Norfloxacin Loaded Lipid Polymer Hybrid Nanoparticles for Oral Administration: Fabrication, Characterization, In Silico Modelling and Toxicity Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
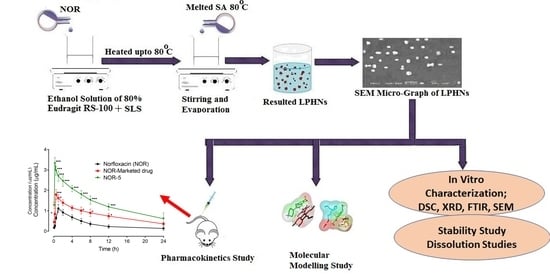
2.2.1. Preparation of Unloaded LPHNs
2.2.2. Preparation of Loaded NOR LPHNs
2.2.3. Lyophilization
2.2.4. Entrapment Efficiency and Drug Loading Capacity
2.2.5. Characterization
Dynamic Light Scattering
Drug-Excipients Interaction
Scanning Electron Microscopy (SEM)
Powder X-ray Diffraction (P-XRD)
Differential Scanning Calorimetry (DSC)
Stability Study
In Vitro Release of NOR from LPHNs
Pharmacokinetic Evaluation
Acute Toxicity
Molecular Modelling
Statistical Analysis
3. Results
3.1. Preparation of LPHNs
3.2. Entrapment Efficiency (EE %) and Drug Loading Capacity (DLC %)
3.3. Drug Excipients Interaction
3.4. Scanning Electron Microscopy (SEM)
3.5. X-ray Diffraction
3.6. Differential Scanning Calorimetry (DSC)
3.7. Stability Study
3.8. In Vitro Drug Release
3.9. Kinetic Modeling
3.10. In Vivo Study
3.11. Acute Toxicity
3.12. Molecular Modelling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McArthur, D.B. Emerging infectious diseases. Nurs. Clin. North Am. 2019, 54, 297. [Google Scholar] [CrossRef] [PubMed]
- Luepke, K.H.; Mohr, J.F. The antibiotic pipeline: Reviving research and development and speeding drugs to market. Expert Rev. Anti-Infect. Ther. 2017, 15, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Fraunfelder, F.W.; Fraunfelder, F.T. Diplopia and Fluoroquinolones. Ophthalmology 2009, 116, 1814–1817. [Google Scholar] [CrossRef]
- Malik, O.A.A. Role of antimicrobials in the treatment of adult patients presenting to the emergency department with acute gastroenteritis - A mini review. Pak. J. Med. Sci. 2017, 33, 488–492. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, T.; Bao, C.; Liu, Z.; Fan, J.; Yang, R.; Qin, S. Design and synthesis of new norfloxacin-1,3,4-oxadiazole hybrids as antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2019, 136, 104966. [Google Scholar] [CrossRef]
- Sood, D.; Kumar, N.; Singh, A.; Sakharkar, M.K.; Tomar, V.; Chandra, R. Antibacterial and Pharmacological Evaluation of Fluoroquinolones: A Chemoinformatics Approach. Genom. Inform. 2018, 16, 44–51. [Google Scholar] [CrossRef]
- Marín, P.; García-Martínez, F.; Hernándis, V.; Escudero, E. Pharmacokinetics of norfloxacin after intravenous, intramuscular and subcutaneous administration to rabbits. J. Veter. Pharmacol. Ther. 2017, 41, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Mehta, M.U.; Uppoor, R.S.; Conner, D.P.; Seo, P.; Vaidyanathan, J.; Volpe, D.A.; Stier, E.; Chilukuri, D.; Dorantes, A.; Ghosh, T.; et al. Impact of the US FDA “Biopharmaceutics Classification System” (BCS) Guidance on Global Drug Development. Mol. Pharm. 2017, 14, 4334–4338. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Mondal, A.; Soni, S.R.; Das, S.; Bhunia, S.; Raju, K.B.; Ghosh, A.; Reddy, C.M. Multidrug salt forms of norfloxacin with non-steroidal anti-inflammatory drugs: Solubility and membrane permeability studies. CrystEngComm 2018, 20, 6420–6429. [Google Scholar] [CrossRef]
