Marine Polysaccharides for Wound Dressings Application: An Overview
Abstract
:1. Introduction
2. MPs for Wound Dressings
2.1. Chitosan
2.2. Marine Glycosaminoglycans
| GAGs Types | Sources | Properties and Applications | Refs |
|---|---|---|---|
| Heparan sulfate | Amussium pleuronectus | Anti-thrombin A more bio-safe source of heparan sulphate | [107] |
| Heparan sulphate | Portunus pelagicus | Highly attenuated anticoagulant activity Treatment of Alzheimer’s disease | [108] |
| Heparan sulfate | Ascidian Phallusia nigra | Low anticoagulant and antithrombotic activity Effective in preventing metastasis of cancerous tissue | [109] |
| Chondroitin sulfate | Ludwigothurea grisea | Anti-inflammatory Blocking cancer metastasis | [110] |
| Chondroitin sulfate | Oncorhynch | Promotes collagen fibre formation Anti-ageing | [99] |
| Chondroitin sulfate | Raja clavata | Cheap raw material cost | [111] |
| Chondroitin sulfate | Echinodermata Ophiuroidea | Promoting fibroblast growth factor 2-induced cell signalling | [112] |
| Dermatan sulfate | Echinodermata Ophiuroidea | Promoting fibroblast growth factor 2-induced cell signalling | [112] |
| Dermatan sulfate | Mitsukurina owstoniPrionace glauca | Neurite outgrowth-promoting | [100] |
2.3. Alginate
2.4. Fucoidan
2.5. Laminarin
2.6. Carrageenan
2.7. Agar
2.8. Ulvan
2.9. Marine Microorganisms Exopolysaccharides
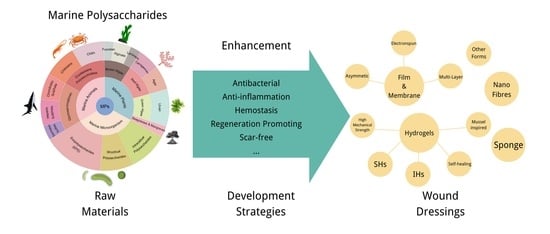
3. Enhancement Strategies for MPs Wound Dressings
3.1. Development of Activities-Enhanced MPs Wound Dressings
3.2. Development of Different Forms of MPs Wound Dressings
3.2.1. MPs Hydrogel
3.2.2. MPs Nanofibrous
3.2.3. MPs Film/Membrane
3.2.4. MPs Sponge
3.2.5. Other Types of MPs Dressings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines 2020, 8, 301. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-Based Composite Materials for Wound Dressing Application: A Mini Review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Dumville, J.C.; Owens, G.L.; Crosbie, E.J.; Peinemann, F.; Liu, Z. Negative Pressure Wound Therapy for Treating Surgical Wounds Healing by Secondary Intention. Cochrane Database Syst. Rev. 2015, 6, CD011278. [Google Scholar] [CrossRef]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Badeleh, S.M.; Maleki, Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Int. J. Low. Extrem. Wounds 2020, 153473462092485. [Google Scholar] [CrossRef]
- Haalboom, M. Chronic Wounds: Innovations in Diagnostics and Therapeutics. Curr. Med. Chem. 2018, 25, 5772–5781. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Zhang, X.; Chen, Z.; Zhao, D.; Ma, J. A Comparative Study of Two Porous Sponge Scaffolds Prepared by Collagen Derived from Porcine Skin and Fish Scales as Burn Wound Dressings in a Rabbit Model. Regen. Biomater. 2020, 7, 63–70. [Google Scholar] [CrossRef]
- Guadarrama-Acevedo, M.C.; Mendoza-Flores, R.A.; Del Prado-Audelo, M.L.; Urban-Morlan, Z.; Giraldo-Gomez, D.M.; Magana, J.J.; Gonzalez-Torres, M.; Reyes-Hernandez, O.D.; Figueroa-Gonzalez, G.; Caballero-Floran, I.H.; et al. Development and Evaluation of Alginate Membranes with Curcumin-Loaded Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics 2019, 11, 389. [Google Scholar] [CrossRef] [Green Version]
- Silvestro, I.; Lopreiato, M.; Scotto d’Abusco, A.; Di Lisio, V.; Martinelli, A.; Piozzi, A.; Francolini, I. Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings. Int. J. Mol. Sci. 2020, 21, 2070. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Fu, R.; Yu, C.; Li, Z.; Guan, H.; Hu, D.; Zhao, D.; Lu, L. Silver Nanoparticle/Chitosan Oligosaccharide/Poly(Vinyl Alcohol) Nanofibers as Wound Dressings: A Preclinical Study. Int. J. Nanomedicine 2013, 8, 4131–4145. [Google Scholar] [CrossRef] [PubMed]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging Innovative Wound Dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Dermatol. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound Dressings—A Review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of Hydrogel Dressing for Diabetic Wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef]
- Guarderas, F.; Leavell, Y.; Sengupta, T.; Zhukova, M.; Megraw, T.L. Assessment of Chicken-Egg Membrane as a Dressing for Wound Healing. Adv. Skin Wound Care 2016, 29, 131–134. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun Polymeric Nanofibres as Wound Dressings: A Review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef]
- Bombin, A.D.J.; Dunne, N.J.; McCarthy, H.O. Electrospinning of Natural Polymers for the Production of Nanofibres for Wound Healing Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 110994. [Google Scholar] [CrossRef]
- Meuleneire, F. A Vapour-Permeable Film Dressing Used on Superficial Wounds. Br. J. Nurs. 2014, 23, S36–S43. [Google Scholar] [CrossRef]
- Bombaldi de Souza, R.; Bombaldi de Souza, F.C.; Bierhalz, A.; Pires, A.L.; Moraes, Â. Chapter 7—Biopolymer-based films and membranes as wound dressings. In Biopolymer Membranes and Films Health, Food, Environment, and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–194. ISBN 978-0-12-818134-8. [Google Scholar]
- Colobatiu, L.; Gavan, A.; Potarniche, A.-V.; Rus, V.; Diaconeasa, Z.; Mocan, A.; Tomuta, I.; Mirel, S.; Mihaiu, M. Evaluation of Bioactive Compounds-Loaded Chitosan Films as a Novel and Potential Diabetic Wound Dressing Material. React. Funct. Polym. 2019, 145, 104369. [Google Scholar] [CrossRef]
- Constantin, V.D.; Carâp, A.; Bobic, S.; Budu, V.; Albu Kaya, M.; Marin, Ş.; Marin, M.M.; Socea, B. Tissue Engineering—Collagen Sponge Dressing for Chronic Wounds. In Proceedings of the ICAMS 7th International Conference on Advanced Materials and Systems, Bucharest, Romania, 18 October 2018; pp. 63–68. [Google Scholar]
- Gustaite, S.; Kazlauske, J.; Bobokalonov, J.; Perni, S.; Dutschk, V.; Liesiene, J.; Prokopovich, P. Characterization of Cellulose Based Sponges for Wound Dressings. Colloids Surf. Physicochem. Eng. Asp. 2015, 480, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Khorshidi, S.; Mohebbali, M.; Imani, R.; Mahmoodi, M.; Solouk, A. Electrospun Fibroin/Graphene Oxide Nanocomposite Mats: An Optimization for Potential Wound Dressing Applications. Fibers Polym. 2020, 21, 480–488. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Hsu, W.-S.; Chung, W.-Y.; Ko, T.-H.; Lin, J.-H. Silver-Based Wound Dressings Reduce Bacterial Burden and Promote Wound Healing: Silver-Containing Dressing for Accelerated Wound Healing. Int. Wound J. 2016, 13, 505–511. [Google Scholar] [CrossRef]
- Woo, H.-D.; Park, K.-T.; Kim, E.-H.; Heo, Y.; Jeong, J.-H.; Pyun, D.-G.; Choi, C.-S.; Lee, J.-G.; Han, D.-K.; Nah, J.-W.; et al. Preparation of UV-Curable Gelatin Derivatives for Drug Immobilization on Polyurethane Foam: Development of Wound Dressing Foam. Macromol. Res. 2015, 23, 994–1003. [Google Scholar] [CrossRef]
- Benskin, L. Commentary: First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence. Front. Pharmacol. 2020, 11, 1272. [Google Scholar] [CrossRef]
- Nagasawa, F.; Yoshikawa, Y.; Tanida, I.; Osawa, S. Development of Wound Dressing with Sustained-Release of Drug Using Natural Polymer. J. Soc. Mater. Sci. Jpn. 2018, 67, 918–923. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, B.; Shekhar, S.; Jain, P. Natural Polymer-Based Composite Wound Dressings; AMRT Book Series; Springer: New York, NY, USA, 2021; pp. 401–423. ISBN 978-3-030-70265-6. [Google Scholar]
- Xiao, R.; Grinstaff, M.W. Chemical Synthesis of Polysaccharides and Polysaccharide Mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- Borro, B.C.; Malmsten, M. Complexation between Antimicrobial Peptides and Polyelectrolytes. Adv. Colloid Interface Sci. 2019, 270, 251–260. [Google Scholar] [CrossRef]
- Evangelista, T.F.S.; Andrade, G.R.S.; Nascimento, K.N.S.; dos Santos, S.B.; de Fátima Costa Santos, M.; Da Ros Montes D’Oca, C.; dos S. Estevam, C.; Gimenez, I.F.; Almeida, L.E. Supramolecular Polyelectrolyte Complexes Based on Cyclodextrin-Grafted Chitosan and Carrageenan for Controlled Drug Release. Carbohydr. Polym. 2020, 245, 116592. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-Based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohydr. Polym. 2020, 238, 116126. [Google Scholar] [CrossRef]
- Jing, X.; Sun, Y.; Ma, X.; Hu, H. Marine Polysaccharides: Green and Recyclable Resources as Wound Dressings. Mater. Chem. Front. 2021, 5, 5595–5616. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [Green Version]
- Manivasagan, P.; Oh, J. Marine Polysaccharide-Based Nanomaterials as a Novel Source of Nanobiotechnological Applications. Int. J. Biol. Macromol. 2016, 82, 315–327. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic Acid and Chitosan-Based Nanosystems: A New Dressing Generation for Wound Care. Expert Opin. Drug Deliv. 2019, 16, 715–740. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [Green Version]
- Xin, S.; Li, Y.; Li, W.; Du, J.; Huang, R.; Du, Y.; Deng, H. Carboxymethyl Chitin/Organic Rectorite Composites Based Nanofibrous Mats and Their Cell Compatibility. Carbohydr. Polym. 2012, 90, 1069–1074. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl Chitosan: Properties and Biomedical Applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Li, J.; Jiang, Z.; Tong, R.; Duan, X.; Bai, L.; Shi, J. Chitosan, N,N,N-Trimethyl Chitosan (TMC) and 2-Hydroxypropyltrimethyl Ammonium Chloride Chitosan (HTCC): The Potential Immune Adjuvants and Nano Carriers. Int. J. Biol. Macromol. 2020, 154, 339–348. [Google Scholar] [CrossRef]
- Khan, Z.A.; Jamil, S.; Akhtar, A.; Bashir, M.M.; Yar, M. Chitosan Based Hybrid Materials Used for Wound Healing Applications- A Short Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 419–436. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun Chitosan Membranes Containing Bioactive and Therapeutic Agents for Enhanced Wound Healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Al-Jbour, N.D.; Beg, M.D.; Gimbun, J.; Alam, A.K.M.M. An Overview of Chitosan Nanofibers and Their Applications in the Drug Delivery Process. Curr. Drug Deliv. 2019, 16, 272–294. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Chen, Q.; Fu, L.; Tao, L.; Wei, Y. Injectable and Self-Healing Chitosan Hydrogel Based on Imine Bonds: Design and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 2198. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, Z.; Huang, J.; Zhao, M.; Wu, J. In Situformation of Injectable Hydrogels for Chronic Wound Healing. J. Mater. Chem. B 2020, 8, 8768–8780. [Google Scholar] [CrossRef]
- Mude, L.; Sanapalli, B.K.R.; Narayanan, A.; Singh, S.K.; Karri, V.V.S.R. Overview of in Situ Gelling Injectable Hydrogels for Diabetic Wounds. Drug Dev. Res. 2021, 82, 503–522. [Google Scholar] [CrossRef]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D Printed Chitosan-Pectin Hydrogel Wound Dressing for Lidocaine Hydrochloride Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109873. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Liu, J.; Xu, Y.; Wang, Y.; Ren, H.; Li, X. Acetate Chitosan with CaCO3 Doping Form Tough Hydrogel for Hemostasis and Wound Healing. Polym. Adv. Technol. 2019, 30, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Concalves Ferreira, M.O.; de Lima, I.S.; Sousa Morais, A.I.; Silva, S.O.; Fonseca de Carvalho, R.B.; Ribeiro, A.B.; Osajima, J.A.; Silva Filho, E.C. Chitosan Associated with Chlorhexidine in Gel Form: Synthesis, Characterization and Healing Wounds Applications. J. Drug Deliv. Sci. Technol. 2019, 49, 375–382. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Sigroha, S.; Khatkar, A. Chitosan- A Naturally Derived Antioxidant Polymer with Diverse Applications. Curr. Org. Chem. 2017, 21, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Gao, T.; Wang, Y.; Liu, J.; Zhang, J.; Yao, R.; Wu, F. Modulating Cationicity of Chitosan Hydrogel to Prevent Hypertrophic Scar Formation during Wound Healing. Int. J. Biol. Macromol. 2020, 154, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Vinsova, J.; Vavrikova, E. Chitosan Derivatives with Antimicrobial, Antitumour and Antioxidant Activities—A Review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, D.-Y.; Lu, S.-T.; Li, P.-W.; Li, S.-D. Chitosan-Based Composite Materials for Prospective Hemostatic Applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlusca, I.P.; Matiut, D.S.; Lisa, G.; Silion, M.; Gradinaru, L.; Oprea, S.; Popa, I.M. Preparation and Characterization of Chitosan-Poly(Vinyl Alcohol)-Neomycin Sulfate Films. Polym. Bull. 2018, 75, 3971–3986. [Google Scholar] [CrossRef]
- Hedayatyanfard, K.; Bagheri-Khoulenjani, S.; Hashemia, A.; Ziai, S.A. Semi-IPN Films and Electrospun Nanofibers Based on Chitosan/PVA as an Antibacterial Wound Dressing. Iran. J. Pharm. Res. 2019, 18, 1156–1167. [Google Scholar]
- Kalantari, K.; Mostafavi, E.; Saleh, B.; Soltantabar, P.; Webster, T.J. Chitosan/PVA Hydrogels Incorporated with Green Synthesized Cerium Oxide Nanoparticles for Wound Healing Applications. Eur. Polym. J. 2020, 134, 109853. [Google Scholar] [CrossRef]
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound Healing with Alginate/Chitosan Hydrogel Containing Hesperidin in Rat Model. J. Drug Deliv. Sci. Technol. 2020, 55, 101379. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Hong, Y.-L.; Wu, T.-L. Novel Silver and Nanoparticle-Encapsulated Growth Factor Co-Loaded Chitosan Composite Hydrogel with Sustained Antimicrobility and Promoted Biological Properties for Diabetic Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111385. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A Review on Chitosan and Its Nanocomposites in Drug Delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Basha, M.; AbouSamra, M.M.; Awad, G.A.; Mansy, S.S. A Potential Antibacterial Wound Dressing of Cefadroxil Chitosan Nanoparticles in Situ Gel: Fabrication, in Vitro Optimization and in Vivo Evaluation. Int. J. Pharm. 2018, 544, 129–140. [Google Scholar] [CrossRef]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Farahani, M.S.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of Curcumin Loaded Chitosan Nanoparticle within Poly (Epsilon-Caprolactone) and Gelatin Fiber Mat for Wound Healing and Layered Dermal Reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, Y.; Shen, Y.; Shi, Y.; Li, F.; Su, C.; Zhao, L. Chitosan Nanoparticles Loaded Hydrogels Promote Skin Wound Healing through the Modulation of Reactive Oxygen Species. Artif. Cells Nanomed. Biotechnol. 2018, 46, S138–S149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehkordi, N.K.; Minaiyan, M.; Talebi, A.; Akbari, V.; Taheri, A. Nanocrystalline Cellulose-Hyaluronic Acid Composite Enriched with GM-CSF Loaded Chitosan Nanoparticles for Enhanced Wound Healing. Biomed. Mater. 2019, 14, 035003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, G.; Wang, D.; Zheng, Y.; Li, Y.; Meng, W.; Zhang, X.; Du, F.; Lee, S. Ag@MOF-Loaded Chitosan Nanoparticle and Polyvinyl Alcohol/Sodium Alginate/Chitosan Bilayer Dressing for Wound Healing Applications. Int. J. Biol. Macromol. 2021, 175, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Rastogi, K.; Tyagi, P.; Rawat, H.; Mittal, G.; Jamini, A.; Singh, H.; Tyagi, A. Comparative Analysis of Collagen and Chitosan-Based Dressing for Haemostatic and Wound Healing Application. AAPS PharmSciTech 2021, 22, 1–5. [Google Scholar] [CrossRef]
- Cao, Z.; Shen, Z.; Luo, X.; Zhang, H.; Liu, Y.; Cai, N.; Xue, Y.; Yu, F. Citrate-Modified Maghemite Enhanced Binding of Chitosan Coating on Cellulose Porous Membranes for Potential Application as Wound Dressing. Carbohydr. Polym. 2017, 166, 320–328. [Google Scholar] [CrossRef]
- Monteiro, C.; Fernandes, H.; Oliveira, D.; Vale, N.; Barbosa, M.; Gomes, P.; Martins, M.C.L. AMP-Chitosan Coating with Bactericidal Activity in the Presence of Human Plasma Proteins. Molecules 2020, 25, 3046. [Google Scholar] [CrossRef]
- Cao, Z.; Luo, X.; Zhang, H.; Fu, Z.; Shen, Z.; Cai, N.; Xue, Y.; Yu, F. A Facile and Green Strategy for the Preparation of Porous Chitosan-Coated Cellulose Composite Membranes for Potential Applications as Wound Dressing. Cellulose 2016, 23, 1349–1361. [Google Scholar] [CrossRef]
- Millner, R.W.J.; Lockhart, A.S.; Bird, H.; Alexiou, C. A New Hemostatic Agent: Initial Life-Saving Experience with Celox (Chitosan) in Cardiothoracic Surgery. Ann. Thorac. Surg. 2009, 87, e13–e14. [Google Scholar] [CrossRef]
- Devlin, J.J.; Kircher, S.; Kozen, B.G.; Littlejohn, L.F.; Johnson, A.S. Comparison of ChitoFlex®, CELOXTM, and QuikClot® in Control of Hemorrhage. J. Emerg. Med. 2009, 41, 237–245. [Google Scholar] [CrossRef]
- Williams, C. Tegasorb Hydrocolloid Dressing: Advanced Formulation. Br. J. Nurs. Mark Allen Publ. 1996, 5, 1271–1272. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. Rsc Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan Based Hydrogels and Their Applications for Drug Delivery in Wound Dressings: A Review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, X. Injectable and Degradable PH-Responsive Hydrogels via Spontaneous Amino-Yne Click Reaction. Acs Appl. Mater. Interfaces 2018, 10, 361–370. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H.; Wang, X. Synthesis and Characterization of an Injectable Epsilon-Polylysine/Carboxymethyl Chitosan Hydrogel Used in Medical Application. Mater. Chem. Phys. 2020, 248, 122902. [Google Scholar] [CrossRef]
- Acute Toxicity of High Dosage Carboxymethyl Chitosan and Its Effect on the Blood Parameters in Rats | SpringerLink. Available online: https://link.springer.com/article/10.1007%2Fs10856-011-4467-4 (accessed on 24 August 2021).
