Quantification of Fluid Volume and Distribution in the Paediatric Colon via Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Processing
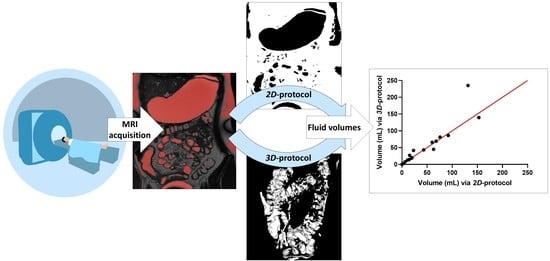
2.2.1. D-Protocol
- Horos [44] to identify and highlight the fluid pockets in the MRI dataset;
- ImageJ to calculate the area of the marked fluid regions.
2.2.2. Three-Dimensional Protocol
- Horos to identify and highlight the fluid pockets in the MRI dataset;
- Blender to remove artefacts and isolate and compute the fluid pockets.
2.3. Statistical Analysis
3. Results
3.1. Participant Demographics
3.2. Colonic Fluid Volume and Number of Pockets
3.3. Robustness of Protocol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Batchelor, H.K.; Fotaki, N.; Klein, S. Paediatric oral biopharmaceutics: Key considerations and current challenges. Adv. Drug Deliv. Rev. 2014, 73, 102–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batchelor, H.K.; Marriott, J.F. Paediatric pharmacokinetics: Key considerations. Br. J. Clin. Pharmacol. 2015, 79, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Murray, K.; Hoad, C.L.; Mudie, D.M.; Wright, J.; Heissam, K.; Abrehart, N.; Pritchard, S.E.; Al Atwah, S.; Gowland, P.A.; Garnett, M.C.; et al. Magnetic Resonance Imaging Quantification of Fasted State Colonic Liquid Pockets in Healthy Humans. Mol. Pharm. 2017, 14, 2629–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadatou-Soulou, E.; Mason, J.; Parsons, C.; Oates, A.; Thyagarajan, M.; Batchelor, H.K. Magnetic Resonance Imaging Quantification of Gastrointestinal Liquid Volumes and Distribution in the Gastrointestinal Tract of Children. Mol. Pharm. 2019, 16, 3896–3903. [Google Scholar] [CrossRef] [PubMed]
- Vertzoni, M.; Augustijns, P.; Grimm, M.; Koziolek, M.; Lemmens, G.; Parrott, N.J.; Pentafragka, C.; Reppas, C.; Rubbens, J.; Abeele, J.V.D.; et al. Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An UNGAP review. Eur. J. Pharm. Sci. 2019, 134, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Mudie, D.M.; Amidon, G.L.; Amidon, G.E. Physiological parameters for oral delivery and in vitro testing. Mol. Pharm. 2010, 7, 1388–1405. [Google Scholar] [CrossRef] [Green Version]
- Stamatopoulos, K.; Karandikar, S.; Goldstein, M.; O’Farrell, C.; Marciani, L.; Sulaiman, S.; Hoad, C.L.; Simmons, M.J.H.; Batchelor, H.K. Dynamic Colon Model (DCM): A Cine-MRI Informed Biorelevant In Vitro Model of the Human Proximal Large Intestine Characterized by Positron Imaging Techniques. Pharmaceutics 2020, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-Targeted Oral Drug Delivery Systems: Design Trends and Approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef]
- Karalis, V.; Magklara, E.; Shah, V.P.; Macheras, P. From Drug Delivery Systems to Drug Release, Dissolution, IVIVC, BCS, BDDCS, Bioequivalence and Biowaivers. Pharm. Res. 2010, 27, 2018–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Corte, T.; Janssens, E.; D’Hondt, A.; Thorrez, K.; Arts, J.; Dejaegher, K.; D’Heygere, F.; Holvoet, A.; Van Besien, B.; Harlet, L.; et al. Beclomethasone dipropionate in microscopic colitis: Results of an exploratory open-label multicentre study (COLCO). United Eur. Gastroenterol. J. 2019, 7, 1183–1188. [Google Scholar] [CrossRef] [Green Version]
- Philip, A.; Philip, B. Colon Targeted Drug Delivery Systems: A Review on Primary and Novel Approaches. Oman Med. J. 2010, 25, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Salladini, C.; Di Marco, G.; Pisciella, F.; Chiarelli, F. Extended-Release Formulations in Epilepsy. J. Child Neurol. 2007, 22, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, G.; Klein, S. Dissolution testing of oral modified-release dosage forms. J. Pharm. Pharmacol. 2012, 64, 944–968. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Parab, S.; Dabholkar, N.; Agrawal, M.; Singhvi, G.; Alexander, A.; Bapat, R.A.; Kesharwani, P. Oral peptide delivery: Challenges and the way ahead. Drug Discov. