fluorescence microscopy | - | [42] |
| Agarose disc covered with a Parafilm® layer (2% w/v agarose disc, thickness: 6 mm, Parafilm® layer: 127 µm) | NI | SEM | Equipment: TA.XT plus texture analyzer, insertion speed 1.00 mm/s | [53] | |
| Aluminum foil | Manual force | Observation of the holes in the aluminum foil | - | [88] | |
| Gelatin hydrogel (5% w/v) | NI | Optical microscopy | - | [65] | |
| Parafilm® (8 layers, ~1 mm) | 10.0 to 50.0 N | OCT | Equipment: TA-XT2 Texture Analyser, insertion speed 0.50 mm/s | [44] | |
| 32.0 N, held for 30 s | Digital microscopy (number of holes/layer) OCT | Equipment: TA-XT2 Texture Analyser, insertion speed 1.19 mm/s or 0.50 mm/s | [22,38,39,41,51,55,56,69,70] | ||
| Manual force, held for 5 min | SEM | - | [93] | ||
| Manual force (~30 N) vs. 50 N | OCT | Equipment: Instron universal testing instrument model 5567, insertion speed 0.50 mm/s | [48] | ||
| Parafilm® (10 layers, ~1 mm) | 30 N, held for 5 min | Digital microscopy (number of holes/layer) | Equipment: XT plus Texture Analyzer, insertion speed 1 mm/s | [52] |
| Dissolution Model/Apparatus | Matrix/Medium | Temp. | Time of Insertion | Insertion Force | Dissolution Measurement | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Skin model | Porcine skin | 37 °C NI | 0.5–60 min NI | Manual force + weight 5.0 g Manual force + weight 13.0 g Manual force NI | Digital microscopy Fluorescence microscopy OCT Optical microscopy SEM Stereoscopic microscopy | [22,38,41,50,51,55,58,62,69,71,75,77,78,79,87,88] | ||
| Mouse skin (Balb-c) | NI | 30–120 min | NI | CLSM | [76] | |||
| Rat skin (Sprague–Dawley) | NI | 0.5–30 min | NI | Bright field microscopy CLSM 3D-CLSM SEM | [47,90,91] Health and diabetics rats [74,80,82] | |||
| Glass plate/beaker and vial | PBS | 37 °C NI | <0.5–10 min | NA | Confocal microscopy Optical microscopy | [59,93] | ||
| Water | NI | 0.33–1 min | NA | Optical microscopy | [66] | |||
| Gelatin block | Gelatin block (5% w/v) | NI | 0.33–1 min | NI | Optical microscopy | [65] | ||
| Gelatin block (35% w/v) | NI | 10 min | NI | Digital microscopy | [35] | |||
| Release Assay/ Apparatus | Matrix/Medium | rpm | Temp. | Time Assay Range | Insertion Force | Quantification Methods | Ref. | |
| Agarose gel | 1% w/v agarose gel containing different concentrations of glucose | NA | NI | 10–180 min | NI | Fluorescence stereomicroscopy QIAquick gel extraction kit (ELISA) | [60] | |
| Dialysis bags | PBS (pH 7.4) | NI | NI | 4–72 h | NA | HPLC | [63] | |
| 30% (v/v) PEG 400 in saline | 250 | 32 ± 1 °C | 1–24 h | NA | HPLC | [52] | ||
| Dialysis membranes (Franz-type diffusion cell) | 30 % v/v ethanol solution in distilled water | NI | NI | 10–1440 min | NA | HPLC | [77,78] | |
| Glass plate/beaker and vial | PBS (pH 5.5) | 500 | 37 °C | 1–120 min | NA | Fluorescence spectroscopy | [50] | |
| PBS (pH 7.4) | 50–100 NI | 37 °C | 1–1440 min | NA | Fluorescence spectroscopy CLSM UV–vis spectroscopy | [23,53,85,93] | ||
| PBS (pH 7.5) | NI | 37 °C | <3 days | NA | Fluorescence spectroscopy | [58] | ||
| Distilled water PBS (pH 6.8) containing 1% tween 80 | 200 | 37 °C | 15–1440 min | NA | HPLC | [64] | ||
| Glucose solutions at different concentrations (5.5, 11.1, and 22.2 mM) Saline | NI | 37 °C | 10–240 min | NA | Bradford protein assay kit | [46] | ||
| USP dissolution apparatus 5 (Paddle-over disc method) | PBS (pH 5.5) | NI | 37.5 °C | 5–1980 min | NA | UV–vis spectroscopy | [54] | |
| NI | DPBS with or without collagenase (2 U/mL) | NI | NI | <120 h | NA | Picogreen kit (quantification of DNA release) | [83] | |
| PBS | 80 | 37 °C | 1–60 min | NA | Picogreen kit (quantification of DNA release) UV spectrophotometry | [96] | ||
| PBS + glutathione | NI | 37 °C | 4–72 h | NA | Standard bicinchoninic acid assay (BCA) HPLC | [40] | ||
| Water | NI | NI | 20–60 s | NA | UV spectrophotometry | [66] | ||
| Skin model | Mouse skin | NA | NI | 3 min | NI | UV spectrophotometry | [33] | |
External Stimulus Application
Stability Assays
3.3.4. In Vivo Assays
4. Discussion
4.1. Summary of Evidence and Characteristics of MNs Associated with Nanostructured Systems
4.2. Assays Employed in MN Analysis
4.3. Limitations
4.4. Future Possibilities and Potential of Polymeric MNs
- Development of specific alternative methods for the evaluation of TDDS as well as the validation of in vitro methods to characterize the dissolution and release profiles of substances from MNs containing nanosystems; development of specific equipment and apparatus to assess these parameters more reliably against physiological skin conditions;