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from Marine Sources as Therapeutic Agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catarina Vale, A.; Pereira, P.; Margarida Barbosa, A.; Torrado, E.; Mano, J.F.; Alves, N.M. Antibacterial Free-Standing Polysaccharide Composite Films Inspired by the Sea. Int. J. Biol. Macromol. 2019, 133, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Vale, A.C.; Reis, R.L.; Alves, N.M. Bioactive and Adhesive Properties of Multilayered Coatings Based on Catechol-Functionalized Chitosan/Hyaluronic Acid and Bioactive Glass Nanoparticles. Int. J. Biol. Macromol. 2020, 157, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Wu, H.; Wang, X.; Yu, W.; Han, F. Biochemical Characterization of a Thermophilic Hyaluronate Lyase TcHly8C from Thermasporomyces Composti DSM22891. Int. J. Biol. Macromol. 2020, 165, 1211–1218. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernandez, N.; Matias, A.A.; do Rosario Bronze, M. Hyaluronic Acid and Chondroitin Sulfate from Marine and Terrestrial Sources: Extraction and Purification Methods. Carbohydr. Polym. 2020, 243, 116411. [Google Scholar] [CrossRef]
- Van Hove, A.H.; Benoit, D.S.W. Depot-Based Delivery Systems for Pro-Angiogenic Peptides: A Review. Front. Bioeng. Biotechnol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Middeldorp, S. Heparin: From Animal Organ Extract to Designer Drug. Thromb. Res. 2008, 122, 753–762. [Google Scholar] [CrossRef]
- Pal, D.; Saha, S. Chondroitin: A Natural Biomarker with Immense Biomedical Applications. RSC Adv. 2019, 9, 28061–28077. [Google Scholar] [CrossRef] [Green Version]
- Pomin, V.H.; Vignovich, W.P.; Gonzales, A.V.; Vasconcelos, A.A.; Mulloy, B. Galactosaminoglycans: Medical Applications and Drawbacks. Molecules 2019, 24, 2803. [Google Scholar] [CrossRef] [Green Version]
- Gulati, K.; Meher, M.K.; Poluri, K.M. Glycosaminoglycan-Based Resorbable Polymer Composites in Tissue Refurbishment. Regen. Med. 2017, 12, 431–457. [Google Scholar] [CrossRef]
- Lima, M.; Rudd, T.; Yates, E. New Applications of Heparin and Other Glycosaminoglycans. Molecules 2017, 22, 749. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Yates, E.A.; Skidmore, M.A. Marine Glycosaminoglycan-like Carbohydrates as Potential Drug Candidates for Infectious Disease. Biochem. Soc. Trans. 2018, 46, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Pavão, M.S.G. Glycosaminoglycans Analogs from Marine Invertebrates: Structure, Biological Effects, and Potential as New Therapeutics. Front. Cell. Infect. Microbiol. 2014, 4, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatara, Y.; Kakizaki, I.; Suto, S.; Ishioka, H.; Negishi, M.; Endo, M. Chondroitin Sulfate Cluster of Epiphycan from Salmon Nasal Cartilage Defines Binding Specificity to Collagens. Glycobiology 2015, 25, 557–569. [Google Scholar] [CrossRef] [Green Version]
- Higashi, K.; Takeuchi, Y.; Mukuno, A.; Tomitori, H.; Miya, M.; Linhardt, R.J.; Toida, T. Composition of Glycosaminoglycans in Elasmobranchs Including Several Deep-Sea Sharks: Identification of Chondroitin/Dermatan Sulfate from the Dried Fins of Isurus Oxyrinchus and Prionace Glauca. PLoS ONE 2015, 10, e0120860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, E. Natural Collagenic Skeleton of Marine Sponges in Pharmaceutics: Innovative Biomaterial for Topical Drug Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 710–720. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Z.; Luo, L.; Tao, M.; Chang, X.; Yang, L.; Huang, X.; Hu, L.; Wu, M. A Non-Anticoagulant Heparin-like Snail Glycosaminoglycan Promotes Healing of Diabetic Wound. Carbohydr. Polym. 2020, 247, 116682. [Google Scholar] [CrossRef]
- Lohmann, N.; Schirmer, L.; Atallah, P.; Wandel, E.; Ferrer, R.A.; Werner, C.; Simon, J.C.; Franz, S.; Freudenberg, U. Glycosaminoglycan-Based Hydrogels Capture Inflammatory Chemokines and Rescue Defective Wound Healing in Mice. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Sandri, G.; Miele, D.; Faccendini, A.; Bonferoni, M.C.; Rossi, S.; Grisoli, P.; Taglietti, A.; Ruggeri, M.; Bruni, G.; Vigani, B.; et al. Chitosan/Glycosaminoglycan Scaffolds: The Role of Silver Nanoparticles to Control Microbial Infections in Wound Healing. Polymers 2019, 11, 1207. [Google Scholar] [CrossRef] [Green Version]
- Volpi, N.; Maccari, F. Structural Characterization and Antithrombin Activity of Dermatan Sulfate Purified from Marine Clam Scapharca Inaequivalvis. Glycobiology 2009, 19, 356–367. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, R.; Shanmugam, A. Isolation and Characterization of Low Molecular Weight Glycosaminoglycans from Marine Mollusc Amussium Pleuronectus (Linne) Using Chromatography. Appl. Biochem. Biotechnol. 2010, 160, 791–799. [Google Scholar] [CrossRef]
- Saravanan, R.; Shanmugam, A. Is Isolation and Characterization of Heparan Sulfate from Marine Scallop Amussium Pleuronectus (Linne.) an Alternative Source of Heparin? Carbohydr. Polym. 2011, 86, 1082–1084. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Cooper, L.C.; Devlin, A.J.; Procter, P.; Guimond, S.E.; Guerrini, M.; Fernig, D.G.; Lima, M.A.; Yates, E.A.; Skidmore, M.A. A Glycosaminoglycan Extract from Portunus Pelagicus Inhibits BACE1, the β Secretase Implicated in Alzheimer’s Disease. Mar. Drugs 2019, 17, 293. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.F.S.; Motta, J.M.; Teixeira, F.C.O.B.; Gomes, A.M.; Vilanova, E.; Kozlowski, E.O.; Borsig, L.; Pavão, M.S.G. Non-Anticoagulant Heparan Sulfate from the Ascidian Phallusia Nigra Prevents Colon Carcinoma Metastasis in Mice by Disrupting Platelet-Tumor Cell Interaction. Cancers 2020, 12, 1353. [Google Scholar] [CrossRef]
- Borsig, L.; Wang, L.; Cavalcante, M.C.M.; Cardilo-Reis, L.; Ferreira, P.L.; Mourąo, P.A.S.; Esko, J.D.; Pavąo, M.S.G. Selectin Blocking Activity of a Fucosylated Chondroitin Sulfate Glycosaminoglycan from Sea Cucumber: EFFECT ON TUMOR METASTASIS AND NEUTROPHIL RECRUITMENT. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murado, M.A.; Fraguas, J.; Montemayor, M.I.; Vázquez, J.A.; González, P. Preparation of Highly Purified Chondroitin Sulphate from Skate (Raja Clavata) Cartilage by-Products. Process Optimization Including a New Procedure of Alkaline Hydroalcoholic Hydrolysis. Biochem. Eng. J. 2010, 49, 126–132. [Google Scholar] [CrossRef]
- Ramachandra, R.; Namburi, R.B.; Ortega-Martinez, O.; Shi, X.; Zaia, J.; Dupont, S.T.; Thorndyke, M.C.; Lindahl, U.; Spillmann, D. Brittlestars Contain Highly Sulfated Chondroitin Sulfates/Dermatan Sulfates That Promote Fibroblast Growth Factor 2-Induced Cell Signaling. Glycobiology 2014, 24, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial Alginate Production, Modification and Its Applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwon, S.H.; Yoon, J.; Seok, H.K.; Oh, K.H.; Sun, J.-Y. Gelation Dynamics of Ionically Crosslinked Alginate Gel with Various Cations. Macromol. Res. 2015, 23, 1112–1116. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and Mechanical Properties of Alginate Based Composite Gels. Trends Food Sci. Technol. 2020, 106, 150–159. [Google Scholar] [CrossRef]
- Severino, P.; da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, L.; Xu, G.K.; Ma, C.; Yang, X.G.; Yao, J.M. Potential of Alginate Fibers Incorporated with Drug-Loaded Nanocapsules as Drug Delivery Systems. J. Mater. Chem. B 2014, 2, 7596–7604. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kucharska, M.K.; Struszczyk, M.H.; Niekraszewicz, A.; Ciechańska, D.; Witczak, E.; Tarkowska, S.; Fortuniak, K.; Gulbas-Diaz, A.; Rogaczewska, A.; Płoszaj, I.; et al. Tromboguard—First Aid Wound Dressing. Prog. Chem. Appl. Chitin Its Deriv. 2011, 16, 121–130. [Google Scholar]
- Summa, M.; Russo, D.; Penna, I.; Margaroli, N.; Bayer, I.S.; Bandiera, T.; Athanassiou, A.; Bertorelli, R. A Biocompatible Sodium Alginate/Povidone Iodine Film Enhances Wound Healing. Eur. J. Pharm. Biopharm. 2018, 122, 17–24. [Google Scholar] [CrossRef]
- Pereira, R.; Mendes, A.; Bartolo, P. Evaluating the properties of an alginate wound dressing for skin repair. In Advanced Ma-terials and Engineering Materials Ii; Kida, K., Ed.; Trans Tech Publications Ltd.: Durnten-Zurich, Switzerland, 2013; Volume 683, pp. 141–144. ISBN 978-3-03785-666-6. [Google Scholar]
- Barros, N.R.; Ahadian, S.; Tebon, P.; Cunha Rudge, M.V.; Pascon Barbosa, A.M.; Herculano, R.D. Highly Absorptive Dressing Composed of Natural Latex Loaded with Alginate for Exudate Control and Healing of Diabetic Wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111589. [Google Scholar] [CrossRef]
- Chitrambalam, T.G.; Christopher, P.J.; Sundaraj, J.; Paladugu, R.; Selvamuthukumaran, S. Comparison of Efficacy of Alginate Filler Dressings with Conventional Saline Dressings for Cavity Wounds in Diabetic Foot Ulcer- A Prospective Cohort Study. J. Clin. Diagn. Res. 2020, 14, PC1–PC4. [Google Scholar]
- Bonilla, P.; Arias, E.M.; Solans, C.; García-Celma, M.J. Influence of Crosslinked Alginate on Drug Release from Highly Concentrated Emulsions. Colloids Surf. Physicochem. Eng. Asp. 2018, 536, 148–155. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.; Chen, K.; Yipeng, Z.; Zhang, H. Enhanced Physical and Antimicrobial Properties of Alginate/Chitosan Composite Aerogels Based on Electrostatic Interactions and Noncovalent Crosslinking. Carbohydr. Polym. 2021, 266, 118102. [Google Scholar] [CrossRef]
- Park, G.Y.; Yeum, J.H.; Yang, D.J.; Park, G.O.; Kim, Y.H.; Jeon, S.; Kim, T.J.; Oh, E.J.; Chung, H.Y.; Choi, J.H. Moisture Wound Healing Characteristics of Alginate Sponge and Hydrogel. Polym. Korea 2018, 42, 112–118. [Google Scholar]
- Ahmad, F.; Mushtaq, B.; Butt, F.A.; Rasheed, A.; Ahmad, S. Preparation and Characterization of Wool Fiber Reinforced Nonwoven Alginate Hydrogel for Wound Dressing. Cellulose 2021, 28, 7941–7951. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, Y.; Yan, J.; Zhang, W. Fabrication of ZnO Nanoparticle-Coated Calcium Alginate Nonwoven Fabric by Ion Exchange Method Based on Amino Hyperbranched Polymer. Mater. Lett. 2020, 270, 127624. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles–A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [Green Version]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. In Vitro Antioxidant and Antibacterial Activity of Sulfated Polysaccharides Isolated from Spatoglossum Asperum. Carbohydr. Polym. 2017, 170, 296–304. [Google Scholar] [CrossRef]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A Review about the Development of Fucoidan in Antitumor Activity: Progress and Challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel Antiviral Fucoidan from Sporophyll of Undaria Pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, W.; Quanbin, Z.; Zhongshan, Z.; Yun, H.; Hong, Z. In-Vitro Anticoagulant Activity of Fucoidan Derivatives from Brown Seaweed Laminaria Japonica. Chin. J. Oceanol. Limnol. 2011, 29, 679–685. [Google Scholar] [CrossRef]
- O’Leary, R.; Rerek, M.; Wood, E.J. Fucoidan Modulates the Effect of Transforming Growth Factor (TGF)-β1 on Fibroblast Proliferation and Wound Repopulation in in Vitro Models of Dermal Wound Repair. Biol. Pharm. Bull. 2004, 27, 266–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaltin, K.; Vargun, E.; Di Martino, A.; Capakova, Z.; Lehocky, M.; Humpolicek, P.; Kazantseva, N.; Saha, P. Cell Response to PLA Scaffolds Functionalized with Various Seaweed Polysaccharides. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–8. [Google Scholar] [CrossRef]
- Sezer, A.D.; Hatipoglu, F.; Cevher, E.; Ogurtan, Z.; Bas, A.L.; Akbuga, J. Chitosan Film Containing Fucoidan as a Wound Dressing for Dermal Burn Healing: Preparation and in Vitro/In Vivo Evaluation. Aaps Pharmscitech 2007, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugapriya, K.; Kim, H.; Lee, Y.W.; Kang, H.W. Multifunctional Heteropolysaccharide Hydrogel under Photobiomodulation for Accelerated Wound Regeneration. Ceram. Int. 2020, 46, 7268–7278. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P.; et al. Bio-Multifunctional Alginate/Chitosan/Fucoidan Sponges with Enhanced Angiogenesis and Hair Follicle Regeneration for Promoting Full-Thickness Wound Healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel Blends of Chitin/Chitosan, Fucoidan and Alginate as Healing-Impaired Wound Dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kordjazi, M.; Shabanpour, B.; Zabihi, E.; Faramarzi, M.; Gavlighi, H.; Feghhi, S.; Hosseini, S. Investigation of Effects of Fucoidan Polysaccharides Extracted from Two Species of Padina on the Wound-Healing Process in the Rat. Turk. J. Vet. Anim. Sci. 2017, 41, 106–117. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, J.; Li, P. Synthesized Phosphorylated and Aminated Derivatives of Fucoidan and Their Potential Antioxidant Activity in Vitro. Int. J. Biol. Macromol. 2009, 44, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, S.; Park, S.; Lee, Y.; Park, J.; Song, P.; Cho, C.; Ku, S.; Song, C. Promoting Wound Healing Using Low Molecular Weight Fucoidan in a Full-Thickness Dermal Excision Rat Model. Mar. Drugs 2017, 15, 112. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, Y. Basic Fibroblast Growth Factor Released from Fucoidan-Modified Chitosan/Alginate Scaffolds for Promoting Fibroblasts Migration. J. Polym. Res. 2018, 25, 83. [Google Scholar] [CrossRef]
- Park, H.; Baek, S.; Kang, H.; Lee, D. Biomaterials to Prevent Post-Operative Adhesion. Mater. Basel Switz. 2020, 13, 3056. [Google Scholar] [CrossRef]
- Cashman, J.D.; Kennah, E.; Shuto, A.; Winternitz, C.; Springate, C.M.K. Fucoidan Film Safely Inhibits Surgical Adhesions in a Rat Model. J. Surg. Res. 2011, 171, 495–503. [Google Scholar] [CrossRef]