- Bueno, M.S.; Chierentin, L.; Bongioanni, A.; Salgado, H.R.N.; Longhi, M.R.; Garnero, C. β-cyclodextrin complexation as an approach to enhance the biopharmaceutical properties of Norfloxacin B Hydrate. Carbohydr. Res. 2019, 485, 107818. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, M.; Jia, L.; Chen, M.; Du, S.; Gong, J. Investigation of Drug–Polymer Miscibility, Molecular Interaction, and Their Effects on the Physical Stabilities and Dissolution Behaviors of Norfloxacin Amorphous Solid Dispersions. Cryst. Growth Des. 2020, 20, 2952–2964. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, J. Solid Dispersion Techniques: A Review. Int. J. Res. Eng. Sci. Manag. 2021, 4, 104–111. [Google Scholar]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin Based Nanoparticles for Drug Delivery and Theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndlovu, S.T.; Ullah, N.; Khan, S.; Ramharack, P.; Soliman, M.; De Matas, M.; Shahid, M.; Sohail, M.; Imran, M.; Shah, S.W.A.; et al. Domperidone nanocrystals with boosted oral bioavailability: Fabrication, evaluation and molecular insight into the polymer-domperidone nanocrystal interaction. Drug Deliv. Transl. Res. 2019, 9, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Pep-Lipid Cubosomes and Vesicles Compartmentalized by Micelles from Self-Assembly of Multiple Neuroprotective Building Blocks Including a Large Peptide Hormone PACAP-DHA. ChemNanoMat 2019, 5, 1381–1389. [Google Scholar] [CrossRef]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid Crystalline Nanostructures as PEGylated Reservoirs of Omega-3 Polyunsaturated Fatty Acids: Structural Insights toward Delivery Formulations against Neurodegenerative Disorders. ACS Omega 2018, 3, 3235–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Sonje, A.; Chandra, A. A comprehensive review on Eudragit polymer. Int. Res. J. Pharmacy 2013, 4, 71–74. [Google Scholar] [CrossRef]
- Xie, F.-M.; Zeng, K.; Li, G.-F.; Lin, Z.-F.; Sun, L.-D. Preparation of stearic acid solid lipid nanoparticles containing podophyllotoxin. Acad. J. First Med Coll. PLA 2005, 25, 99–101. [Google Scholar]
- Rahim, H.; Sadiq, A.; Khan, S.; Amin, F.; Ullah, R.; Shahat, A.A.; Mahmood, M.H. Fabrication and characterization of glimepiride nanosuspension by ultrasonication-assisted precipitation for improvement of oral bioavailability and in vitro α-glucosidase inhibition. Int. J. Nanomed. 2019, 14, 6287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Racault, C.; Langlais, F.; Naslain, R. Solid-state synthesis and characterization of the ternary phase Ti3SiC2. J. Mater. Sci. 1994, 29, 3384–3392. [Google Scholar] [CrossRef]
- Ullah, N.; Khan, S.; Ahmed, S.; Govender, T.; Faidah, H.S.; de Matas, M.; Shahid, M.; Minhas, M.U.; Sohail, M.; Khurram, M. Dexibuprofen nanocrystals with improved therapeutic performance: Fabrication, characterization, in silico modeling, and in vivo evaluation. Int. J. Nanomed. 2018, 13, 1677–1692. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, U.; Burgess, D.J. A novel USP apparatus 4 based release testing method for dispersed systems. Int. J. Pharm. 2010, 388, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Moffat, A.C.; Osseston, M.D.; Widdop, B.; Watts, J. Clarke’s Analysis of Drugs and Poisons. In Pharmaceuticals, Body Fluids and Postmortem Materials; Pharmaceutical Press: London, UK, 2011; Volume 12004. [Google Scholar]
- Roohullah, Z.I.; Nasir, F.; Akhlaq, M.; Sadozai, S.K.; Zada, A.; Khan, A. Sustained release carbamezapine matrix tablets prepared by solvent-evaporation technique using different polymers. Middle-East J. Sci. Res. 2013, 15, 1368–1374. [Google Scholar]