- Evaluation of aspects that directly or indirectly impact the product profile, for example, the mechanical force required for the insertion of the device into the skin. One way to evaluate this parameter, indicative of future self-administration success rate, is to evaluate the mechanical characteristics and in vitro or in vivo skin insertion. Since different individuals have distinct hand strengths, the validation and standardization of these assays is critical to understanding and predicting the consequences of this variability;
- The standardization of quality methods will boost the growth of polymeric MNs in the market as well as allow the evaluation of systems in line with the trend of personalized medicine, especially for the treatment of chronic diseases and associated comorbidities;
- The association of nanostructured systems and polymeric matrices for the transcutaneous administration of substances will be enhanced if different strategies that modulate drug release are combined (different systems, different polymeric layers, or where the combination of substances in free form and those associated with nanosystems are introduced in the same matrix). However, control methods will have to be developed to characterize these systems;
- The lack of investment in stability studies that prove the maintenance of nanometric characteristics after inclusion in the polymeric matrix may represent a breakpoint in the process of scaling up from the bench to market.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Benson, H.A.E.; Watkinson, A.C. Transdermal and Topical Drug Delivery: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 9780470450291. [Google Scholar]
- Mishra, D.K.; Pandey, V.; Maheshwari, R.; Ghode, P.; Tekade, R.K. Cutaneous and transdermal drug delivery: Techniques and delivery systems. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 595–650. ISBN 9780128179093. [Google Scholar]
- Jepps, O.G.; Dancik, Y.; Anissimov, Y.G.; Roberts, M.S. Modeling the human skin barrier—Towards a better understanding of dermal absorption. Adv. Drug Deliv. Rev. 2013, 65, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Lan, X.; She, J.; Lin, D.A.; Xu, Y.; Li, X.; Yang, W.F.; Lui, V.W.Y.; Jin, L.; Xie, X.; Su, Y.X. Microneedle-mediated delivery of lipid-coated cisplatin nanoparticles for efficient and safe cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 33060–33069. [Google Scholar] [CrossRef]
- Pireddu, R.; Schlich, M.; Marceddu, S.; Valenti, D.; Pini, E.; Fadda, A.M.; Lai, F.; Sinico, C. Nanosuspensions and microneedles roller as a combined approach to enhance diclofenac topical bioavailability. Pharmaceutics 2020, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Kovaliov, M.; Li, S.; Korkmaz, E.; Cohen-Karni, D.; Tomycz, N.; Ozdoganlar, O.B.; Averick, S. Extended-release of opioids using fentanyl-based polymeric nanoparticles for enhanced pain management. RSC Adv. 2017, 7, 47904–47912. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Xu, B.; Xu, J.; Shou, D.; Liu, E.; Gao, J.; Liang, W.; Huang, Y. Microneedle-assisted dendritic cell-targeted nanoparticles for transcutaneous DNA immunization. Polym. Chem. 2015, 6, 373–379. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, H.S.; Hwang, Y.H.; Kim, J.J.; Kang, K.Y.; Kim, S.J.; Kim, H.K.; Kim, J.D.; Jeong, D.H.; Paik, M.J.; et al. Enhanced anti-tumor immunotherapy by dissolving microneedle patch loaded ovalbumin. PLoS ONE 2019, 14, e0220382. [Google Scholar] [CrossRef] [Green Version]
- An, M.; Liu, H. Dissolving Microneedle Arrays for Transdermal Delivery of Amphiphilic Vaccines. Small 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.H.; Chen, M.C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013, 9, 8952–8961. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets Global Transdermal Drug Delivery System Market (2020 to 2027)—COVID-19 Impact and Analysis. Available online: https://www.prnewswire.com/news-releases/global-transdermal-drug-delivery-system-market-2020-to-2027---covid-19-impact-and-analysis-301146587.html (accessed on 5 May 2021).
- Future Market Insights Microneedle Drug Delivery Systems Market by product type-Solid Microneedles, Hollow Microneedles, and Dissolving Microneedles for 2020–2030. Available online: https://www.futuremarketinsights.com/reports/microneedle-drug-delivery-systems-market (accessed on 5 May 2021).