- Yao, Y.; Zaw, A.M.; Anderson, D.E.J.; Hinds, M.T.; Yim, E.K.F. Fucoidan Functionalization on Poly(Vinyl Alcohol) Hydrogels for Improved Endothelialization and Hemocompatibility. Biomaterials 2020, 249, 120011. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, A.; Jung, W.-K.; Jeon, T.J. Effects of Fucoidan on Cell Morphology and Migration in Osteoblasts. Food Sci. Biotechnol. 2015, 24, 699–704. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Liu, J.; Song, J.; Yu, P.; Chen, P.; Liao, Z.; Wu, M.; Tong, H. Physicochemical Characterization of Sargassum Fusiforme Fucoidan Fractions and Their Antagonistic Effect against P-Selectin-Mediated Cell Adhesion. Int. J. Biol. Macromol. 2019, 133, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.R.; Nayak, S.; Sahu, B.B.; Bhutia, S.K.; Jena, M. Multifunctional Role of Fucoidan, Sulfated Polysaccharides in Human Health and Disease: A Journey under the Sea in Pursuit of Potent Therapeutic Agents. Int. J. Biol. Macromol. 2020, 164, 4263–4278. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Nakazato, K.; Tomioka, S.; Iha, M.; Nakajima, K. Intestinal Absorption of Fucoidan Extracted from the Brown Seaweed, Cladosiphon Okamuranus. Mar. Drugs 2014, 13, 48–64. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, Structure and Biofunctional Activities of Laminarin from Brown Algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Miao, H.; Ishaimichaeli, R.; Peretz, T.; Vlodavsky, I. Laminarin Sulfate Mimics the Effects of Heparin on Smooth-Muscle Cell-Proliferation and Basic Fibroblast Growth Factor-Receptor Binding and Mitogenic Activity. J. Cell. Physiol. 1995, 164, 482–490. [Google Scholar] [CrossRef]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [Green Version]
- Can, M.; Sahiner, N. A Facile One-Pot Synthesis of Microgels and Nanogels of Laminarin for Biomedical Applications. J. Colloid Interface Sci. 2021, 588, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, Antibacterial and in Vivo Wound Healing Properties of Laminaran Purified from Cystoseira Barbata Seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Wu, Y.; Li, H.; Liu, W. A High Strength Semi-Degradable Polysaccharide-Based Hybrid Hydrogel for Promoting Cell Adhesion and Proliferation. J. Mater. Sci. 2018, 53, 6302–6312. [Google Scholar] [CrossRef]
- Kim, Y.-E.; Kim, Y.-J. Effects of Nanofibrous Membranes Containing Low Molecular Weight Beta-Glucan on Normal and Cancer Cells. J. Nanosci. Nanotechnol. 2017, 17, 3597–3605. [Google Scholar] [CrossRef]
- Calagna, G.; Maranto, M.; Paola, C.; Capra, G.; Perino, A.; Chiantera, V.; Cucinella, G. ‘Secondary Prevention’ against Female HPV Infection: Literature Review of the Role of Carrageenan. Expert Rev. Anti Infect. Ther. 2020, 18, 865–874. [Google Scholar] [CrossRef]
- Lokhande, G.; Carrow, J.K.; Thakur, T.; Xavier, J.R.; Parani, M.; Bayless, K.J.; Gaharwar, A.K. Nanoengineered Injectable Hydrogels for Wound Healing Application. Acta Biomater. 2018, 70, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Li, L. Thermoreversible Gelation and Scaling Behavior of Ca 2+ -Induced κ-Carrageenan Hydrogels. Food Hydrocoll. 2016, 61, 793–800. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A Review. Veterinární Medicína 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- Tytgat, L.; Van Damme, L.; Arevalo, M.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; Van Vlierberghe, S. Extrusion-Based 3D Printing of Photo-Crosslinkable Gelatin and κ-Carrageenan Hydrogel Blends for Adipose Tissue Regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joy, R.; Vigneshkumar, P.N.; John, F.; George, J. Hydrogels Based on Carrageenan; Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2021; pp. 293–325. ISBN 978-0-12-821649-1. [Google Scholar]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan Based Hydrogels for Drug Delivery, Tissue Engineering and Wound Healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Kim, D.; Banerjee, I.; Pal, K. Carrageenan: A Wonder Polymer from Marine Algae for Potential Drug Delivery Applications. Curr. Pharm. Des. 2019, 25, 1172–1186. [Google Scholar] [CrossRef]
- Lim, H.-P.; Ooi, C.-W.; Tey, B.-T.; Chan, E.-S. Controlled Delivery of Oral Insulin Aspart Using PH-Responsive Alginate/κ-Carrageenan Composite Hydrogel Beads. React. Funct. Polym. 2017, 120, 20–29. [Google Scholar] [CrossRef]
- Pettinelli, N.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Feijoo-Bandín, S.; Lago, F. Carrageenan-Based Physically Crosslinked Injectable Hydrogel for Wound Healing and Tissue Repairing Applications. Int. J. Pharm. 2020, 589, 119828. [Google Scholar] [CrossRef]
- Mokhtari, H.; Tavakoli, S.; Safarpour, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. Recent Advances in Chemically-Modified and Hybrid Carrageenan-Based Platforms for Drug Delivery, Wound Healing, and Tissue Engineering. Polymers 2021, 13, 1744. [Google Scholar] [CrossRef]
- Nair, A.V.; Raman, M.; Doble, M. Cyclic Beta-(1 -> 3) (1 -> 6) Glucan/Carrageenan Hydrogels for Wound Healing Applications. RSC Adv. 2016, 6, 98545–98553. [Google Scholar] [CrossRef]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; da Silva Jeremias, T.; de Carvalho, R.G.; Iucif Vieira, D.G.; Oliveira, L.F.C.; Fernandes Maia, L.; Trentin, A.G.; et al. Carrageenan Hydrogel as a Scaffold for Skin-Derived Multipotent Stromal Cells Delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef]
- Barba, B.J.D.; Tranquilan-Aranilla, C.; Abad, L.V. Hemostatic Potential of Natural/Synthetic Polymer Based Hydrogels Crosslinked by Gamma Radiation. Radiat. Phys. Chem. 2016, 118, 111–113. [Google Scholar] [CrossRef]
- Wang, F.F.; Yao, Z.; Wu, H.G.; Zhang, S.X.; Zhu, N.N.; Gai, X. Antibacterial Activities of Kappa-Carrageenan Oligosaccharides. Appl. Mech. Mater. 2011, 108, 194–199. [Google Scholar] [CrossRef]
- El-Fawal, G. Preparation, Characterization and Antibacterial Activity of Biodegradable Films Prepared from Carrageenan. J. Food Sci. Technol. 2014, 51, 2234–2239. [Google Scholar] [CrossRef] [Green Version]
- Cregut, M.; Rondags, E. New Insights in Agar Biorefinery with Arylsulphatase Activities. Process Biochem. 2013, 48, 1861–1871. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-Based Edible Films for Food Packaging Applications—A Review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Gioele, C.; Marilena, S.; Valbona, A.; Nunziacarla, S.; Andrea, S.; Antonio, M. Gracilaria Gracilis, Source of Agar: A Short Review. Curr. Org. Chem. 2017, 21, 380–386. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Fazita, M.R.N.; Paridah, M.T. A Review of Extractions of Seaweed Hydrocolloids: Properties and Applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, B.; Zhao, S.; Qiao, D.; Xie, F. Plasticized Starch/Agar Composite Films: Processing, Morphology, Structure, Mechanical Properties and Surface Hydrophilicity. Coatings 2021, 11, 311. [Google Scholar] [CrossRef]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive Chitosan–Agarose Hydrogel for Skin Regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-Based Biomaterials for Advanced Drug Delivery. J. Controlled Release 2020, 326, 523–543. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, Z.; Cai, R.; Yang, W.; He, H.; Wang, Y. Rational Design of Ag/ZnO Hybrid Nanoparticles on Sericin/Agarose Composite Film for Enhanced Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Wang, Y.; Hao, J. Rapid-Forming and Self-Healing Agarose-Based Hydrogels for Tissue Adhesives and Potential Wound Dressings. Biomacromolecules 2018, 19, 980–988. [Google Scholar] [CrossRef]
- Wolska, J.; Setkowicz, J.; Maliszewska, I.H. Preparation and Characterization of Chistosan-Agar Films. Prog. Chem. Appl. Chitin ITS Deriv. 2020, 25, 210–226. [Google Scholar] [CrossRef]
- Rivadeneira, J.; Audisio, M.C.; Gorustovich, A. Films Based on Soy Protein-Agar Blends for Wound Dressing: Effect of Different Biopolymer Proportions on the Drug Release Rate and the Physical and Antibacterial Properties of the Films. J. Biomater. Appl. 2018, 32, 1231–1238. [Google Scholar] [CrossRef]
- Uppuluri, V.N.V.A.; Shanmugarajan, T.S. Icariin-Loaded Polyvinyl Alcohol/Agar Hydrogel: Development, Characterization, and In Vivo Evaluation in a Full-Thickness Burn Model. Int. J. Low. Extrem. Wounds 2019, 18, 323–335. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, M.; Liu, M.; Yu, Y.; Xu, X.; Li, J. Fabrication of Double-Network Hydrogels with Universal Adhesion and Superior Extensibility and Cytocompatibility by One-Pot Method. Biomacromolecules 2020, 21, 4699–4708. [Google Scholar] [CrossRef]
- Sulastri, E.; Lesmana, R.; Zubair, M.S.; Elamin, K.M.; Wathoni, N. A Comprehensive Review on Ulvan Based Hydrogel and Its Biomedical Applications. Chem. Pharm. Bull. 2021, 69, 432–443. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a Bioactive Marine Sulphated Polysaccharide as a Key Constituent of Hybrid Biomaterials: A Review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated Seaweed Polysaccharides as Multifunctional Materials in Drug Delivery Applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Alves, A.; Pinho, E.D.; Neves, N.M.; Sousa, R.A.; Reis, R.L. Processing Ulvan into 2D Structures: Cross-Linked Ulvan Membranes as New Biomaterials for Drug Delivery Applications. Int. J. Pharm. 2012, 426, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yue, Z.; Winberg, P.C.; Dinoro, J.N.; Hayes, P.; Beirne, S.; Wallace, G.G. Development of Rhamnose-Rich Hydrogels Based on Sulfated Xylorhamno-Uronic Acid toward Wound Healing Applications. Biomater. Sci. 2019, 7, 3497–3509. [Google Scholar] [CrossRef] [PubMed]
- Mariia, K.; Arif, M.; Shi, J.; Song, F.; Chi, Z.; Liu, C. Novel Chitosan-Ulvan Hydrogel Reinforcement by Cellulose Nanocrystals with Epidermal Growth Factor for Enhanced Wound Healing: In Vitro and in Vivo Analysis. Int. J. Biol. Macromol. 2021, 183, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Kikionis, S.; Ioannou, E.; Toskas, G.; Roussis, V. Electrospun Biocomposite Nanofibers of Ulvan/PCL and Ulvan/PEO. J. Appl. Polym. Sci. 2015, 132, 42153. [Google Scholar] [CrossRef]
- Wang, J.; Salem, D.R.; Sani, R.K. Extremophilic Exopolysaccharides: A Review and New Perspectives on Engineering Strategies and Applications. Carbohydr. Polym. 2019, 205, 8–26. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural Features of Microbial Exopolysaccharides in Relation to Their Antioxidant Activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Tabernero, A.; Cardea, S. Microbial Exopolysaccharides as Drug Carriers. Polymers 2020, 12, 2142. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kim, S.-K. Extracellular polysaccharides produced by marine bacteria. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 72, pp. 79–94. ISBN 978-0-12-800269-8. [Google Scholar]
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M. Exopolysaccharides from Marine and Marine Extremophilic Bacteria: Structures, Properties, Ecological Roles and Applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Suresh Kumar, A.; Mody, K.; Jha, B. Bacterial Exopolysaccharides—A Perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.H.; El Awady, M.E.; Nasr-Eldin, M.A.; Ibrahim, H.M.M.; Al Bahnasy, M.E. Production and Assessment of Antioxidant Activity of Exopolysaccharide from Marine Streptomyces Globisporus BU2018. Egypt. J. Bot. 2019, 59, 645–655. [Google Scholar] [CrossRef]
- Abinaya, M.; Vaseeharan, B.; Divya, M.; Vijayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Khaled, J.M.; Al-anbr, M.N.; Benelli, G. Structural Characterization of Bacillus Licheniformis Dahb1 Exopolysaccharide-Antimicrobial Potential and Larvicidal Activity on Malaria and Zika Virus Mosquito Vectors. Environ. Sci. Pollut. Res. 2018, 25, 18604–18619. [Google Scholar] [CrossRef] [PubMed]
- Athmika; Ghate, S.D.; Arun, A.B.; Rao, S.S.; Kumar, S.T.A.; Kandiyil, M.K.; Saptami, K.; Rekha, P.D. Genome Analysis of a Halophilic Bacterium Halomonas Malpeensis YU-PRIM-29(T) Reveals Its Exopolysaccharide and Pigment Producing Capabilities. Sci. Rep. 2021, 11, 1–14. [Google Scholar]
- Almutairi, M.H.; Helal, M.M.I. Exopolysaccharide Production from Isolated Enterobacter Sp. Strain ACD2 from the Northwest of Saudi Arabia. J. King Saud Univ. Sci. 2021, 33, 101318. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J. Bacterial Exopolysaccharides: Chemical Structures, Gene Clusters and Genetic Engineering. Int. J. Biol. Macromol. 2021, 173, 481–490. [Google Scholar] [CrossRef]
- Aullybux, A.A.; Puchooa, D.; Bahorun, T.; Jeewon, R. Phylogenetics and Antibacterial Properties of Exopolysaccharides from Marine Bacteria Isolated from Mauritius Seawater. Ann. Microbiol. 2019, 69, 957–972. [Google Scholar] [CrossRef]
- Viju, N.; Satheesh, S.; Punitha, S.M.J. Antibiofilm and Antifouling Activities of Extracellular Polymeric Substances Isolated from the Bacteria Associated with Marine Gastropod Turbo Sp. Oceanol. Hydrobiol. Stud. 2016, 45, 11–19. [Google Scholar] [CrossRef]
- Almutairi, M.H.; Helal, M.M. Biological and Microbiological Activities of Isolated Enterobacter Sp. ACD2 Exopolysaccharides from Tabuk Region of Saudi Arabia. J. King Saud Univ. Sci. 2021, 33, 101328. [Google Scholar] [CrossRef]
- Sun, M.-L.; Zhao, F.; Chen, X.-L.; Zhang, X.-Y.; Zhang, Y.-Z.; Song, X.-Y.; Sun, C.-Y.; Yang, J. Promotion of Wound Healing and Prevention of Frostbite Injury in Rat Skin by Exopolysaccharide from the Arctic Marine Bacterium Polaribacter Sp. SM1127. Mar. Drugs 2020, 18, 48. [Google Scholar] [CrossRef] [Green Version]
- Sivasankar, P.; Seedevi, P.; Poongodi, S.; Sivakumar, M.; Murugan, T.; Sivakumar, L.; Sivakumar, K.; Balasubramanian, T. Characterization, Antimicrobial and Antioxidant Property of Exopolysaccharide Mediated Silver Nanoparticles Synthesized by Streptomyces Violaceus MM72. Carbohydr. Polym. 2018, 181, 752–759. [Google Scholar] [CrossRef]
- Sran, K.S.; Bisht, B.; Mayilraj, S.; Choudhury, A.R. Structural Characterization and Antioxidant Potential of a Novel Anionic Exopolysaccharide Produced by Marine Microbacterium Aurantiacum FSW-25. Int. J. Biol. Macromol. 2019, 131, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Youssif, A.M.; Hamed, M.M.; Abdrabo, M.A.A. Production and Characterization of Extracellular Polymeric Substances by Marine Halomonas Sp. NASH Isolated from Wadi El-Natroun. J. Pure Appl. Microbiol. 2020, 14, 2745–2756. [Google Scholar] [CrossRef]