- Kamble, R.; Sharma, S.; Mehta, P. Norfloxacin mixed solvency based solid dispersions: An in-vitro and in-vivo investigation. J. Taibah Univ. Sci. 2017, 11, 512–522. [Google Scholar] [CrossRef]
- OECD. OECD (Organization for Economic Co-Operation and Development) Revised Draft Guidelines 423, OECD Guidelines for the Testing of Chemicals 2001; Organization for Economic Co-operation and Development: Paris, France, 2001. [Google Scholar]
- Krieger, E.; Vriend, G. YASARA View molecular graphics for all devices from smart phones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [Green Version]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Zur Mühlen, A.; Mehnert, W. Drug release and release mechanism of prednisolone loaded solid lipid nanoparticles. Pharmazie 1998, 53, 552–555. [Google Scholar]
- Sadiq, A.A.; Abdul Rassol, A. Formulation and evaluation of silibinin loaded solid lipid nanoparticles for peroral use targeting lower part of gastrointestinal tract. Int. J. Pharm. Pharm. Sci. 2014, 6, 55–67. [Google Scholar]
- Barzegar-Jalali, M. Kinetic Analysis of Drug Release from Nanoparticles. J. Pharm. Pharm. Sci. 2008, 11, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Gao, F.; Bu, H.; Xiao, J.; Li, Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: In vitro characteristics and absorption mechanism in rats. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 740–747. [Google Scholar] [CrossRef]
- Ahmed, S.; Govender, T.; Khan, I.; Rehman, N.U.; Ali, W.; Shah, S.M.H.; Khan, S.; Hussain, Z.; Ullah, R.; Alsaid, M.S. Experimental and molecular modeling approach to optimize suitable polymers for fabrication of stable fluticasone nanoparticles with enhanced dissolution and antimicrobial activity. Drug Des. Dev. Ther. 2018, 12, 255–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, H.A.; Khan, S.; Shahid, M.; Ullah, R.; Bari, A.; Ali, S.S.; Hussain, Z.; Sohail, M.; Khan, S.U.; Htar, T.T. Engineering of Naproxen Loaded Polymer Hybrid Enteric Microspheres for Modified Release Tablets: Development, Characterization, in silico Modelling and in vivo Evaluation. Drug Des. Dev. Ther. 2020, 14, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Khan, S.; Shahid, M.; Raza, A.; Hussain, Z.; Sohali, M.; Minhas, M.U.; Shah, S.W.A.; Khan, N.; Khan, J.; et al. Clarithromycin loaded lipid polymer hybrid nanoparticles: Fabrication, in vitro and in vivo evaluation. Pak. J. Pharm. Sci. 2020, 33, 1303–1313. [Google Scholar]
- Wu, X.; Zhang, L.; Zhang, X.; Zhu, Y.; Wu, Y.; Li, Y.; Li, B.; Liu, S.; Zhao, J.; Ma, Z. Ethyl cellulose nanodispersions as stabilizers for oil in water Pickering emulsions. Sci. Rep. 2017, 7, 12079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matteucci, M.E.; Hotze, M.A.; Johnston, K.P.; Williams, R.O. Drug Nanoparticles by Antisolvent Precipitation: Mixing Energy versus Surfactant Stabilization. Langmuir 2006, 22, 8951–8959. [Google Scholar] [CrossRef]
- Khan, S.; De Matas, M.; Zhang, J.; Anwar, J. Nanocrystal Preparation: Low-Energy Precipitation Method Revisited. Cryst. Growth Des. 2013, 13, 2766–2777. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Shape and Aggregation Control of Nanoparticles: Not Shaken, Not Stirred. J. Am. Chem. Soc. 2006, 128, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Dora, C.P.; Singh, S.K.; Kumar, S.; Datusalia, A.K.; Deep, A. Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol. Pharm. Drug Res. 2010, 67, 283–290. [Google Scholar]