- Donnelly, R.F.; Moffatt, K.; Alkilani, A.Z.; Vicente-Pérez, E.M.; Barry, J.; McCrudden, M.T.C.; Woolfson, A.D. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: A pilot study centred on pharmacist intervention and a patient information leaflet. Pharm. Res. 2014, 31, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Jeong, J.H.; Lee, K.M.; Jeong, K.H.; Yang, H.; Kim, M.; Jung, H.; Lee, S.; Choi, Y.W. Nanostructured lipid carrier-loaded hyaluronic acid microneedles for controlled dermal delivery of a lipophilic molecule. Int. J. Nanomed. 2013, 9, 289–299. [Google Scholar] [CrossRef] [Green Version]
- McCaffrey, J.; McCrudden, C.M.; Ali, A.A.; Massey, A.S.; McBride, J.W.; McCrudden, M.T.C.; Vicente-Perez, E.M.; Coulter, J.A.; Robson, T.; Donnelly, R.F.; et al. Transcending epithelial and intracellular biological barriers; A prototype DNA delivery device. J. Control. Release 2016, 226, 238–247. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z.; Ho, D. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.; Larrañeta, E.; Mccrudden, M.T.C.; Mccrudden, C.M.; Brady, A.J.; Fallows, S.J.; Mccarthy, H.O.; Kissenpfennig, A.; Donnelly, R.F. In vivo studies investigating biodistribution of nanoparticle-encapsulated rhodamine B delivered via dissolving microneedles. J. Control. Release 2017, 265, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Permana, A.D.; Tekko, I.A.; McCrudden, M.T.C.; Anjani, Q.K.; Ramadon, D.; McCarthy, H.O.; Donnelly, R.F. Solid lipid nanoparticle-based dissolving microneedles: A promising intradermal lymph targeting drug delivery system with potential for enhanced treatment of lymphatic filariasis. J. Control. Release 2019, 316, 34–52. [Google Scholar] [CrossRef]
- Justin, R.; Román, S.; Chen, D.; Tao, K.; Geng, X.; Grant, R.T.; MacNeil, S.; Sun, K.; Chen, B. Biodegradable and conductive chitosan-graphene quantum dot nanocomposite microneedles for delivery of both small and large molecular weight therapeutics. RSC Adv. 2015, 5, 51934–51946. [Google Scholar] [CrossRef]
- Ita, K. Transdermal delivery of drugs with microneedles—Potential and challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef] [Green Version]
- Paredes, A.J.; McKenna, P.E.; Ramöller, I.K.; Naser, Y.A.; Volpe-Zanutto, F.; Li, M.; Abbate, M.T.A.; Zhao, L.; Zhang, C.; Abu-Ershaid, J.M.; et al. Microarray patches: Poking a hole in the challenges faced when delivering poorly soluble drugs. Adv. Funct. Mater. 2021, 31, 1–27. [Google Scholar] [CrossRef]
- Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Rahiminezhad, Z.; Abedi, M.; Tamaddon, A.; Ahadian, S. Microneedle arrays combined with nanomedicine approaches for transdermal delivery of therapeutics. J. Clin. Med. 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Arya, J.; Henry, S.; Kalluri, H.; McAllister, D.V.; Pewin, W.P.; Prausnitz, M.R. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials 2017, 128, 1–7. [Google Scholar] [CrossRef]
- Caffarel-Salvador, E.; Kim, S.; Soares, V.; Tian, R.Y.; Stern, S.R.; Minahan, D.; Yona, R.; Lu, X.; Zakaria, F.R.; Collins, J.; et al. A microneedle platform for buccal macromolecule delivery. Sci. Adv. 2021, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.R.; Munni, M.N.; Campbell, L.; Mostofa, G.; Dobson, L.; Shittu, M.; Pattanayek, S.K.; Uddin, M.J.; Bhusan Das, D. Translation of polymeric microneedles for treatment of human diseases: Recent trends, Progress, and Challenges. Pharmaceutics 2021, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Wang, J.; Hu, Q.; Hochu, G.M.; Xin, H.; Wang, C.; Gu, Z. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors. ACS Nano 2016, 10, 8956–8963. [Google Scholar] [CrossRef]
- Wei, S.; Quan, G.; Lu, C.; Pan, X.; Wu, C. Dissolving microneedles integrated with pH-responsive micelles containing AIEgen with ultra-photostability for enhancing melanoma photothermal therapy. Biomater. Sci. 2020, 8, 5739–5750. [Google Scholar] [CrossRef]