- Xiao, R.; Yang, X.; Li, M.; Li, X.; Wei, Y.; Cao, M.; Ragauskas, A.; Thies, M.; Ding, J.; Zheng, Y. Investigation of Composition, Structure and Bioactivity of Extracellular Polymeric Substances from Original and Stress-Induced Strains of Thraustochytrium Striatum. Carbohydr. Polym. 2018, 195, 515–524. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, Y.-K.; Choi, T.-R.; Kim, H.; Song, H.-S.; Lee, S.M.; Park, S.L.; Lee, H.S.; Kim, Y.-G.; et al. Bioprospecting of Exopolysaccharide from Marine Sphingobium Yanoikuyae BBL01: Production, Characterization, and Metal Chelation Activity. Bioresour. Technol. 2021, 324, 124674. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rekha, P.D. A Bioactive Exopolysaccharide from Marine Bacteria Alteromonas Sp. PRIM-28 and Its Role in Cell Proliferation and Wound Healing in Vitro. Int. J. Biol. Macromol. 2019, 131, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rekha, P.D. A Novel Exopolysaccharide from Marine Bacterium Pantoea Sp. YU16-S3 Accelerates Cutaneous Wound Healing through Wnt/Beta-Catenin Pathway. Carbohydr. Polym. 2020, 238, 116191. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, G.; Liu, R.; Wei, M.; Zhang, J.; Sun, C. EPS364, a Novel Deep-Sea Bacterial Exopolysaccharide, Inhibits Liver Cancer Cell Growth and Adhesion. Mar. Drugs 2021, 19, 171. [Google Scholar] [CrossRef]
- Sardari, R.R.R.; Kulcinskaja, E.; Ron, E.Y.C.; Bjornsdottir, S.; Fridjonsson, O.H.; Hreggvidsson, G.O.; Karlsson, E.N. Evaluation of the Production of Exopolysaccharides by Two Strains of the Thermophilic Bacterium Rhodothermus Marinus. Carbohydr. Polym. 2017, 156, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Finore, I.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Lo Giudice, A. Production and Biotechnological Potential of Extracellular Polymeric Substances from Sponge-Associated Antarctic Bacteria. Appl. Environ. Microbiol. 2018, 84, e01624-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, P.; Shah, A.A.; Hasan, F.; Hertkorn, N.; Gonsior, M.; Sajjad, W.; Chen, F. A Glacier Bacterium Produces High Yield of Cryoprotective Exopolysaccharide. Front. Microbiol. 2020, 10, 3096. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, A.D.; Kuo, Y.-H.; Wang, S.-L. Biosynthesis of α-Glucosidase Inhibitors by a Newly Isolated Bacterium, Paenibacillus Sp. TKU042 and Its Effect on Reducing Plasma Glucose in a Mouse Model. Int. J. Mol. Sci. 2017, 18, 700. [Google Scholar] [CrossRef]
- Hassan, S.W.M.; Ibrahim, H.A.H. Production, Characterization and Valuable Applications of Exopolysaccharides from Marine Bacillus Subtilis SH1. Pol. J. Microbiol. 2017, 66, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Zhu, W.; Song, R.; Yan, F.; Wang, Y. Exopolysaccharide from Bacillus Vallismortis WF4 as an Emulsifier for Antifungal and Antipruritic Peppermint Oil Emulsion. Int. J. Biol. Macromol. 2019, 125, 436–444. [Google Scholar] [CrossRef]
- Alejandra Lopez-Ortega, M.; Chavarria-Hernandez, N.; del Rocio Lopez-Cuellar, M.; Ines Rodriguez-Hernandez, A. A Review of Extracellular Polysaccharides from Extreme Niches: An Emerging Natural Source for the Biotechnology. From the Adverse to Diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Mansour, M.K.; Sedeek, M.S.; Habib, M.H.; Ulber, R.; Farag, M.A. Rediscovering Bacterial Exopolysaccharides of Terrestrial and Marine Origins: Novel Insights on Their Distribution, Biosynthesis, Biotechnological Production, and Future Perspectives. Crit. Rev. Biotechnol. 2021, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Decho, A.W.; Gutierrez, T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front. Microbiol. 2017, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Deepika, M.S.; Thangam, R.; Vijayakumar, T.S.; Sasirekha, R.; Vimala, R.T.V.; Sivasubramanian, S.; Arun, S.; Babu, M.D.; Thirumurugan, R. Antibacterial Synergy between Rutin and Florfenicol Enhances Therapeutic Spectrum against Drug Resistant Aeromonas Hydrophila. Microb. Pathog. 2019, 135, 103612. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Ibrahim, A.Y.; Asker, M.S.; Mahmoud, M.G.; El Awady, M.E. Production, Characterization and Biological Activities of Acidic Exopolysaccharide from Marine Bacillus Amyloliquefaciens 3MS 2017. Asian Pac. J. Trop. Med. 2017, 10, 715–725. [Google Scholar] [CrossRef]
- Cortes, H.; Caballero-Florán, I.H.; Mendoza-Muñoz, N.; Escutia-Guadarrama, L.; Figueroa-González, G.; Reyes-Hernández, O.D.; González-Del Carmen, M.; Varela-Cardoso, M.; González-Torres, M.; Florán, B.; et al. Xanthan Gum in Drug Release. Cell. Mol. Biol. Noisy--Gd. Fr. 2020, 66, 199–207. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of Xanthan Gum as Polysaccharide in Tissue Engineering: A Review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, G.-H.; Tian, M.P.; Wang, Y.-N.; Qu, C.-C.; Cheng, X.-J.; Feng, C.; Chen, X.-G. Mussel-Inspired Antibacterial Polydopamine/Chitosan/Temperature-Responsive Hydrogels for Rapid Hemostasis. Int. J. Biol. Macromol. 2019, 138, 321–333. [Google Scholar] [CrossRef]
- Choudhary, P.; Ramalingam, B.; Das, S.K. Fabrication of Chitosan-Reinforced Multifunctional Graphene Nanocomposite as Antibacterial Scaffolds for Hemorrhage Control and Wound-Healing Application. ACS Biomater. Sci. Eng. 2020, 6, 5911–5929. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Xu, K.; Mei, L.; Wu, C.; Liu, J.; Liu, Z.; Wan, L.; Zhong, W. Co-Assembled Supramolecular Hydrogels of Cell Adhesive Peptide and Alginate for Rapid Hemostasis and Efficacious Wound Healing. Soft Matter 2019, 15, 8603–8610. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, L.; Shi, X. Injectable Drug-Loaded Polysaccharide Hybrid Hydrogels for Hemostasis. RSC Adv. 2019, 9, 36858–36866. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Q.; Hou, T.; Li, C.; Hu, Z.; Liang, L.; Li, S.; Zhong, Q.; Li, P. Construction of a Composite Sponge Containing Tilapia Peptides and Chitosan with Improved Hemostatic Performance. Int. J. Biol. Macromol. 2019, 139, 719–729. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Zhao, J.-Y.; Hu, W.-Z.; Ma, K.; Chao, Y.; Sun, P.-J.; Fu, X.-B.; Zhang, H. Synthetic Poly(Vinyl Alcohol)-Chitosan as a New Type of Highly Efficient Hemostatic Sponge with Blood-Triggered Swelling and High Biocompatibility. J. Mater. Chem. B 2019, 7, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Hiep, N.T.; Khon, H.C.; Niem, V.V.T.; Toi, V.V.; Quyen, T.N.; Hai, N.D.; Anh, M.N.T. Microwave-Assisted Synthesis of Chitosan/Polyvinyl Alcohol Silver Nanoparticles Gel for Wound Dressing Applications. Int. J. Polym. Sci. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y.; Sun, T.; et al. Evaluation of Genipin-Crosslinked Chitosan Hydrogels as a Potential Carrier for Silver Sulfadiazine Nanocrystals. Colloids Surf. B Biointerfaces 2016, 148, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rescignano, N.; Hernandez, R.; Lopez, L.; Calvillo, I.; Kenny, J.; Mijangos, C. Preparation of Alginate Hydrogels Containing Silver Nanoparticles: A Facile Approach for Antibacterial Applications. Polym. Int. 2016, 65, 921–926. [Google Scholar] [CrossRef]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium Alginate and Gum Acacia Hydrogels of ZnO Nanoparticles Show Wound Healing Effect on Fibroblast Cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef]
- Abd El-Malek, F.F.; Yousef, A.S.; El-Assar, S.A. Hydrogel Film Loaded with New Formula from Manuka Honey for Treatment of Chronic Wound Infections. J. Glob. Antimicrob. Resist. 2017, 11, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory PH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Khamrai, M.; Banerjee, S.L.; Kundu, P.P. Modified Bacterial Cellulose Based Self-Healable Polyeloctrolyte Film for Wound Dressing Application. Carbohydr. Polym. 2017, 174, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Hari, N.; Nair, A.J. Development and Characterization of Chitosan-Based Antimicrobial Films Incorporated with Streptomycin Loaded Starch Nanoparticles. NEW Horiz. Transl. Med. 2016, 3, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes-Parra, J. Physical, Mechanical and Antibacterial Properties of Alginate Film: Effect of the Crosslinking Degree and Oregano Essential Oil Concentration. J. Food Eng.—J Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring Mechanical and Antibacterial Properties of Chitosan/Gelatin Nanofiber Membranes with Fe3O4 Nanoparticles for Potential Wound Dressing Application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Doostan, M.; Maleki, H.; Doostan, M.; Khoshnevisan, K.; Faridi-Majidi, R.; Arkan, E. Effective Antibacterial Electrospun Cellulose Acetate Nanofibrous Patches Containing Chitosan/Erythromycin Nanoparticles. Int. J. Biol. Macromol. 2021, 168, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized Chitosan-Matrigel-Polyacrylamide Hydrogels as Wound Dressing for Wound Repair and Regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.; Borse, V.; Thakkar, R.; Srivastava, R. Dual-Purpose Injectable Doxorubicin Conjugated Alginate Gel Containing Polycaprolactone Microparticles for Anti-Cancer and Anti-Inflammatory Therapy. Curr. Drug Deliv. 2017, 14, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Bras, T.; Rosa, D.; Goncalves, A.C.; Gomes, A.C.; Alves, V.D.; Crespo, J.G.; Duarte, M.F.; Neves, L.A. Development of Bioactive Films Based on Chitosan and Cynara Cardunculus Leaves Extracts for Wound Dressings. Int. J. Biol. Macromol. 2020, 163, 1707–1718. [Google Scholar] [CrossRef]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen Loaded PVA/Chitosan Membranes: A Highly Efficient Strategy towards an Improved Skin Wound Healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial Anti-Oxidant Electroactive Injectable Hydrogel as Self-Healing Wound Dressing with Hemostasis and Adhesiveness for Cutaneous Wound Healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Fan, C.; Yang, Y.; Lee, B.H.; Wei, K. 5-Hydroxymethylfurfural-Embedded Poly (Vinyl Alcohol)/Sodium Alginate Hybrid Hydrogels Accelerate Wound Healing. Int. J. Biol. Macromol. 2019, 138, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A Composite Hydrogel of Chitosan/Heparin/Poly (Gamma-Glutamic Acid) Loaded with Superoxide Dismutase for Wound Healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel Electrospun Chitosan/Polyvinyl Alcohol/Zinc Oxide Nanofibrous Mats with Antibacterial and Antioxidant Properties for Diabetic Wound Healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Mei, L.; Fan, R.; Li, X.; Wang, Y.; Han, B.; Gu, Y.; Zhou, L.; Zheng, Y.; Tong, A.; Guo, G. Nanofibers for Improving the Wound Repair Process: The Combination of a Grafted Chitosan and an Antioxidant Agent. Polym. Chem. 2017, 8, 1664–1671. [Google Scholar] [CrossRef]
- Pan, H.; Fan, D.; Cao, W.; Zhu, C.; Duan, Z.; Fu, R.; Li, X.; Ma, X. Preparation and Characterization of Breathable Hemostatic Hydrogel Dressings and Determination of Their Effects on Full-Thickness Defects. Polymers 2017, 9, 727. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.H.; Seo, D.I.; Lee, D.-W.; Bhang, S.H.; Park, K.; Jang, G.; Kim, C.H.; Chun, H.J. Preparation and Evaluation of Visible-Light Cured Glycol Chitosan Hydrogel Dressing Containing Dual Growth Factors for Accelerated Wound Healing. J. Ind. Eng. Chem. 2017, 53, 360–370. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.; He, J.; Zhang, H.; Guo, B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing to Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, Y.; Peng, J.; Xu, C.; Xu, Q.; Xing, M.; Chang, J. A Novel Dual-Adhesive and Bioactive Hydrogel Activated by Bioglass for Wound Healing. NPG Asia Mater. 2019, 11, 66. [Google Scholar] [CrossRef]
- Yar, M.; Gigliobianco, G.; Shahzadi, L.; Dew, L.; Siddiqi, S.A.; Khan, A.F.; Chaudhry, A.A.; Rehman, I.U.; MacNeil, S. Production of Chitosan PVA PCL Hydrogels to Bind Heparin and Induce Angiogenesis. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 466–476. [Google Scholar] [CrossRef]
- Ciriza, J.; Rodríguez-Romano, A.; Nogueroles, I.; Cabezuelo, R.; Pedraz, J.; Rico, P. Borax-Loaded Injectable Alginate Hydrogels Promote Muscle Regeneration in Vivo after an Injury. Mater. Sci. Eng. C 2021, 123, 112003. [Google Scholar] [CrossRef] [PubMed]
- Antunes, B.P.; Moreira, A.F.; Gaspar, V.M.; Correia, I.J. Chitosan/Arginine-Chitosan Polymer Blends for Assembly of Nanofibrous Membranes for Wound Regeneration. Carbohydr. Polym. 2015, 130, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Han, .; Li, Y.; Zeng, Q.; Li, H.; Peng, J.; Xu, Y.; Chang, J. Injectable Bioactive Akermanite/Alginate Composite Hydrogels for in Situ Skin Tissue Engineering. J. Mater. Chem. B 2017, 5, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Nooshabadi, V.T.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome Loaded Alginate Hydrogel Promotes Tissue Regeneration in Full-Thickness Skin Wounds: An in Vivo Study. J. Biomed. Mater. Res. A 2020, 108, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Arora, A.; Alam, M.S.; Gupta, B. Development of Antimicrobial and Scar Preventive Chitosan Hydrogel Wound Dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef]
- Chen, L.-C.; Lin, S.-Y.; Sheu, M.-T.; Su, C.-H.; Lin, H.-L.; Hsieh, C.-M. Fabrication and Characterization of Rhizochitosan and Its Incorporation with Platelet Concentrates to Promote Wound Healing. Carbohydr. Polym. 2021, 268, 118239. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.; Gupta, B. Scar Free Healing Mediated by the Release of Aloe Vera and Manuka Honey from Dextran Bionanocomposite Wound Dressings. Int. J. Biol. Macromol. 2018, 120, 1581–1590. [Google Scholar] [CrossRef]
- Choudhary, M.; Chhabra, P.; Tyagi, A.; Singh, H. Scar Free Healing of Full Thickness Diabetic Wounds: A Unique Combination of Silver Nanoparticles as Antimicrobial Agent, Calcium Alginate Nanoparticles as Hemostatic Agent, Fresh Blood as Nutrient/Growth Factor Supplier and Chitosan as Base Matrix. Int. J. Biol. Macromol. 2021, 178, 41–52. [Google Scholar] [CrossRef]