- Lestari, M.L.A.D.; Müller, R.H.; Möschwitzer, J.P. Systematic Screening of Different Surface Modifiers for the Production of Physically Stable Nanosuspensions. J. Pharm. Sci. 2015, 104, 1128–1140. [Google Scholar] [CrossRef]
- Seedat, N.; Kalhapure, R.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid–polymer hybrid nanoparticles: In vitro and in silico studies. Mater. Sci. Eng. C 2016, 61, 616–630. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Rambharose, S.; Mocktar, C.; Vepuri, S.B.; Soliman, M.; Govender, T. Ultra-small lipid-dendrimer hybrid nanoparticles as a promising strategy for antibiotic delivery: In vitro and in silico studies. Int. J. Pharm. 2016, 504, 1–10. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Al-Thamarani, S.; Gardouh, A. Enhanced oral bioavailability and gastroprotective effect of ibuprofen through mixed polymer–lipid nanoparticles. Ther. Deliv. 2021, 12, 363–374. [Google Scholar] [CrossRef]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid lipid nanoparticles for targeted brain drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef]
- Weyhers, H.; Ehlers, S.; Hahn, H.; Souto, E.B.; Müller, R.H. Solid lipid nanoparticles (SLN)–Effects of lipid composition on in vitro degradation and in vivo toxicity. Die Pharm. 2006, 61, 539–544. [Google Scholar]
- Suh, W.H.; Suslick, K.; Stucky, G.D.; Suh, Y.-H. Nanotechnology, nanotoxicology, and neuroscience. Prog. Neurobiol. 2009, 87, 133–170. [Google Scholar] [CrossRef] [Green Version]
- Dang, L.; Yang, H.; Black, S.; Wei, H. The Effect of Temperature and Solvent Composition on Transformation of β- to α-Glycine as Monitored in Situ by FBRM and PVM. Org. Process. Res. Dev. 2009, 13, 1301–1306. [Google Scholar] [CrossRef]
- Abbaspour, M.; Makhmalzadeh, B.; Arastoo, Z.; Jahangiri, A.; Shiralipour, R. Effect of Anionic Polymers on Drug Loading and Release from Clindamycin Phosphate Solid Lipid Nanoparticles. Trop. J. Pharm. Res. 2013, 12, 477–482. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, R.; Kaur, I.P. Pharmacokinetics, tissue distribution and relative bioavailability of isoniazid-solid lipid nanoparticles. Int. J. Pharm. 2013, 441, 202–212. [Google Scholar] [CrossRef]
- Yang, S.C.; Lu, L.F.; Cai, Y.; Zhu, J.B.; Liang, B.W.; Yang, C.Z. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Control. Release 1999, 59, 299–307. [Google Scholar] [CrossRef]











| Formulation Code | Stearic Acid (gm) | Eudragit (gm) | Sonication (Hz) | SLS | Sonication Time (min) | Stirring Time (min) |
|---|---|---|---|---|---|---|
| BF-1 | 0.5 | 1.0 | 30% | 0.2 | 2 | 20 |
| BF-2 | 0.5 | 1.0 | 30% | 0.3 | 2 | 20 |
| BF-3 | 0.5 | 1.0 | 30% | 0.5 | 5 | 20 |
| BF-4 | 0.5 | 1.0 | 30% | 0.6 | 8 | 20 |
| BF-5 | 0.5 | 1.0 | 30% | 0.8 | 8 | 40 |
| BF-6 | 0.5 | 1.0 | 30% | 1.0 | 8 | 60 |
| Formulation Code | Oleic Acid (mL) | Ethyl Cellulose (gm) |
|---|---|---|
| NOR-1 | 0 | 0 |
| NOR-2 | 0.1 | 0 |
| NOR-3 | 0.2 | 0 |
| NOR-4 | 0.2 | 0.3 |
| NOR-5 | 0.2 | 0.5 |
| Formulation Code | Stearic Acid (gm) | Eudragit (gm) | Sonication (Hz) | SLS | Sonication Time (min) | Stirring Time (min) | Particle Size (nm) ± SD |
|---|---|---|---|---|---|---|---|
| BF-1 | 0.5 | 1.0 | 30% | 0.2 | 2 | 20 | 605.41 ± 5.0 |
| BF-2 | 0.5 | 1.0 | 30% | 0.3 | 2 | 20 | 445.34 ± 4.5 |
| BF-3 | 0.5 | 1.0 | 30% | 0.5 | 5 | 20 | 310.76 ± 5.0 |