- Peng, T.; Huang, Y.; Feng, X.; Zhu, C.; Ma, X.; Wang, X.; Bai, X.; Pan, X.; Wu, C. Dissolving microneedles loading TPGS biphasic functionalized PLGA nanoparticles for efficient chemo-photothermal combined therapy of melanoma. Adv. Ther. 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Qin, W.; Quan, G.; Sun, Y.; Chen, M.; Yang, P.; Feng, D.; Wen, T.; Hu, X.; Pan, X.; Wu, C. Dissolving microneedles with spatiotemporally controlled pulsatile release nanosystem for synergistic chemo-photothermal therapy of Melanoma. Theranostics 2020, 10, 8179–8196. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.W.; He, X.L.; Yang, F.; Han, R.X.; Yang, C.L.; Li, W.; Qian, Z.Y. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020, 5, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Danehy, R.; Cai, H.; Ao, Z.; Pu, M.; Nusawardhana, A.; Rowe-Magnus, D.; Guo, F. Microneedle patch-mediated treatment of bacterial biofilms. ACS Appl. Mater. Interfaces 2019, 11, 14640–14646. [Google Scholar] [CrossRef]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. X 2020, 2, 100047. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Anjani, Q.K.; Sartini; Utomo, E.; Volpe-Zanutto, F.; Paredes, A.J.; Evary, Y.M.; Mardikasari, S.A.; Pratama, M.R.; Tuany, I.N.; et al. Selective delivery of silver nanoparticles for improved treatment of biofilm skin infection using bacteria-responsive microparticles loaded into dissolving microneedles. Mater. Sci. Eng. C 2021, 120, 111786. [Google Scholar] [CrossRef]
- Wan, T.; Pan, Q.; Ping, Y. Microneedle-assisted genome editing: A transdermal strategy of targeting NLRP3 by CRISPR-Cas9 for synergistic therapy of inflammatory skin disorders. Sci. Adv. 2021, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tekko, I.A.; Permana, A.D.; Vora, L.; Hatahet, T.; McCarthy, H.O.; Donnelly, R.F. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: Potential for enhanced treatment of psoriasis. Eur. J. Pharm. Sci. 2020, 152, 105469. [Google Scholar] [CrossRef]
- Ramalheiro, A.; Paris, J.L.; Silva, B.F.B.; Pires, L.R. Rapidly dissolving microneedles for the delivery of cubosome-like liquid crystalline nanoparticles with sustained release of rapamycin. Int. J. Pharm. 2020, 591, 119942. [Google Scholar] [CrossRef]
- Wu, B.; Fu, J.; Zhou, Y.; Luo, S.; Zhao, Y.; Quan, G.; Pan, X.; Wu, C. Tailored core‒shell dual metal–organic frameworks as a versatile nanomotor for effective synergistic antitumor therapy. Acta Pharm. Sin. B 2020, 10, 2198–2211. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; McCrudden, M.T.C.; Donnelly, R.F. Enhanced intradermal delivery of nanosuspensions of antifilariasis drugs using dissolving microneedles: A proof of concept study. Pharmaceutics 2019, 11, 346. [Google Scholar] [CrossRef] [Green Version]
- Devineni, J.; Pravallika, C.D.; Rani, B.S.; Nalluri, B.N. Effective single drug treatment of lymphatic filariasis through enhanced transdermal delivery of ivermectin liposomes using solid and dissolving microneedles. Indian J. Pharm. Educ. Res. 2020, 54, S492–S504. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Tan, D.; Liu, Q.; Xia, R.; Chen, M.; Liu, Y.; Xue, L.; Lei, Y. A dissolving and glucose-responsive insulin-releasing microneedle patch for type 1 diabetes therapy. J. Mater. Chem. B 2021, 9, 648–657. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Zhang, Y.; Zhou, J.; Sun, S.; Liu, Y. H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J. Mater. Chem. B 2017, 5, 8200–8208. [Google Scholar] [CrossRef]
- Angkawinitwong, U.; Courtenay, A.J.; Rodgers, A.M.; Larrañeta, E.; Mccarthy, H.O.; Brocchini, S.; Donnelly, R.F.; Williams, G.R. A novel transdermal protein delivery strategy via electrohydrodynamic coating of PLGA microparticles onto microneedles. ACS Appl. Mater. Interfaces 2020, 12, 12478–12488. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Morrow, D.I.J.; Fay, F.; Scott, C.J.; Abdelghany, S.; Singh, R.R.T.; Garland, M.J.; David Woolfson, A. Microneedle-mediated intradermal nanoparticle delivery: Potential for enhanced local administration of hydrophobic pre-formed photosensitisers. Photodiagnosis Photodyn. Ther. 2010, 7, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.J.; Lin, Y.J.; Hu, Y.C.; Chiang, W.L.; Chen, K.J.; Yang, W.C.; Liu, H.L.; Fu, C.C.; Sung, H.W. Multidrug release based on microneedle arrays filled with pH-responsive PLGA hollow microspheres. Biomaterials 2012, 33, 5156–5165. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.; Tekko, I.A.; Vora, L.; Larrañeta, E.; Permana, A.D.; Donnelly, R.F. Nanosuspension-based dissolving microneedle arrays for intradermal delivery of curcumin. Pharmaceutics 2019, 11, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Wang, Y.; Wang, M.; Yang, X.; Tang, Y.; Pang, M.; Wang, W.; Chen, L.; Wu, C.; Xu, Y. Microneedles mediated bioinspired lipid nanocarriers for targeted treatment of alopecia. J. Control. Release 2021, 329, 1–15. [Google Scholar] [CrossRef]
- El-Sayed, N.; Vaut, L.; Schneider, M. Customized fast-separable microneedles prepared with the aid of 3D printing for nanoparticle delivery. Eur. J. Pharm. Biopharm. 2020, 154, 166–174. [Google Scholar] [CrossRef]
- Pineda-Álvarez, R.A.; Bernad-Bernad, M.J.; Rodríguez-Cruz, I.M.; Escobar-Chávez, J.J. Development and characterization of starch/gelatin microneedle arrays loaded with lecithin–gelatin nanoparticles of losartan for transdermal delivery. J. Pharm. Innov. 2020. [Google Scholar] [CrossRef]
- Volpe-Zanutto, F.; Ferreira, L.T.; Permana, A.D.; Kirkby, M.; Paredes, A.J.; Vora, L.K.; Bonfanti, A.P.; Charlie-Silva, I.; Raposo, C.; Figueiredo, M.C.; et al. Artemether and lumefantrine dissolving microneedle patches with improved pharmacokinetic performance and antimalarial efficacy in mice infected with Plasmodium yoelii. J. Control. Release 2021, 333, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Donnelly, R.F.; Larrañeta, E.; González-Vázquez, P.; Thakur, R.R.S.; Vavia, P.R. Novel bilayer dissolving microneedle arrays with concentrated PLGA nano-microparticles for targeted intradermal delivery: Proof of concept. J. Control. Release 2017, 265, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, G.; McCaffrey, J.; Ali, A.A.; McBride, J.W.; McCrudden, C.M.; Vincente-Perez, E.M.; Donnelly, R.F.; McCarthy, H.O. Dissolving microneedles for DNA vaccination: Improving functionality via polymer characterization and RALA complexation. Hum. Vaccines Immunother. 2017, 13, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Han, Y.; Yu, X.; Liang, L.; Chang, H.; Yeo, D.C.; Wiraja, C.; Wee, M.L.; Liu, L.; Liu, X.; et al. Upconversion nanoparticle powered microneedle patches for transdermal delivery of siRNA. Adv. Healthc. Mater. 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Demuth, P.C.; Garcia-Beltran, W.F.; Ai-Ling, M.L.; Hammond, P.T.; Irvine, D.J. Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv. Funct. Mater. 2013, 23, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.L.; Huang, C.Y.; Hsu, Y.P.; Hwang, T.L.; Chang, S.H.; Wang, H.Y.J.; Feng, L.Y.; Tzou, S.J.; Wei, K.C.; Yang, H.W. On-skin glucose-biosensing and on-demand insulin-zinc hexamers delivery using microneedles for syringe-free diabetes management. Chem. Eng. J. 2020, 398, 125536. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Qian, C.; Lu, Y.; Kahkoska, A.R.; Xie, Z.; Jing, X.; Buse, J.B.; Gu, Z.; States, U.; et al. H2O2-Responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano 2017, 11, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Lanza, J.S.; Vucen, S.; Flynn, O.; Donadei, A.; Cojean, S.; Loiseau, P.M.; Fernandes, A.P.S.M.; Frézard, F.; Moore, A.C. A TLR9-adjuvanted vaccine formulated into dissolvable microneedle patches or cationic liposomes protects against leishmaniasis after skin or subcutaneous immunization. Int. J. Pharm. 2020, 586, 119390. [Google Scholar] [CrossRef]
- Lima, A.F.; Amado, I.R.; Pires, L.R. Poly(d,l-lactide-co-glycolide) (PLGA) nanoparticles Loaded with proteolipid protein (PLP)—Exploring a new administration route. Polymers 2020, 12, 3063. [Google Scholar] [CrossRef]
- Pawar, S.; Shende, P. 22 factorial design-based biocompatible microneedle arrays containing artemether co-loaded with lumefantrine nanoparticles for transepidermal delivery. Biomed. Microdevices 2020, 22, 1–15. [Google Scholar] [CrossRef]
- González García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Ninan, N.; Cavallaro, A.A.; Trinidad, A.D.; Zhao, Y.; Hayball, A.J.D.; Vasilev, K. Self-sterilizing antibacterial silver-loaded microneedles. Chem. Commun. 2019, 55, 171–174. [Google Scholar] [CrossRef]
- Fang, J.H.; Liu, C.H.; Hsu, R.S.; Chen, Y.Y.; Chiang, W.H.; Wang, H.M.D.; Hu, S.H. Transdermal composite microneedle composed of mesoporous iron oxide nanoraspberry and PVA for androgenetic alopecia treatment. Polymers 2020, 12, 1392. [Google Scholar] [CrossRef] [PubMed]