- Hangge, P.; Stone, J.; Albadawi, H.; Zhang, Y.S.; Khademhosseini, A.; Oklu, R. Hemostasis and Nanotechnology. Cardiovasc. Diagn. Ther. 2017, 7, S267–S275. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.; Mo, X.; Pan, J. In Situ Forming Hydrogel of Natural Polysaccharides through Schiff Base Reaction for Soft Tissue Adhesive and Hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef]
- Hattori, H.; Amano, Y.; Nogami, Y.; Takase, B.; Ishihara, M. Hemostasis for Severe Hemorrhage with Photocrosslinkable Chitosan Hydrogel and Calcium Alginate. Ann. Biomed. Eng. 2010, 38, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wang, S.; Jiang, Z.; Chi, J.; Yu, S.; Li, H.; Zhang, Y.; Li, L.; Zhou, C.; Liu, W.; et al. Hemostatic Performance of Chitosan-Based Hydrogel and Its Study on Biodistribution and Biodegradability in Rats. Carbohydr. Polym. 2021, 264, 117965. [Google Scholar] [CrossRef] [PubMed]
- Taskin, A.K.; Yasar, M.; Ozaydin, I.; Kaya, B.; Bat, O.; Ankarali, S.; Yildirim, U.; Aydin, M. The Hemostatic Effect of Calcium Alginate in Experimental Splenic Injury Model. Turk. J. Trauma Emerg. Surg. 2013, 19, 195–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najar, M.H.; Minaiyan, M.; Taheri, A. Preparation and Invivo Evaluation of a Novel Gel-Based Wound Dressing Using Arginine-Alginate Surface-Modified Chitosan Nanofibers. J. Biomater. Appl. 2018, 32, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wang, Y.; Zhang, Y.; Ren, X.; Qiu, Y.; Huang, T.-S. Novel Quaternarized N-Halamine Chitosan and Polyvinyl Alcohol Nanofibrous Membranes as Hemostatic Materials with Excellent Antibacterial Properties. Carbohydr. Polym. 2020, 232, 115823. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Wang, F.; Meng, W.; Yang, X.; Li, P.; Jiang, J.; Tan, H.; Zheng, Y. Preparation of Porous Carboxymethyl Chitosan Grafted Poly (Acrylic Acid) Superabsorbent by Solvent Precipitation and Its Application as a Hemostatic Wound Dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 18–29. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and Characterization of Chitosan/Gelatin/PVA Hydrogel for Wound Dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of Sandwich-like Chitosan/Polycaprolactone/Gelatin Scaffolds for Guided Tissue Regeneration Membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110618. [Google Scholar] [CrossRef] [PubMed]
- Atashgahi, M.; Ghaemi, B.; Valizadeh, A.; Moshiri, A.; Hossein Nekoofar, M.; Amani, A. Epinephrine-Entrapped Chitosan Nanoparticles Covered by Gelatin Nanofibers: A Bi-Layer Nano-Biomaterial for Rapid Hemostasis. Int. J. Pharm. 2021, 608, 121074. [Google Scholar] [CrossRef]
- Qiao, Z.; Lv, X.; He, S.; Bai, S.; Liu, X.; Hou, L.; He, J.; Tong, D.; Ruan, R.; Zhang, J.; et al. A Mussel-Inspired Supramolecular Hydrogel with Robust Tissue Anchor for Rapid Hemostasis of Arterial and Visceral Bleedings. Bioact. Mater. 2021, 6, 2829–2840. [Google Scholar] [CrossRef]
- Shou, Y.; Zhang, J.; Yan, S.; Xia, P.; Xu, P.; Li, G.; Zhang, K.; Yin, J. Thermoresponsive Chitosan/DOPA-Based Hydrogel as an Injectable Therapy Approach for Tissue-Adhesion and Hemostasis. ACS Biomater. Sci. Eng. 2020, 6, 3619–3629. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Yuan, Z.; He, Y.; Chen, M.; Li, K.; Hu, J.; Yang, Y.; Xia, Z.; Cai, K. Near Infrared Light-Triggered on-Demand Cur Release from Gel-PDA@Cur Composite Hydrogel for Antibacterial Wound Healing. Chem. Eng. J. 2021, 403, 126182. [Google Scholar] [CrossRef]
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, Self-Healing, Antibacterial, and Hemostatic N,O-Carboxymethyl Chitosan/Oxidized Chondroitin Sulfate Composite Hydrogel for Wound Dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111324. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Przekora, A. Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Appl. Sci.-Basel 2021, 11, 4114. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, D.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Biomaterials Based on N,N,N-Trimethyl Chitosan Fibers in Wound Dressing Applications. Int. J. Biol. Macromol. 2016, 89, 471–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufato, K.; Souza, P.; de Oliveira, A.; Berton, S.; Sabino, R.; Muniz, E.; Popat, K.; Radovanovic, E.; Kipper, M.; Martins, A. Antimicrobial and Cytocompatible Chitosan, N,N,N-Trimethyl Chitosan, and Tanfloc-Based Polyelectrolyte Multilayers on Gellan Gum Films. Int. J. Biol. Macromol. 2021, 183, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Dai, N.-T.; Hong, P.-D. Combination of High Efficiency Nano-Silver and Alginate for Wound Infection Control. Int. J. Nanotechnol. 2013, 10, 905–915. [Google Scholar] [CrossRef]
- Lu, B.; Ye, H.; Shang, S.; Xiong, Q.; Yu, K.; Li, Q.; Xiao, Y.; Dai, F.; Lan, G. Novel Wound Dressing with Chitosan Gold Nanoparticles Capped with a Small Molecule for Effective Treatment of Multiantibiotic-Resistant Bacterial Infections. Nanotechnology 2018, 29, 425603. [Google Scholar] [CrossRef] [PubMed]
- Lemraski, E.G.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, S.; Rafiee-Moghaddam, R.; Webster, T.J. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In Vitro and in Vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, H.-I. Characterization and Antibacterial Properties of Genipin-Crosslinked Chitosan/Poly(Ethylene Glycol)/ZnO/Ag Nanocomposites. Carbohydr. Polym. 2012, 89, 111–116. [Google Scholar] [CrossRef]
- Melnikova, N.; Knyazev, A.; Nikolskiy, V.; Peretyagin, P.; Belyaeva, K.; Nazarova, N.; Liyaskina, E.; Malygina, D.; Revin, V. Wound Healing Composite Materials of Bacterial Cellulose and Zinc Oxide Nanoparticles with Immobilized Betulin Diphosphate. Nanomaterials 2021, 11, 713. [Google Scholar] [CrossRef]
- Du, S.; Chen, X.; Chen, X.; Li, S.; Yuan, G.; Zhou, T.; Li, J.; Jia, Y.; Xiong, D.; Tan, H. Covalent Chitosan-Cellulose Hydrogels via Schiff-Base Reaction Containing Macromolecular Microgels for PH-Sensitive Drug Delivery and Wound Dressing. Macromol. Chem. Phys. 2019, 220, 116191. [Google Scholar] [CrossRef]
- Kalaycioglu, Z.; Kahya, N.; Adimcilar, V.; Kaygusuz, H.; Torlak, E.; Akin-Evingur, G.; Erim, F.B. Antibacterial Nano Cerium Oxide/Chitosan/Cellulose Acetate Composite Films as Potential Wound Dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Xue, J.; Wu, J.; Chang, J.; Wu, C. Defective Black Nano-Titania Thermogels for Cutaneous Tumor-Induced Therapy and Healing. NANO Lett. 2019, 19, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, M.; Aly, M.M.; Sheshtawi, R.M. Antimicrobial Activities of Different Novel Chitosan-Collagen Nanocomposite Films Against Some Bacterial Pathogens. Int. J. Pharm. Phytopharm. Res. 2020, 10, 114–121. [Google Scholar]
- Ryan, C.; Alcock, E.; Buttimer, F.; Schmidt, M.; Clarke, D.; Pemble, M.; Bardosova, M. Synthesis and Characterisation of Cross-Linked Chitosan Composites Functionalised with Silver and Gold Nanoparticles for Antimicrobial Applications. Sci. Technol. Adv. Mater. 2017, 18, 528–540. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Wu, X.; Ren, Y.; Li, Z.; Ren, J. Controlled Release of Silver Ions from AgNPs Using a Hydrogel Based on Konjac Glucomannan and Chitosan for Infected Wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Sha, D.; Liu, Y.; Xu, J.; Shi, K.; Yu, C.; Ji, X. Injectable and Antibacterial Epsilon-Poly(L-Lysine)-Modified Poly(Vinyl Alcohol)/Chitosan/AgNPs Hydrogels as Wound Healing Dressings. Polymer 2021, 212, 123155. [Google Scholar] [CrossRef]
- Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Jami, M.; Bidgoli, M.R.; Vossoughi, M.; Ramazani, A.; Kamyabhesari, K. Fabrication and Evaluation of Chitosan/Gelatin/PVA Hydrogel Incorporating Honey for Wound Healing Applications: An in Vitro, in Vivo Study. Int. J. Pharm. 2021, 592, 120068. [Google Scholar] [CrossRef]
- Rezaei, N.; Hamidabadi, H.G.; Khosravimelal, S.; Zahiri, M.; Ahovan, Z.A.; Bojnordi, M.N.; Eftekhari, B.S.; Hashemi, A.; Ganji, F.; Darabi, S.; et al. Antimicrobial Peptides-Loaded Smart Chitosan Hydrogel: Release Behavior and Antibacterial Potential against Antibiotic Resistant Clinical Isolates. Int. J. Biol. Macromol. 2020, 164, 855–862. [Google Scholar] [CrossRef]
- Jull, A.; Walker, N.; Parag, V.; Molan, P.; Rodgers, A. Randomized Clinical Trial of Honey-Impregnated Dressings for Venous Leg Ulcers. Br. J. Surg. 2008, 95, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Thenmozhi, R.; Rathinamoorthy, R.; Thilagavathi, G. Optimisation of Chitosan-Honey Composite Film for Wound Dressing Application. Indian J. Chem. Technol. 2016, 23, 279–288. [Google Scholar]
- Zhang, D.; Zhou, W.; Wei, B.; Wang, X.; Tang, R.; Nie, J.; Wang, J. Carboxyl-Modified Poly(Vinyl Alcohol)-Crosslinked Chitosan Hydrogel Films for Potential Wound Dressing. Carbohydr. Polym. 2015, 125, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; et al. Gel Characterisation and in Vivo Evaluation of Minocycline-Loaded Wound Dressing with Enhanced Wound Healing Using Polyvinyl Alcohol and Chitosan. Int. J. Pharm. 2010, 392, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Hurler, J.; Berg, O.A.; Skar, M.; Conradi, A.H.; Johnsen, P.J.; Skalko-Basnet, N. Improved Burns Therapy: Liposomes-in-Hydrogel Delivery System for Mupirocin. J. Pharm. Sci. 2012, 101, 3906–3915. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Wang, Y.; Wu, Z.; An, J.; Lu, Z.; Mei, L.; Li, C. In Situ Cross-Linked Polysaccharide Hydrogel as Extracellular Matrix Mimics for Antibiotics. Carbohydr. Polym. 2014, 105, 63–69. [Google Scholar] [CrossRef]
- Vasile, B.S.; Oprea, O.; Voicu, G.; Ficai, A.; Andronescu, E.; Teodorescu, A.; Holban, A. Synthesis and Characterization of a Novel Controlled Release Zinc Oxide/Gentamicin-Chitosan Composite with Potential Applications in Wounds Care. Int. J. Pharm. 2014, 463, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Takami, T.; Uchida, Y.; Murakami, Y. Chitosan Gel Sheet Containing Drug Carriers with Controllable Drug-Release Properties. Colloids Surf. B Biointerfaces 2018, 163, 257–265. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, B.; Tian, J.; Wu, J. Anti-Inflammation Biomaterial Platforms for Chronic Wound Healing. Biomater. Sci. 2021, 9, 4388–4409. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A.; Michalska-Sionkowska, M.; Sionkowska, A. The Characterization of Thin Films Based on Chitosan and Tannic Acid Mixture for Potential Applications as Wound Dressings. Polym. Test. 2019, 78, 106007. [Google Scholar] [CrossRef]
- Ge, Y.; Ge, M. Sustained Broad-Spectrum Antimicrobial and Haemostatic Chitosan-Based Film with Immerged Tea Tree Oil Droplets. Fibers Polym. 2015, 16, 308–318. [Google Scholar] [CrossRef]
- Akrami-Hasan-Kohal, M.; Tayebi, L.; Ghorbani, M. Curcumin-Loaded Naturally-Based Nanofibers as Active Wound Dressing Mats: Morphology, Drug Release, Cell Proliferation, and Cell Adhesion Studies. New J. Chem. 2020, 44, 10343–10351. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lin, J.-H.; Hong, Y.-S. Development of Chitosan/Poly-Gamma-Glutamic Acid/Pluronic/Curcumin Nanoparticles in Chitosan Dressings for Wound Regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, Y.; Huang, H.; Fan, S.; Yang, C.; Wang, L.; Li, W.; Niu, W.; Liao, J. ROS-Eliminating Carboxymethyl Chitosan Hydrogel to Enhance Burn Wound-Healing Efficacy. Front. Pharmacol. 2021, 12, 679580. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable Conductive Injectable Hydrogels as Novel Antibacterial, Anti-Oxidant Wound Dressings for Wound Healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Zheng, Z.; Bian, S.; Li, Z.; Zhang, Z.; Liu, Y.; Zhai, X.; Pan, H.; Zhao, X. Catechol Modified Quaternized Chitosan Enhanced Wet Adhesive and Antibacterial Properties of Injectable Thermo-Sensitive Hydrogel for Wound Healing. Carbohydr. Polym. 2020, 249, 116826. [Google Scholar] [CrossRef]
- Huber, D.; Grzelak, A.; Baumann, M.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Anti-Inflammatory and Anti-Oxidant Properties of Laccase-Synthesized Phenolic-O-Carboxymethyl Chitosan Hydrogels. New Biotechnol. 2018, 40, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Najafi-Soulari, S.; Shekarchizadeh, H.; Kadivar, M. Encapsulation Optimization of Lemon Balm Antioxidants in Calcium Alginate Hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 1631–1644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, G.; Song, G.; Liu, T.; Cao, C.; Yang, Y.; Zhang, Y.; Hong, W. Incorporation of ZnO/Bioactive Glass Nanoparticles into Alginate/Chitosan Composite Hydrogels for Wound Closure. ACS Appl. Bio Mater. 2019, 2, 5042–5052. [Google Scholar] [CrossRef]
- Chen, S.; Cui, S.; Hu, J.; Zhou, Y.; Liu, Y. Pectinate Nanofiber Mat with High Absorbency and Antibacterial Activity: A Potential Superior Wound Dressing to Alginate and Chitosan Nanofiber Mats. Carbohydr. Polym. 2017, 174, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qu, X.; Kim, E.; Lei, M.; Dai, K.; Tan, X.; Xu, M.; Li, J.; Liu, Y.; Shi, X.; et al. Bio-Inspired Redox-Cycling Antimicrobial Film for Sustained Generation of Reactive Oxygen Species. Biomaterials 2018, 162, 109–122. [Google Scholar] [CrossRef]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Ke, Q.-F.; Tao, S.-C.; Guo, S.-C.; Rui, B.-Y.; Guo, Y.-P. Fabrication of Hydroxyapatite/Chitosan Composite Hydrogels Loaded with Exosomes Derived from MiR-126-3p Overexpressed Synovial Mesenchymal Stem Cells for Diabetic Chronic Wound Healing. J. Mater. Chem. B 2016, 4, 6830–6841. [Google Scholar] [CrossRef]
- Nooshabadi, V.T.; Khanmohamadi, M.; Valipour, E.; Mahdipour, S.; Salati, A.; Malekshahi, Z.V.; Shafei, S.; Amini, E.; Farzamfar, S.; Ai, J. Impact of Exosome-Loaded Chitosan Hydrogel in Wound Repair and Layered Dermal Reconstitution in Mice Animal Model. J. Biomed. Mater. Res. A 2020, 108, 2138–2149. [Google Scholar] [CrossRef]