| BF-4 | 0.5 | 1.0 | 30% | 0.6 | 8 | 20 | 140.82 ± 4.0 |
| BF-5 | 0.5 | 1.0 | 30% | 0.8 | 8 | 40 | 129.53 ± 3.0 |
| BF-6 | 0.5 | 1.0 | 30% | 1.0 | 8 | 60 | 115.25 ± 2.5 |
| Formulation Code | Oleic Acid (mL) | Ethyl Cellulose (gm) | EE (%) | DLC (%) |
|---|---|---|---|---|
| NOR-1 | 0 | 0 | 65± 2.08 | 0.258 |
| NOR-2 | 0.1 | 0 | 71 ± 2.51 | 0.272 |
| NOR-3 | 0.2 | 0 | 79 ± 1.52 | 0.291 |
| NOR-4 | 0.2 | 0.3 | 89 ± 0.1 | 0.295 |
| NOR-5 | 0.2 | 0.5 | 97 ± 1.52 | 0.302 |
| Formulation | Zero Order (R2) | First Order (R2) | Higuchi Model (R2) | Korsmeyar-Peppas | |
|---|---|---|---|---|---|
| (n) | (R2) | ||||
| NOR-1 | 0.935 | 0.891 | 0.920 | 0.683664 | 0.941 |
| NOR-2 | 0.946 | 0.975 | 0.934 | 0.747877 | 0.943 |
| NOR-3 | 0.953 | 0.941 | 0.945 | 0.807612 | 0.945 |
| NOR-4 | 0.977 | 0.953 | 0.969 | 0.878347 | 0.955 |
| NOR-5 | 0.981 | 0.948 | 0.980 | 0.903442 | 0.967 |
| Sample | Pharmacokinetic Parameter | |||
|---|---|---|---|---|
| T1/2 (h) | Tmax (h) | Cmax (µg/mL) | AUC0–t (µgh/mL) | |
| Norfloxacin | 4.037 ± 2.024 | 1.10 ± 0.654 | 1.133 ± 0.1856 | 8.600 ± 2.511 |
| NOR-Nano | 26.07 ± 3.273 *** | 0.31 ± 0.874 ** | 3.333 ± 0.2963 *** | 33.23 ± 4.486 ** |
| NOR-Marketed drug | 8.64 ± 1.497 * | 0.54 ± 1.021 | 1.833 ± 0.2404 | 19.30 ± 3.118 * |
| Dose (mg/kg) | No. of Dead Mice | Percent Lethality | LD50 (mg/kg) |
|---|---|---|---|
| 50 | 0 | 00.00 | >1600 |
| 100 | 0 | 00.00 | |
| 200 | 0 | 00.00 | |
| 400 | 0 | 00.00 | |
| 800 | 1 | 16.66 | |
| 1600 | 2 | 33.33 |
| S. No | Co-Polymer Complex | Binding Energies (kcal/mol) |
|---|---|---|
| 1 | Stearic Acid-NOR | −2.4 |
| 2 | SLS-NOR | −2.4 |
| 3 | Eudragit-NOR | −2.3 |
| 4 | Oleic Acid-NOR | −2.5 |
| 5 | Ethyl Cellulose-NOR | −3.4 |
| 6 | Stearic Acid-Eudragit-SLS-NOR | −5.2 |
| 7 | Stearic Acid-Eudragit-SLS- OA-EC-NOR | −5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; Khan, S.; Kazi, M.; Alshehri, S.M.; Shahid, M.; Khan, S.U.; Hussain, Z.; Sohail, M.; Shafique, M.; Hamid, H.A.; et al. Norfloxacin Loaded Lipid Polymer Hybrid Nanoparticles for Oral Administration: Fabrication, Characterization, In Silico Modelling and Toxicity Evaluation. Pharmaceutics 2021, 13, 1632. https://doi.org/10.3390/pharmaceutics13101632
Khan MA, Khan S, Kazi M, Alshehri SM, Shahid M, Khan SU, Hussain Z, Sohail M, Shafique M, Hamid HA, et al. Norfloxacin Loaded Lipid Polymer Hybrid Nanoparticles for Oral Administration: Fabrication, Characterization, In Silico Modelling and Toxicity Evaluation. Pharmaceutics. 2021; 13(10):1632. https://doi.org/10.3390/pharmaceutics13101632
Chicago/Turabian StyleKhan, Muhammad Asghar, Shahzeb Khan, Mohsin Kazi, Sultan M. Alshehri, Muhammad Shahid, Shafi Ullah Khan, Zahid Hussain, Muhammad Sohail, Muhammad Shafique, Hajra Afeera Hamid, and et al. 2021. "Norfloxacin Loaded Lipid Polymer Hybrid Nanoparticles for Oral Administration: Fabrication, Characterization, In Silico Modelling and Toxicity Evaluation" Pharmaceutics 13, no. 10: 1632. https://doi.org/10.3390/pharmaceutics13101632
APA StyleKhan, M. A., Khan, S., Kazi, M., Alshehri, S. M., Shahid, M., Khan, S. U., Hussain, Z., Sohail, M., Shafique, M., Hamid, H. A., Kamran, M., Elhissi, A., Wasim, M., & Thu, H. E. (2021). Norfloxacin Loaded Lipid Polymer Hybrid Nanoparticles for Oral Administration: Fabrication, Characterization, In Silico Modelling and Toxicity Evaluation. Pharmaceutics, 13(10), 1632. https://doi.org/10.3390/pharmaceutics13101632