- Su, L.C.; Chen, M.C. Efficient delivery of nanoparticles to deep skin layers using dissolvable microneedles with an extended-length design. J. Mater. Chem. B 2017, 5, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y.; Deng, L.; Zhang, Z.R.; Lin, Y. A fast-dissolving microneedle array loaded with chitosan nanoparticles to evoke systemic immune responses in mice. J. Mater. Chem. B 2020, 8, 216–225. [Google Scholar] [CrossRef]
- Mir, M.; Permana, A.D.; Ahmed, N.; Khan, G.M.; ur Rehman, A.; Donnelly, R.F. Enhancement in site-specific delivery of carvacrol for potential treatment of infected wounds using infection responsive nanoparticles loaded into dissolving microneedles: A proof of concept study. Eur. J. Pharm. Biopharm. 2020, 147, 57–68. [Google Scholar] [CrossRef]
- Rojekar, S.; Vora, L.K.; Tekko, I.A.; Volpe-Zanutto, F.; McCarthy, H.O.; Vavia, P.R.; Ryan, R.F. Etravirine-loaded dissolving microneedle arrays for long-acting delivery. Eur. J. Pharm. Biopharm. 2021, 165, 41–51. [Google Scholar] [CrossRef]
- Guo, L.; Chen, J.; Qiu, Y.; Zhang, S.; Xu, B.; Gao, Y. Enhanced transcutaneous immunization via dissolving microneedle array loaded with liposome encapsulated antigen and adjuvant. Int. J. Pharm. 2013, 447, 22–30. [Google Scholar] [CrossRef]
- Yang, H.W.; Ye, L.; Guo, X.D.; Yang, C.; Compans, R.W.; Prausnitz, M.R. Ebola Vaccination Using a DNA Vaccine Coated on PLGA-PLL/γPGA Nanoparticles Administered Using a Microneedle Patch. Adv. Healthc. Mater. 2017, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.; Ali, A.A.; McCrudden, C.M.; McBride, J.W.; McCaffrey, J.; Robson, T.; Kett, V.L.; Dunne, N.J.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer: Strategic optimisation of RALA mediated gene delivery from a biodegradable microneedle system. Eur. J. Pharm. Biopharm. 2018, 127, 288–297. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Cao, Q.; Zhang, Y.; Yu, W.; Zhu, J.; Liu, D.; Jiang, G. Microneedles integrated with ZnO quantum-dot capped mesoporous bioactive glasses for glucose-mediated insulin delivery. ACS Biomater. Sci. Eng. 2018, 4, 2473–2483. [Google Scholar] [CrossRef]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; A novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 921–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattarabhiran, S.P.; Saju, A.; Sonawane, K.R.; Manimaran, R.; Bhatnagar, S.; Roy, G.; Kulkarni, R.B.; Venuganti, V.V.K. Dissolvable microneedle-mediated transcutaneous delivery of tetanus toxoid elicits effective immune response. AAPS PharmSciTech 2019, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; Thakkar, H.P. Vinpocetine loaded ultradeformable liposomes as fast dissolving microneedle patch: Tackling treatment challenges of dementia. Eur. J. Pharm. Biopharm. 2020, 156, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; Thakkar, H.P. QbD-driven development of dissolving microneedle patch loaded with ultradeformable liposomes encapsulated Noopept: Exploring a patient friendly, once-daily option to manage dementia. Eur. J. Pharm. Sci. 2021, 164, 105909. [Google Scholar] [CrossRef]
- Liao, J.F.; Lee, J.C.; Lin, C.K.; Wei, K.C.; Chen, P.Y.; Yang, H.W. Self-assembly DNA polyplex vaccine inside dissolving microneedles for high-potency intradermal vaccination. Theranostics 2017, 7, 2593–2605. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, B.; Zhu, J.; Zhang, Y.; Liu, T.; Song, G. Polymer microneedles integrated with glucose-responsive mesoporous bioactive glass nanoparticles for transdermal delivery of insulin. Biomed. Phys. Eng. Express 2019, 5, 045038. [Google Scholar] [CrossRef]
- Liu, D.; Yu, B.; Jiang, G.; Yu, W.; Zhang, Y.; Xu, B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater. Sci. Eng. C 2018, 90, 180–188. [Google Scholar] [CrossRef]
- Tong, Z.; Zhou, J.; Zhong, J.; Tang, Q.; Lei, Z.; Luo, H.; Ma, P.; Liu, X. Glucose- and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats. ACS Appl. Mater. Interfaces 2018, 10, 20014–20024. [Google Scholar] [CrossRef]