- Bari, E.; Di Silvestre, D.; Mastracci, L.; Grillo, F.; Grisoli, P.; Marrubini, G.; Nardini, M.; Mastrogiacomo, M.; Sorlini, M.; Rossi, R.; et al. GMP-Compliant Sponge-like Dressing Containing MSC Lyo-Secretome: Proteomic Network of Healing in a Murine Wound Model. Eur. J. Pharm. Biopharm. 2020, 155, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.E.; Grumezescu, A.M.; Hermenean, A.O.; Andronescu, E.; Vasile, B.S. Scar-Free Healing: Current Concepts and Future Perspectives. Nanomaterials 2020, 10, 2179. [Google Scholar] [CrossRef]
- Halim, A.S.; Nor, F.M.; Saad, A.Z.M.; Nasir, N.A.M.; Norsa’adah, B.; Ujang, Z. Efficacy of Chitosan Derivative Films versus Hydrocolloid Dressing on Superficial Wounds. J. Taibah Univ. Med. Sci. 2018, 13, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Polymer-Based Scaffolds Loaded with Aloe Vera Extract for the Treatment of Wounds. Pharmaceutics 2021, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Koga, A.Y.; Felix, J.C.; Marques Silvestre, R.G.; Lipinski, L.C.; Carletto, B.; Kawahara, F.A.; Pereira, A.V. Evaluation of Wound Healing Effect of Alginate Film Containing Aloe Vera Gel and Cross-Linked with Zinc Chlorides. Acta Cirúrgica Brasileira 2020, 35, e202000507. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Milan, P.B.; Amoupour, M. Enhanced Antimicrobial and Full-Thickness Wound Healing Efficiency of Hydrogels Loaded with Heparinized ZnO Nanoparticles: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2021, 166, 200–212. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.; Sharma, D.; Gupta, B. Dextran Based Herbal Nanobiocomposite Membranes for Scar Free Wound Healing. Int. J. Biol. Macromol. 2018, 113, 227–239. [Google Scholar] [CrossRef]
- Mehta, M.; Branford, O.A.; Rolfe, K.J. The Evidence for Natural Therapeutics as Potential Anti-Scarring Agents in Burn-Related Scarring. Burns Trauma 2016, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.-J.; Hyun, H.; Lee, D.-W.; Yang, D.H. Visible Light-Cured Glycol Chitosan Hydrogel Containing a Beta-Cyclodextrin-Curcumin Inclusion Complex Improves Wound Healing In Vivo. Molecules 2017, 22, 1513. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.; Ye, H.; Wu, D. Recent Advances on Polymeric Hydrogels as Wound Dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, M.; Woo, M.W.; Li, Y.; Han, W.; Dang, X. High-Mechanical Strength Carboxymethyl Chitosan-Based Hydrogel Film for Antibacterial Wound Dressing. Carbohydr. Polym. 2021, 256, 117590. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Wu, L.; Yang, Y. Multifunctional Double Network Hydrogel Film for Skin Wound Healing. Mater. Express 2021, 11, 1084–1091. [Google Scholar] [CrossRef]
- Rao, Z.; Liu, S.; Wu, R.; Wang, G.; Sun, Z.; Bai, L.; Wang, W.; Chen, H.; Yang, H.; Wei, D.; et al. Fabrication of Dual Network Self-Healing Alginate/Guar Gum Hydrogels Based on Polydopamine-Type Microcapsules from Mesoporous Silica Nanoparticles. Int. J. Biol. Macromol. 2019, 129, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, X.; Luo, J.; Wen, Q.; Wu, Z.; Ding, Q.; Zhao, L.; Yang, L.; Wang, B.; Fu, S. Naproxen Nanoparticle-Loaded Thermosensitive Chitosan Hydrogel for Prevention of Postoperative Adhesions. ACS Biomater. Sci. Eng. 2019, 5, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Arafa, A.A.; Nada, A.A.; Ibrahim, A.Y.; Sajkiewicz, P.; Zahran, M.K.; Hakeim, O.A. Preparation and Characterization of Smart Therapeutic PH-Sensitive Wound Dressing from Red Cabbage Extract and Chitosan Hydrogel. Int. J. Biol. Macromol. 2021, 182, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A Near-Infrared Light-Responsive Multifunctional Nanocomposite Hydrogel for Efficient and Synergistic Antibacterial Wound Therapy and Healing Promotion. J. Mater. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, R.; Li, Q.; Dai, F.; Lan, G.; Shang, S.; Lu, F. A Self-Adapting Hydrogel Based on Chitosan/Oxidized Konjac Glucomannan/AgNPs for Repairing Irregular Wounds. Biomater. Sci. 2020, 8, 1910–1922. [Google Scholar] [CrossRef]
- Xuan, X.; Zhou, Y.; Chen, A.; Zheng, S.; An, Y.; He, H.; Huang, W.; Chen, Y.; Yang, Y.; Li, S.; et al. Silver Crosslinked Injectable BFGF-Eluting Supramolecular Hydrogels Speed up Infected Wound Healing. J. Mater. Chem. B 2020, 8, 1359–1370. [Google Scholar] [CrossRef]
- Li, W.; Wang, B.; Zhang, M.; Wu, Z.; Wei, J.; Jiang, Y.; Sheng, N.; Liang, Q.; Zhang, D.; Chen, S. All-Natural Injectable Hydrogel with Self-Healing and Antibacterial Properties for Wound Dressing. Cellulose 2020, 27, 2637–2650. [Google Scholar] [CrossRef]
- Cho, S.; Lim, S.; Han, D.; Yuk, S.; Im, G.-I.; Jh, L. Time-Dependent Alginate/Polyvinyl Alcohol Hydrogels as Injectable Cell Carriers. J. Biomater. Sci. Polym. Ed. 2009, 20, 863–876. [Google Scholar] [CrossRef]
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-Inspired Electroactive and Antioxidative Scaffolds with Incorporation of Polydopamine-Reduced Graphene Oxide for Enhancing Skin Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-Inspired, Antibacterial, Conductive, Antioxidant, Injectable Composite Hydrogel Wound Dressing to Promote the Regeneration of Infected Skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, K.; Darabi, M.A.; Yuan, Q.; Xing, M. Mussel-Inspired Alginate Gel Promoting the Osteogenic Differentiation of Mesenchymal Stem Cells and Anti-Infection. Mater. Sci. Eng. C 2016, 69, 496–504. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Niu, D.; Wu, N.; Yun, W.; Wang, W.; Zhang, K.; Li, G.; Yan, S.; Xu, G.; et al. Mussel-Inspired Bisphosphonated Injectable Nanocomposite Hydrogels with Adhesive, Self-Healing, and Osteogenic Properties for Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 32673–32689. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver Nanoparticle Impregnated Chitosan-PEG Hydrogel Enhances Wound Healing in Diabetes Induced Rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and Characterization of Chitosan-PVP-Nanocellulose Composites for in-Vitro Wound Dressing Application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, M.; Zhang, Y.; Cao, Q.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. Novel Lignin-Chitosan-PVA Composite Hydrogel for Wound Dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 110002. [Google Scholar] [CrossRef]
- Lu, B.; Lu, F.; Zou, Y.; Liu, J.; Rong, B.; Li, Z.; Dai, F.; Wu, D.; Lan, G. In Situ Reduction of Silver Nanoparticles by Chitosan-L-Glutamic Acid/Hyaluronic Acid: Enhancing Antimicrobial and Wound-Healing Activity. Carbohydr. Polym. 2017, 173, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Baghaie, S.; Khorasani, M.T.; Zarrabi, A.; Moshtaghian, J. Wound Healing Properties of PVA/Starch/Chitosan Hydrogel Membranes with Nano Zinc Oxide as Antibacterial Wound Dressing Material. J. Biomater. Sci. Polym. Ed. 2017, 28, 2220–2241. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Li, X.; Lin, Z.; Chen, L.; Chen, H.; Jin, Y.; Zhang, T.; Xia, H.; Lu, Y.; et al. Ultrafast In-Situ Forming Halloysite Nanotube-Doped Chitosan/Oxidized Dextran Hydrogels for Hemostasis and Wound Repair. Carbohydr. Polym. 2021, 267, 118155. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Qin, D.; Sun, M.; Wang, T.; Chen, X. Research Status of Self-Healing Hydrogel for Wound Management: A Review. Int. J. Biol. Macromol. 2020, 164, 2108–2123. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An Injectable Self-Healing Hydrogel with Adhesive and Antibacterial Properties Effectively Promotes Wound Healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zou, Y.; Wu, S.; Zou, X. Self-Healing and Tough Hydrogels with Conductive Properties Prepared through an Interpenetrating Polymer Network Strategy. Polymer 2020, 206, 122907. [Google Scholar] [CrossRef]
- Samchenko, Y.; Ulberg, Z.; Korotych, O. Multipurpose Smart Hydrogel Systems. Adv. Colloid Interface Sci. 2011, 168, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, R.; Derakhshankhah, H.; Haghshenas, B.; Massoumi, B.; Abbasian, M.; Jaymand, M. A Bio-Inspired Magnetic Natural Hydrogel Containing Gelatin and Alginate as a Drug Delivery System for Cancer Chemotherapy. Int. J. Biol. Macromol. 2020, 156, 438–445. [Google Scholar] [CrossRef]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable Hydrogel-Based Drug Delivery Systems for Local Cancer Therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Monette, A.; Ceccaldi, C.; Assaad, E.; Lerouge, S.; Lapointe, R. Chitosan Thermogels for Local Expansion and Delivery of Tumor-Specific T Lymphocytes towards Enhanced Cancer Immunotherapies. Biomaterials 2016, 75, 237–249. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, C.; Yang, S.; Wang, Q.; Liang, F.; Liu, C.; Qiu, D.; Qu, X.; Hu, Z.; Yang, Z. Injectable Tissue Adhesive Composite Hydrogel with Fibroblasts for Treating Skin Defects. J. Mater. Chem. B 2017, 5, 2416–2424. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, A.; Liu, T.; Wang, Y.; Gao, Y.; Li, J.; Yang, P. An Injectable Nanocomposite Hydrogel for Potential Application of Vascularization and Tissue Repair. Ann. Biomed. Eng. 2020, 48, 1511–1523. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, T.; Chen, W.; Wang, X.; Li, J.; Chen, Y.; Yu, Y.; Shen, Z.; Yu, Q.; Zhang, Y. Release of VEGF and BMP9 from Injectable Alginate Based Composite Hydrogel for Treatment of Myocardial Infarction. Bioact. Mater. 2021, 6, 520–528. [Google Scholar] [CrossRef]
- Des Rieux, A.; De Berdt, P.; Ansorena, E.; Ucakar, B.; Damien, J.; Schakman, O.; Audouard, E.; Bouzin, C.; Auhl, D.; Simón-Yarza, T.; et al. Vascular Endothelial Growth Factor-Loaded Injectable Hydrogel Enhances Plasticity in the Injured Spinal Cord: Vegf-Loaded Injectable Hydrogel. J. Biomed. Mater. Res. A 2014, 102, 2345–2355. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.; Zhou, Y.; Zhou, F.; Liu, W.; Wang, Z. Mussel-Inspired Hydrogels: From Design Principles to Promising Applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef]
- Pandey, N.; Soto-Garcia, L.F.; Liao, J.; Zimmern, P.; Nguyen, K.T.; Hong, Y. Mussel-Inspired Bioadhesives in Healthcare: Design Parameters, Current Trends, and Future Perspectives. Biomater. Sci. 2020, 8, 1240–1255. [Google Scholar] [CrossRef]
- Han, L.; Li, P.; Tang, P.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-Inspired Cryogels for Promoting Wound Regeneration through Photobiostimulation, Modulating Inflammatory Responses and Suppressing Bacterial Invasion. Nanoscale 2019, 11, 15846–15861. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, R.; Balraj, S.; Ganesh, J.; Milton, M.C.J. Therapeutic Agents Loaded Chitosan-Based Nanofibrous Mats as Potential Wound Dressings: A Review. Mater. Today Chem. 2019, 12, 386–395. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef] [PubMed]
- Coskun, G.; Karaca, E.; Ozyurtlu, M.; Ozbek, S.; Yermezler, A.; Cavusoglu, I. Histological Evaluation of Wound Healing Performance of Electrospun Poly(Vinyl Alcohol)/Sodium Alginate as Wound Dressing in Vivo. Biomed. Mater. Eng. 2014, 24, 1527–1536. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.I.; Kim, M.S.; Chung, G.Y.; Shin, H.S. Spirulina Extract-Impregnated Alginate-PCL Nanofiber Wound Dressing for Skin Regeneration. Biotechnol. Bioprocess Eng. 2017, 22, 679–685. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, M.; Martins, A.; Fonseca, N.A.; Moreira, J.N.; Reis, R.L.; Neves, N.M. Antibacterial Activity of Chitosan Nanofiber Meshes with Liposomes Immobilized Releasing Gentamicin. Acta Biomater. 2015, 18, 196–205. [Google Scholar] [CrossRef]
- Amini, F.; Semnani, D.; Karbasi, S.; Banitaba, S.N. A Novel Bilayer Drug-Loaded Wound Dressing of PVDF and PHB/Chitosan Nanofibers Applicable for Post-Surgical Ulcers. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 772–777. [Google Scholar] [CrossRef]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound Dressing Based on Electrospun PVA/Chitosan/Starch Nanofibrous Mats: Fabrication, Antibacterial and Cytocompatibility Evaluation and in Vitro Healing Assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Xie, X.; Li, D.; Su, C.; Cong, W.; Mo, X.; Hou, G.; Wang, C. Functionalized Biomimetic Composite Nanfibrous Scaffolds with Antibacterial and Hemostatic Efficacy for Facilitating Wound Healing. J. Biomed. Nanotechnol. 2019, 15, 1267–1279. [Google Scholar] [CrossRef]
- Tanha, S.; Rafiee-Tehrani, M.; Abdollahi, M.; Vakilian, S.; Esmaili, Z.; Naraghi, Z.S.; Seyedjafari, E.; Javar, H.A. G-CSF Loaded Nanofiber/Nanoparticle Composite Coated with Collagen Promotes Wound Healing in Vivo. J. Biomed. Mater. Res. A 2017, 105, 2830–2842. [Google Scholar] [CrossRef]
- Mirmajidi, T.; Chogan, F.; Rezayan, A.H.; Sharifi, A.M. In Vitro and in Vivo Evaluation of a Nanofiber Wound Dressing Loaded with Melatonin. Int. J. Pharm. 2021, 596, 120213. [Google Scholar] [CrossRef]
- Golchin, A.; Hosseinzadeh, S.; Jouybar, A.; Staji, M.; Soleimani, M.; Ardeshirylajimi, A.; Khojasteh, A. Wound Healing Improvement by Curcumin-Loaded Electrospun Nanofibers and BFP-MSCs as a Bioactive Dressing. Polym. Adv. Technol. 2020, 31, 1519–1531. [Google Scholar] [CrossRef]
- Lu, W.-C.; Chuang, F.-S.; Venkatesan, M.; Cho, C.-J.; Chen, P.-Y.; Tzeng, Y.-R.; Yu, Y.-Y.; Rwei, S.-P.; Kuo, C.-C. Synthesis of Water Resistance and Moisture-Permeable Nanofiber Using Sodium Alginate-Functionalized Waterborne Polyurethane. Polymers 2020, 12, 2882. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X.B. In Vitro and in Vivo Evaluation of Chitosan-Alginate/Gentamicin Wound Dressing Nanofibrous with High Antibacterial Performance. Polym. Test. 2020, 82, 106298. [Google Scholar] [CrossRef]
- Malgarim Cordenonsi, L.; Faccendini, A.; Rossi, S.; Bonferoni, M.C.; Malavasi, L.; Raffin, R.; Schapoval, E.; Fante, C.; Vigani, B.; Miele, D.; et al. Platelet Lysate Loaded Electrospun Scaffolds: Effect of Nanofiber Types on Wound Healing. Eur. J. Pharm. Biopharm. 2019, 142, 247–257. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, S.; Zhong, L.; Wang, B.; Wang, H.; Hong, F. Zn2+-Loaded TOBC Nanofiber-Reinforced Biomimetic Calcium Alginate Hydrogel for Antibacterial Wound Dressing. Int. J. Biol. Macromol. 2020, 143, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Oksuz, K.E.; Ozkaya, N.K.; Inan, Z.D.S.; Ozer, A. Novel Natural Spider Silk Embedded Electrospun Nanofiber Mats for Wound Healing. Mater. Today Commun. 2021, 26, 101942. [Google Scholar] [CrossRef]