- Qu, M.; Kim, H.J.; Zhou, X.; Wang, C.; Jiang, X.; Zhu, J.; Xue, Y.; Tebon, P.; Sarabi, S.A.; Ahadian, S.; et al. Biodegradable microneedle patch for transdermal gene delivery. Nanoscale 2020, 12, 16724–16729. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, L.; Zhang, S.; Xu, B.; Gao, Y.; Hu, Y.; Hou, J.; Bai, B.; Shen, H.; Mao, P. DNA-based vaccination against hepatitis B virus using dissolving microneedle arrays adjuvanted by cationic liposomes and CpG ODN. Drug Deliv. 2016, 23, 2391–2398. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.X.; Ma, M.; Xue, F.; Shen, S.; Chen, Q.; Kuang, Y.; Liang, K.; Wang, X.; Chen, H. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J. Control. Release 2020, 324, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.Q.; Chen, G.; Xu, W.; Zhou, D.; Li, J.X.; Huang, Y.C.; Lin, R.; Gu, Z.; Du, J.Z. Microneedle-array patch with pH-sensitive formulation for glucose-responsive insulin delivery. Nano Res. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Permana, A.D.; Paredes, A.J.; Volpe-Zanutto, F.; Anjani, Q.K.; Utomo, E.; Donnelly, R.F. Dissolving microneedle-mediated dermal delivery of itraconazole nanocrystals for improved treatment of cutaneous candidiasis. Eur. J. Pharm. Biopharm. 2020, 154, 50–61. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, H.; Wang, Z.; Yang, X.; Zhang, M.; Liu, X.; Wang, B.; Wu, Z.; Chen, D. Preparation and characterization of dissolving hyaluronic acid composite microneedles loaded micelles for delivery of curcumin. Drug Deliv. Transl. Res. 2020, 10, 1520–1530. [Google Scholar] [CrossRef]
- Zhao, J.H.; Zhang, Q.B.; Liu, B.; Piao, X.H.; Yan, Y.L.; Hu, X.G.; Zhou, K.; Zhang, Y.T.; Feng, N.P. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int. J. Nanomed. 2017, 12, 4763–4772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Li, Y.; Chen, X.; Zhou, Z.; Pang, J.; Luo, X.; Kong, M. A surface charge dependent enhanced Th1 antigen-specific immune response in lymph nodes by transfersome-based nanovaccine-loaded dissolving microneedle-assisted transdermal immunization. J. Mater. Chem. B 2019, 7, 4854–4866. [Google Scholar] [CrossRef]
- Guo, T.; Cheng, N.; Zhao, J.; Hou, X.; Zhang, Y.; Feng, N. Novel nanostructured lipid carriers-loaded dissolving microneedles for controlled local administration of aconitine. Int. J. Pharm. 2019, 572, 118741. [Google Scholar] [CrossRef]
- Zhou, Z.; Pang, J.; Wu, X.; Wu, W.; Chen, X.; Kong, M. Reverse immune suppressive microenvironment in tumor draining lymph nodes to enhance anti-PD1 immunotherapy via nanovaccine complexed microneedle. Nano Res. 2020, 13, 1509–1518. [Google Scholar] [CrossRef]
- Tripathy, N.; Wang, J.; Tung, M.; Conway, C.; Chung, E.J. Transdermal delivery of kidney-targeting nanoparticles using dissolvable microneedles. Cell. Mol. Bioeng. 2020, 13, 475–486. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography (OCT). Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, H.T.T.; Yin, Y.; Thambi, T.; Kim, B.S.; Jeong, J.H.; Lee, D.S. Highly potent intradermal vaccination by an array of dissolving microneedle polypeptide cocktails for cancer immunotherapy. J. Mater. Chem. B 2020, 8, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Comparison of body distribution of poly(vinyl alcohol) with other water-soluble polymers after intravenous administration. J. Pharm. Pharmacol. 1995, 47, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Hespe, W.; Meier, A.M.; Blankwater, Y.J. Excretion and distribution studies in rats with two forms of 14carbon-labelled polyvinylpyrrolidone with a relatively low mean molecular weight after intravenous administration. Arzneimittelforschung 1977, 27, 1158–1162. [Google Scholar]
- Wang, M.; Hu, L.; Xu, C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Kaneo, Y.; Hashihama, S.; Kakinoki, A.; Tanaka, T.; Nakano, T.; Ikeda, Y. Pharmacokinetics and biodisposition of poly(vinyl alcohol) in rats and mice. Drug Metab. Pharmacokinet. 2005, 20, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Yin, D.; Liang, W.; Xing, S.; Gao, Z.; Zhang, W.; Guo, Z.; Gao, S. Hepatitis B DNA vaccine-polycation nano-complexes enhancing immune response by percutaneous administration with microneedle. Biol. Pharm. Bull. 2013, 36, 1283–1291. [Google Scholar] [CrossRef] [Green Version]
- Psimadas, D.; Georgoulias, P.; Valotassiou, V.; Loudos, G. Improved dermal delivery of FITC–BSA using a combination of passive and active methods. J. Pharm. Sci. 2012, 101, 2271–2280. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, B.; Tang, R.; Ding, X.; Hou, X.; Wang, X.; Gu, S.; Lu, L.; Zhang, Y.; Gao, S.; et al. Combination of microneedles with PLGA Nanoparticles as a potential strategy for topical drug delivery. Curr. Nanosci. 2011, 7, 545–551. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Guo, L.; Mao, P.; Gao, Y. Dissolving Microneedle Arrays for Intradermal Immunization of Hepatitis B Virus DNA Vaccine. Procedia Vaccinol. 2015, 9, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Loizidou, E.Z.; Inoue, N.T.; Ashton-Barnett, J.; Barrow, D.A.; Allender, C.J. Evaluation of geometrical effects of microneedles on skin penetration by CT scan and finite element analysis. Eur. J. Pharm. Biopharm. 2016, 107, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bal, S.M.; Kruithof, A.C.; Zwier, R.; Dietz, E.; Bouwstra, J.A.; Lademann, J.; Meinke, M.C. Influence of microneedle shape on the transport of a fluorescent dye into human skin in vivo. J. Control. Release 2010, 147, 218–224. [Google Scholar] [CrossRef]
- Cole, G.; Ali, A.A.; McErlean, E.; Mulholland, E.J.; Short, A.; McCrudden, C.M.; McCaffrey, J.; Robson, T.; Kett, V.L.; Coulter, J.A.; et al. DNA vaccination via RALA nanoparticles in a microneedle delivery system induces a potent immune response against the endogenous prostate cancer stem cell antigen. Acta Biomater. 2019, 96, 480–490. [Google Scholar] [CrossRef]
- Lan, X.; Zhu, W.; Huang, X.; Yu, Y.; Xiao, H.; Jin, L.; Pu, J.J.; Xie, X.; She, J.; Lui, V.W.Y.; et al. Microneedles loaded with anti-PD-1-cisplatin nanoparticles for synergistic cancer immuno-chemotherapy. Nanoscale 2020, 12, 18885–18898. [Google Scholar] [CrossRef] [PubMed]
- Master, A.M.; Rodriguez, M.E.; Kenney, M.E.; Oleinick, N.L.; Sen Gupta, A. Influence of array interspacing on the force required for successful microneedle skin penetration: Theoretical and practical approaches. J. Pharm. Sci. 2010, 99, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- USP Dissolution and Drug Release Tests. Available online: https://www.usp.org/chemical-medicines/dissolution (accessed on 25 July 2021).
- Sekkat, N.; Kalia, Y.N.; Guy, R.H. Biophysical study of porcine ear skin in vitro and its comparison to human skin in vivo. J. Pharm. Sci. 2002, 91, 2376–2381. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Sartorelli, P.; Andersen, H.R.; Angerer, J.; Corish, J.; Drexler, H.; Göen, T.; Griffin, P.; Hotchkiss, S.A.M.; Larese, F.; Montomoli, L.; et al. Percutaneous penetration studies for risk assessment. Environ. Toxicol. Pharmacol. 2000, 8, 133–152. [Google Scholar] [CrossRef]
- Meyer, W. Bemerkungen zur eignung der schweinehaut als biologisches modell fur die haut des menschen. Hautarzt 1996, 47, 178–182. [Google Scholar] [CrossRef]
- OCDE 428 OECD—GUIDELINE FOR THE TESTING OF CHEMICALS: Skin Absorption: In vitro Method. Test 2004, 4, 1–8. [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weimer, P.; Rossi, R.C.; Koester, L.S. Dissolving Microneedles Developed in Association with Nanosystems: A Scoping Review on the Quality Parameters of These Emerging Systems for Drug or Protein Transdermal Delivery. Pharmaceutics 2021, 13, 1601. https://doi.org/10.3390/pharmaceutics13101601
Weimer P, Rossi RC, Koester LS. Dissolving Microneedles Developed in Association with Nanosystems: A Scoping Review on the Quality Parameters of These Emerging Systems for Drug or Protein Transdermal Delivery. Pharmaceutics. 2021; 13(10):1601. https://doi.org/10.3390/pharmaceutics13101601
Chicago/Turabian StyleWeimer, Patrícia, Rochele Cassanta Rossi, and Letícia Scherer Koester. 2021. "Dissolving Microneedles Developed in Association with Nanosystems: A Scoping Review on the Quality Parameters of These Emerging Systems for Drug or Protein Transdermal Delivery" Pharmaceutics 13, no. 10: 1601. https://doi.org/10.3390/pharmaceutics13101601
APA StyleWeimer, P., Rossi, R. C., & Koester, L. S. (2021). Dissolving Microneedles Developed in Association with Nanosystems: A Scoping Review on the Quality Parameters of These Emerging Systems for Drug or Protein Transdermal Delivery. Pharmaceutics, 13(10), 1601. https://doi.org/10.3390/pharmaceutics13101601