- Serdar, T.; Acarturk, F.; Arzu, B. Evaluation of Three-Layered Doxycycline-Collagen Loaded Nanofiber Wound Dressing. Int. J. Pharm. 2017, 529, 642–653. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic Acid—Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Bellini, M.Z.; Resende Pires, A.L.; Vasconcelos, M.O.; Moraes, A.M. Comparison of the Properties of Compacted and Porous Lamellar Chitosan-Xanthan Membranes as Dressings and Scaffolds for the Treatment of Skin Lesions. J. Appl. Polym. Sci. 2012, 125, E421–E431. [Google Scholar] [CrossRef]
- Pal, P.; Dadhich, P.; Srivas, P.K.; Das, B.; Maulik, D.; Dhara, S. Bilayered Nanofibrous 3D Hierarchy as Skin Rudiment by Emulsion Electrospinning for Burn Wound Management. Biomater. Sci. 2017, 5, 1786–1799. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chang, Y.-C.; Cheng, L.-C.; Liu, C.-H.; Chang, S.C.; Hsien, T.-Y.; Wang, D.-M.; Hsieh, H.-J. Preparation of Graphene-Embedded Hydroxypropyl Cellulose/Chitosan/Polyethylene Oxide Nanofiber Membranes as Wound Dressings with Enhanced Antibacterial Properties. Cellulose 2020, 27, 2651–2667. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Novel Asymmetric Chitosan/PVP/Nanocellulose Wound Dressing: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2018, 112, 1300–1309. [Google Scholar] [CrossRef]
- Alves, P.; Santos, M.; Mendes, S.; Miguel, S.P.; de Sa, K.D.; Cabral, C.S.D.; Correia, I.J.; Ferreira, P. Photocrosslinkable Nanofibrous Asymmetric Membrane Designed for Wound Dressing. Polymers 2019, 11, 653. [Google Scholar] [CrossRef] [Green Version]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Zhou, M.; Guo, Z.; Li, Y.; Hou, Y.; Wang, D.; Zhang, L. Preparation and Characterization of Thermosensitive Artificial Skin with a Sandwich Structure. Mater. Lett. 2015, 147, 4–7. [Google Scholar] [CrossRef]
- Jung, J.; Li, L.; Yeh, C.-K.; Ren, X.; Sun, Y. Amphiphilic Quaternary Ammonium Chitosan/Sodium Alginate Multilayer Coatings Kill Fungal Cells and Inhibit Fungal Biofilm on Dental Biomaterials. Mater. Sci. Eng. C 2019, 104, 109961. [Google Scholar] [CrossRef]
- Silva, J.; Duarte, A.; Caridade, S.; Picart, C.; Reis, R.L.; Mano, J.F. Tailored Freestanding Multi Layered Membranes Based on Chitosan and Alginate. Biomacromolecules 2014, 15, 3817–3826. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, D.; Carvalho, J.P.F.; Bastos, V.; Oliveira, H.; Moreirinha, C.; Almeida, A.; Silvestre, A.; Vilela, C.; Freire, C. Antibacterial Multi-Layered Nanocellulose-Based Patches Loaded with Dexpanthenol for Wound Healing Applications. Nanomaterials 2020, 10, 2469. [Google Scholar] [CrossRef]
- Ghalei, S.; Nourmohammadi, J.; Solouk, A.; Mirzadeh, H. Enhanced Cellular Response Elicited by Addition of Amniotic Fluid to Alginate Hydrogel-Electrospun Silk Fibroin Fibers for Potential Wound Dressing Application. Colloids Surf. B Biointerfaces 2018, 172, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-Y.; Ng, S.-F. Development of Moringa Oleifera Standardized Leaf Extract Nanofibers Impregnated onto Hydrocolloid Film as A Potential Chronic Wound Dressing. Fibers Polym. 2020, 21, 2462–2472. [Google Scholar] [CrossRef]
- Thu, H.-E.; Zulfakar, M.H.; Ng, S.-F. Alginate Based Bilayer Hydrocolloid Films as Potential Slow-Release Modern Wound Dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.-H.; Lai, C.W.; Gholami, A.; Omidifar, N.; Shokripour, M. Asymmetric Membranes: A Potential Scaffold for Wound Healing Applications. Symmetry 2020, 12, 1100. [Google Scholar] [CrossRef]
- Woo, C.H.; Choi, Y.C.; Choi, J.S.; Lee, H.Y.; Cho, Y.W. A Bilayer Composite Composed of TiO2-Incorporated Electrospun Chitosan Membrane and Human Extracellular Matrix Sheet as a Wound Dressing. J. Biomater. Sci. Polym. Ed. 2015, 26, 841–854. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Liu, Y.; Li, Y.; Zhang, C.; Qi, W.; Yeung, K.W.K.; Wong, T.M.; Zhao, X.; Pan, H. Electrospun Chitosan/PVA/Bioglass Nanofibrous Membrane with Spatially Designed Structure for Accelerating Chronic Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110083. [Google Scholar] [CrossRef]
- Ngece, K.; Aderibigbe, B.A.; Ndinteh, D.T.; Fonkui, Y.T.; Kumar, P. Alginate-Gum Acacia Based Sponges as Potential Wound Dressings for Exuding and Bleeding Wounds. Int. J. Biol. Macromol. 2021, 172, 350–359. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent Advances on Antimicrobial Wound Dressing: A Review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, N.; Meng, G.; He, J.; Wu, F. The Effect of Form of Carboxymethyl-Chitosan Dressings on Biological Properties in Wound Healing. Colloids Surf. B Biointerfaces 2020, 194, 111191. [Google Scholar] [CrossRef]
- Hu, S.; Bi, S.; Yan, D.; Zhou, Z.; Sun, G.; Cheng, X.; Chen, X. Preparation of Composite Hydroxybutyl Chitosan Sponge and Its Role in Promoting Wound Healing. Carbohydr. Polym. 2018, 184, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Sanad, R.A.-B.; Abdel-Bar, H.M. Chitosan-Hyaluronic Acid Composite Sponge Scaffold Enriched with Andrographolide-Loaded Lipid Nanoparticles for Enhanced Wound Healing. Carbohydr. Polym. 2017, 173, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Wu, J.; Wang, S.; Huang, M.; Liu, X.; Zhang, R. Construction of Silver Sulfadiazine Loaded Chitosan Composite Sponges as Potential Wound Dressings. Carbohydr. Polym. 2017, 157, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, A.; Anisha, B.S.; Chennazhi, K.P.; Jayakumar, R. Chitosan-Hyaluronic Acid/VEGF Loaded Fibrin Nanoparticles Composite Sponges for Enhancing Angiogenesis in Wounds. Colloids Surf. B Biointerfaces 2015, 127, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Saporito, F.; Sandri, G.; Rossi, S.; Bonferoni, M.C.; Riva, F.; Malavasi, L.; Caramella, C.; Ferrari, F. Freeze Dried Chitosan Acetate Dressings with Glycosaminoglycans and Traxenamic Acid. Carbohydr. Polym. 2018, 184, 408–417. [Google Scholar] [CrossRef]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef]
- Tamahkar, E.; Ozkahraman, B.; Ozbas, Z.; Izbudak, B.; Yarimcan, F.; Boran, F.; Ozturk, A.B. Aloe Vera-Based Antibacterial Porous Sponges for Wound Dressing Applications. J. Porous Mater. 2021, 28, 741–750. [Google Scholar] [CrossRef]
- Hartrianti, P.; Nguyen, L.T.H.; Johanes, J.; Chou, S.M.; Zhu, P.; Tan, N.S.; Tang, M.B.Y.; Ng, K.W. Fabrication and Characterization of a Novel Crosslinked Human Keratin-Alginate Sponge. J. Tissue Eng. Regen. Med. 2017, 11, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wang, Y.; Qi, H.; Shi, C.; Wei, G.; Xiao, L.; Huang, Z.; Liu, S.; Yu, H.; Teng, C.; et al. Nanocomposite Sponges of Sodium Alginate/Graphene Oxide/Polyvinyl Alcohol as Potential Wound Dressing: In Vitro and in Vivo Evaluation. Compos. Part B Eng. 2019, 167, 396–405. [Google Scholar] [CrossRef]
- Gupta, V.; Khan, Y.; Berkland, C.J.; Laurencin, C.T.; Detamore, M.S. Microsphere-Based Scaffolds in Regenerative Engineering. Annu. Rev. Biomed. Eng. 2017, 19, 135–161. [Google Scholar] [CrossRef]
- Duvnjak Romić, M.; Špoljarić, D.; Šegvić Klarić, M.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin Loaded Lipid Enriched Chitosan Microspheres—Hybrid Dressing for Moderate Exuding Wounds. J. Drug Deliv. Sci. Technol. 2019, 52, 431–439. [Google Scholar] [CrossRef]
- Romic, M.D.; Klaric, M.S.; Lovric, J.; Pepic, I.; Cetina-Cizmek, B.; Filipovic-Grcic, J.; Hafner, A. Melatonin-Loaded Chitosan/Pluronic (R) F127 Microspheres as in Situ Forming Hydrogel: An Innovative Antimicrobial Wound Dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef]
- Gil, J.; Natesan, S.; Li, J.; Valdes, J.; Harding, A.; Solis, M.; Davis, S.C.; Christy, R.J. A PEGylated Fibrin Hydrogel-Based Antimicrobial Wound Dressing Controls Infection without Impeding Wound Healing. Int. Wound J. 2017, 14, 1248–1257. [Google Scholar] [CrossRef]
- Rath, G.; Johal, E.S.; Goyal, A.K. Development of Serratiopeptidase and Metronidazole Based Alginate Microspheres for Wound Healing. Artif. Cells Blood Substit. Biotechnol. 2011, 39, 44–50. [Google Scholar] [CrossRef]
- Revin, V.V.; Nazarova, N.B.; Tsareva, E.E.; Liyaskina, E.V.; Revin, V.D.; Pestov, N.A. Production of Bacterial Cellulose Aerogels with Improved Physico-Mechanical Properties and Antibacterial Effect. Front. Bioeng. Biotechnol. 2020, 8, 603407. [Google Scholar] [CrossRef]
- Bernardes, B.G.; Del Gaudio, P.; Alves, P.; Costa, R.; García-Gonzaléz, C.A.; Oliveira, A.L. Bioaerogels: Promising Nanostructured Materials in Fluid Management, Healing and Regeneration of Wounds. Molecules 2021, 26, 3834. [Google Scholar] [CrossRef]
- Muhammad, A.; Lee, D.; Shin, Y.; Park, J. Recent Progress in Polysaccharide Aerogels: Their Synthesis, Application, and Future Outlook. Polymers 2021, 13, 1347. [Google Scholar] [CrossRef]
- Lovskaya, D.; Menshutina, N.; Mochalova, M.; Nosov, A.; Grebenyuk, A. Chitosan-Based Aerogel Particles as Highly Effective Local Hemostatic Agents. Production Process and In Vivo Evaluations. Polymers 2020, 12, 2055. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gomez-Amoza, J.L.; Garcia-Gonzalez, C.A. Vancomycin-Loaded Chitosan Aerogel Particles for Chronic Wound Applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Kim, H. Preparation of Chitosan Aerogel Crosslinked in Chemical and Ionical Ways by Non-Acid Condition for Wound Dressing. Int. J. Biol. Macromol. 2020, 164, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Valchuk, N.A.; Brovko, O.S.; Palamarchuk, I.A.; Boitsova, T.A.; Bogolitsyn, K.G.; Ivakhnov, A.D.; Chukhchin, D.G.; Bogdanovich, N.I. Preparation of Aerogel Materials Based on Alginate–Chitosan Interpolymer Complex Using Supercritical Fluids. Russ. J. Phys. Chem. B 2019, 13, 1121–1124. [Google Scholar] [CrossRef]
- Keil, C.; Huebner, C.; Richter, C.; Lier, S.; Barthel, L.; Meyer, V.; Subrahmanyam, R.; Gurikov, P.; Smirnova, I.; Haase, H. Ca-Zn-Ag Alginate Aerogels for Wound Healing Applications: Swelling Behavior in Simulated Human Body Fluids and Effect on Macrophages. Polymers 2020, 12, 2741. [Google Scholar] [CrossRef]











| Dressing Types | Advantages | Disadvantages | Suitable Conditions | Refs |
|---|---|---|---|---|
| Hydrogels | Good absorption of exudate Good moisturizing properties Have a cleansing effect No reoccurring mechanical damage Self-adhesive Concealed appearance Good antibacterial properties Accelerated wound healing | Poor ability to absorb exudate Higher costs Possible allergic reaction | Pressure ulcers Surgical wounds Burns Radiation dermatitis Diabetic foot ulcer | [15,16] |
| Nanofibre mats | Good antibacterial properties Effective control of local wound infection Good absorption of exudate Accelerated wound healing | Cytotoxic risk Prone to allergic reactions Higher production cost | Burns and scald Localized trauma infection | [17,18] |
| Films | Good antibacterial properties Good moisturizing properties Self-adhesive | Poor mechanical properties Higher costs | Epithelializing wounds and superficial wounds with limited exudate Chronic venous ulcer Radiation dermatitis | [19] |
| Membranes | Good haemostatic effect Promotes granulation tissue formation and self-decomposition of necrotic tissue Good antibacterial property | Poor ability to absorb ooze Higher production cost | Chronic venous ulcer All kinds of dermatitis and eczema | [20,21] |
| Sponge | Good absorption of exudate Low permeability Good antibacterial properties Thermal insulation | Excessive absorption Higher costs Inconvenient to observe | Infected wounds Diabetic foot ulcer Medium to heavily exuding wounds Venous ulcers | [22,23] |
| Derivatives | Structures | Properties | Refs |
|---|---|---|---|
| Carboxymethyl chitosan |  | Better and more controlled water solubility Inhibits scarring | [43] |
| Alkylation chitosan |  | Better water solubility Enhanced haemostatic efficacy Better mechanical stability | [44,45] |
| Trimethyl chitosan ammonium |  | Water-soluble over a wide pH range Good flocculation and antistatic properties Better antibacterial properties | [46] |
| Bioactivities | Mechanisms and Hypotheses |
|---|---|
| Antibacterial | No definitive conclusion yet. The main hypotheses include: (1) adheres to and electrostatically disrupts bacterial cell walls and cell membranes, (2) chelates trace metal cations leading to potential imbalance, (3) interaction with intracellular targets to inhibit protein synthesis, (4) deposits on bacteria and affects metabolism |
| Anti-inflammatory | Induces increased levels of anti-inflammatory cytokines such as IL-10, TGF-β1 and decreased levels of pro-inflammatory cytokines. |
| Antioxidant | It is achieved by donating hydrogen atoms. The amino and carboxyl groups of CS stabilize free radicals. |
| Promotes tissue regeneration | Modulates growth factors to: promote macrophage transfer to wounds; promote fibroblast proliferation; promote proteoglycan and collagen synthesis; promote angiogenesis. |
| Haemostasis | Promotes the aggregation of platelets and red blood cells and their adhesion to tissues to form clots |
| Scar-free | Dependent on its cationic properties. CS inhibits the production of type I collagen in wounds, promotes the production of granulation and epithelial tissue, as well as reducing wound contraction, thereby reducing scarring. |
| Sources of EPS | Habitat | Functions and Applications | Refs |
|---|---|---|---|
| Sphingobium yanoikuyae BBL01 | Coast | Gelling agent Metal-complexion Antioxidant | [220] |
| Vibrio alginolyticus 364 | deep-sea | Anti-tumour | [223] |
| Rhodothermus marinus DSM 4252T | Shallow marine hot springs | Antioxidant Anti-haemolytic Anti-thrombotic | [224] |
| Winogradsky sp. CAL384 and Shewanella sp. CAL606 | Antarctic Ocean | Emulsifier Chelates heavy metals | [225] |
| Pseudomonas sp. BGI-2 | Glacier ice | Antioxidant Low temperature protection | [226] |
| Paenibacillus sp. TKU042 | Marine chitinous materials | Antioxidant Anti-inflammatory Alpha-glucosidase inhibitor | [227] |
| Bacillus subtilis SH1 | Marine surface sediment | Antiviral Antibacterial Antioxidant | [228] |
| Bacillus vallismortis WF4 | Coast | Anti-fungal Anti-itch | [229] |
| Bioactivities | Dressing Type | Structural Components | Active Agents | Other Features | Refs |
|---|---|---|---|---|---|
| Haemostatic Antibacterial | Hydrogels | Hydroxybutyl CS | Dopamine | Mussel-inspired technology High viscosity High mechanical strength Thermosensitive hydrogel | [238] |
| Haemostatic Antibacterial | Sponge | CS | Graphene-silver-polycationic peptide | -- | [239] |
| Haemostatic | Hydrogels | Alginate Pept-1 | Cross-linked zinc ions Tannic acid | High physical stability | [240] |
| Haemostatic | Hydrogels | Alginate GLE CMC | Cross-linked zinc ions Tannic acid | Effective drug delivery | [241] |
| Haemostatic | Sponge | CS | Tilapia peptides | -- | [242] |
| Haemostatic Antibacterial Anti-inflammatory Promotes tissue regeneration | Sponge | Alginate CS Fucoidan | -- | -- | [144] |
| Haemostatic Promotes tissue regeneration | Sponge | CS PVA | -- | For non-compression wounds | [243] |
| Antibacterial | Hydrogels | CS PVA | Ag NPs | -- | [244] |
| Antibacterial Pro-regenerative Anti-inflammatory | Hydrogels | CS | AgNPs Nanocrystals | High physical stability Effective drug delivery | [245] |
| Antibacterial | Hydrogels | Alginate CaCO3 GDL | AgNPs | -- | [246] |
| Antibacterial Anti-inflammatory | Hydrogels | Alginate Gum acacia | ZnNPs | -- | [247] |
| Antibacterial | Hydrogels | CS Gelatin | Manuka honey | -- | [248] |
| Antibacterial Anti-inflammatory | Hydrogels | Carboxylated Agarose | Zinc ions Tannic acid | pH-sensitive | [249] |
| Antibacterial Promotes tissue regeneration | Film | CS Modified bacterial cellulose | -- | Self-healing High biocompatibility | [250] |
| Antibacterial | Film | CS Starch nanocrystals | Streptomycin | Sustained slow release | [251] |
| Antibacterial | Film | Alginate CaCO3 | Oregano essential oil | High physical stability | [252] |
| Antibacterial | Membranes | CS Gelatin | Fe3O4 NPs | Extremely strong mechanical properties | [253] |
| Antibacterial | Nanofibres mats | Cellulose acetate | CS-Erythromycin NPs | High drug loading capacity High water holding capacity High porosity | [254] |
| Anti-inflammatory Promotes tissue regeneration | Hydrogels | QCS Matrigel Polyacrylamide | -- | Good mechanical properties Good adhesion | [255] |
| Anti-inflammatory | Hydrogels | Alginate Polycaprolactone | Doxorubicin Ibuprofen | -- | [256] |
| Anti-inflammatory | Films | CS | Cynara cardunculus leaves extracts | -- | [257] |
| Anti-inflammatory | Membranes | CS PVA | Ibuprofen | Prepared by supercritical CO2 technology Highly biocompatible | [258] |
| Antioxidant Antibacterial Promotes tissue regeneration | Hydrogels | QCS-polyaniline Glycerol polyethylene glycol copolymer sebacate | -- | Injectable Self-healing Adhesive conductive | [259] |
| Antioxidant Promotes tissue regeneration Anti-inflammatory | Hydrogels | Alginate PVA | Ag NPs hydroxymethylfurfural | -- | [260] |
| Antioxidant Promotes tissue regeneration | Hydrogels | CS Heparin Poly(gamma-glutamic acid) | Superoxide dismutase | Good mechanical properties Adhesion | [261] |
| Antioxidant Antibacterial | Membranes | CS PVA | ZnO | Electrospun membrane | [262] |
| Antioxidant Promotes tissue regeneration | Nanofibres mats | Grafted CS Polypropylene carbonate | Curcumin | Sustained release | [263] |
| Haemostatic Anti-inflammatory Promotes tissue regeneration | Hydrogels | CMC PVA | -- | Physically cross-linked Non-adhesive | [264] |
| Promotes tissue regeneration | Hydrogels | Ethylene glycol CS | GF VEGF PDGF-BB | Effective drug delivery Sustained release | [265] |
| Promotes tissue regeneration Antioxidant Antibacterial | Hydrogels | QCS Poly(N-isopropylacrylamide) | Reduced graphene oxide | Injectable Self-healing Self-contracting for wound healing Conductivity | [266] |
| Promotes tissue regeneration Haemostasis | Hydrogels | Alginate Adipic acid dihydrazide Polyglutamic acid | Bioglass | High physical stability | [267] |
| Promotes tissue regeneration | Hydrogels | CS PVA PCL | Heparin | Promotes angiogenesis | [268] |
| Promotes tissue regeneration | Hydrogels | Alginate | Borax | -- | [269] |
| Promotes tissue regeneration Antibacterial | Membranes | CS Arginine CS | Arginine CS | Similar in structure to ECM Promotes cell adhesion Electrospun membrane | [270] |
| Promotes tissue regeneration | Hydrogels | Alginate Biological ceramics | Biological ceramics | Promotes angiogenesis High physical stability | [271] |
| Promotes tissue regeneration | Hydrogels | Alginate | Exosome | High physical stability High porosity | [272] |
| Scar-free ntibacterial | Hydrogels | CS PVP PEG | Tetracycline hydrochloride | Efficient drug delivery | [273] |
| Scar-free | Hydrogels | CMC | Aloe vera | Aloe vera synergistically enhances the scar-inhibiting activity of CMC | [58] |
| Scar-free Promotes tissue regeneration | Sponge/hydrogels | Rhizo CS | Platelet concentrates | Dressings healed wounds as functional tissue instead of scars | [274] |
| Scar-free Antibacterial | Membranes | CS Dextran Nanosoy Glycerol | Aloe vera Manuka Honey | -- | [275] |
| Scar-free | Hydrogels | Alginate CS | AgNPs | High physical stability | [276] |
| Categories | Structural Components | Functional Components | Bioactivities | Other Features & Responsiveness | Refs |
|---|---|---|---|---|---|
| High mechanical properties | CMC Waterborne polyurethane—gelatine hydrolysate | -- | Antibacterial | High mechanical strength Thermal stability | [348] |
| High mechanical properties | CS Poly (acrylamide) | Carbon nanotubes VEGF | Anti-inflammatory Promotes tissue regeneration | Double-network hydrogels High mechanical strength | [349] |
| Self-healing | Alginate Guar Gum | GA | Promotes tissue regeneration | Thermal stability High mechanical strength | [350] |
| Smart hydrogels | CS | Naproxen | In vivo anti-adhesion Analgesic | Thermosensitive Low side effects | [351] |
| Smart hydrogels | CS Methylenebisacrylamide | Red cabbage extract Curcumin | Not tested | pH-sensitive Dynamic monitoring of wound pH to assess wound recovery status by colourimetry Efficient drug delivery | [352] |
| Smart hydrogels | Dodecyl modified CS | Photothermolysis Ciprofloxacin | Strong, artificially controlled sterilisation Anti-inflammatory Antioxidants | Photosensitive Adherence Injectable | [353] |
| Injectable hydrogels | CMC Chondroitin oxide sulphate | Chondroitin oxide sulphate | Antibacterial Haemostatic | Longer gelation time Low cytotoxicity Self-healing | [291] |
| Injectable hydrogels | CS Oxidized konjac glucomannan | Ag NPs | Antibacterial | Self-adaptive Self-healing Adhesive | [354] |
| Injectable hydrogels | CS | bFGF Ag(crosslinked) | Antibacterial Anti-inflammatory Promotes tissue regeneration | Low cytotoxicity Promotes polarization of M2 macrophages | [355] |
| Injectable hydrogels | CS Bacterial cellulose | -- | Antibacterial | Self-healing Enhanced mechanical properties | [356] |
| Injectable hydrogels | Alginate PVA | CaSO4 | Promotes tissue regeneration | Effective drug delivery High mechanical strength | [357] |
| Mussel-inspired | CS Silk cellulose | Tannic acid (crosslinked) | Haemostasis | Strong wet tissue adhesion High mechanical strength | [241] |
| Mussel-inspired | CS Silk cellulose Dopamine reduced graphene oxide | Dopamine reduced graphene oxide | Antioxidant Promotes tissue regeneration | Strong wet tissue adhesion High mechanical strength Conductivity | [358] |
| Mussel-inspired | CS Gelatin graft-dopamine | Polydopamine-coated carbon nanotubes | Antibacterial Antioxidant Haemostasis Promotes tissue regeneration | Strong wet tissue adhesion High mechanical strength Conductivity Self-healing | [359] |
| Mussel-inspired | Alginate | Dopamine | Antibacterial | Strong wet tissue adhesion High mechanical strength | [360] |
| Mussel-inspired | Alginate nHA/PLGA-Dex | Schiff base | Promotes tissue regeneration Haemostatic | Strong wet tissue adhesion High mechanical strength | [361] |
| MPs Component | Other Main Components | Active Agents | Biological Activities | Other Features | Refs |
|---|---|---|---|---|---|
| CS | Polyvinylidene fluoride Polyhydroxybutyric acid | Gentamicin | Not tested | Double layer drug delivery Efficient drug delivery Strong mechanical properties | [390] |
| CS | PVA Starch | -- | Antibacterial Promotes tissue regeneration | High water vapour transmission rate to provide a moist Well-oxygenated wound healing environment Low cytotoxicity | [391] |
| QCS | Collagen PCL PVA | -- | Haemostatic, antibacterial Anti-inflammatory Promotes tissue regeneration | -- | [392] |
| CS | PCL | Human granulocyte colony-stimulating factor-loaded CS NPs | Anti-inflammatory Promotes tissue regeneration | The stent promotes stem cell adhesion and proliferation, sustained slow release | [393] |
| CS | PCL PVA Polycaprolactone | Melatonin | Anti-inflammatory Promotes tissue regeneration | Three layers of nanofibres Hydrophilic effect | [394] |
| CS | PVA Carbopol Polycaprolactone | Curcumin Mesenchymal stem cells | -- | Promotes tissue regeneration | [395] |
| Alginate | WPU CaCl | -- | Not test | Effective drug delivery High mechanical strength | [396] |
| Alginate CS | Gentamicin | -- | Antibacterial | Effective drug delivery Promotes tissue regeneration | [397] |
| Alginate | PUL | PL | Anti-inflammatory | High mechanical strength | [398] |
| Alginate | TOBC | Zn2+ | Antibacterial | High mechanical strength | [399] |
| Alginate | PVA | Spider silks | Anti-inflammatory | Effective drug delivery Promotes tissue regeneration | [400] |
| Alginate CS | PCL Lumi | Doxycycline, PEO | Not test | Strong wet tissue adhesion High mechanical strength Effective drug delivery | [401] |
| Alginate CS | Glutaraldehyde polylysine | -- | Promotes tissue regeneration | High water vapour transmission rate to provide a moist environment Effective drug delivery | [388] |
| Categories | Structural Components | Functional Components | Bioactivities | Other Features | Refs |
|---|---|---|---|---|---|
| Electrospun membranes | CS PCL | -- | Promotes tissue regeneration | The ECM-like structure facilitates cell adhesion and penetration Promotes compartmentalization and prevents initial cell migration | [404] |
| Electrospun membranes | CS Cellulose Polyethylene oxide | Graphene | Antibacterial | Good water vapour transmission and breathability | [405] |
| Asymmetric membranes | CS PVP Nanocellulose | Stearic acid (coating) | Antibacterial | Unilateral hydrophobic Low cytotoxicity High biocompatibility | [406] |
| Asymmetric membranes | CS Gelatin methacrylate | Polycaprolactone Polylactic acid (dense layer) | Promotes tissue regeneration | Good mechanical properties Provide a moist environment for the wound healing Promotes cell adhesion Electrospun membranes | [407] |
| Asymmetric membranes | CS Aloe vera | Polycaprolactone(dense layer) | Promotes tissue regeneration | Good mechanical properties Promotes cell adhesion Electrospun membranes | [408] |
| Multi-layer membranes | CS Gelatine Poly(N-isopropylacrylamide)-grafted polyurethane | -- | Promotes tissue regeneration | Provide a moist healing environment for the wound healing | [409] |
| Multi-layer membranes | Alginate CS | PMMA | Antibacterial Promotes tissue regeneration | Efficient drug delivery | [410] |
| Multi-layer membranes | Alginate CS | Genipin | Antioxidant | Good mechanical properties High water vapour transmission rate to provide a moist | [411] |
| Multi-layer membranes | Alginate | OBC | Antibacterial | Efficient drug delivery | [412] |
| MPs Composition | Other Main Components | Bioactivities | Other Features | Refs |
|---|---|---|---|---|
| CS Hydroxybutyl CS | -- | Promotes tissue regeneration Antibacterial | Non-cytotoxic Highly absorbent | [422] |
| CS | HA, andrographolide lipid nanocarriers | Promotes tissue regeneration Scar-free | High encapsulation rate Slow release | [423] |
| CS | AgSD NPs | Antibacterial | Low cytotoxicity | [424] |
| CS | HA VEGF-loaded fibrin nanoparticles | Haemostasis Promote tissue regeneration | Proper mechanical properties | [425] |
| CS | GAGs Tranexamic acid | Haemostasis Promote tissue regeneration | Highly synergistic haemostatic | [426] |
| CS | Ag NPs Stearic acid (coating) | Antibacterial Promotes tissue regeneration | The presence of a hydrophobic An anti-adhesive surface allows the inside of the sponge to retain its water-absorbing capacity for a long time | [427] |
| Alginate | AV | Antibacterial | High degree of swelling | [428] |
| Alginate | 1-ethyl-3-dimethyl aminopropyl carbon diimine hydrochloride | Promotes tissue regeneration | Good mechanical properties Considerable water vapour transmittance | [429] |
| Alginate | Graphene oxide | Promotes tissue regeneration | High flexibility and mechanical strength High water absorption | [430] |
| Alginate Fucoidan CS | -- | Haemostasis Antibacterial Anti-inflammatory | Excellent elasticity Good mechanical properties | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. https://doi.org/10.3390/pharmaceutics13101666
Shen S, Chen X, Shen Z, Chen H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics. 2021; 13(10):1666. https://doi.org/10.3390/pharmaceutics13101666
Chicago/Turabian StyleShen, Shenghai, Xiaowen Chen, Zhewen Shen, and Hao Chen. 2021. "Marine Polysaccharides for Wound Dressings Application: An Overview" Pharmaceutics 13, no. 10: 1666. https://doi.org/10.3390/pharmaceutics13101666
APA StyleShen, S., Chen, X., Shen, Z., & Chen, H. (2021). Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics, 13(10), 1666. https://doi.org/10.3390/pharmaceutics13101666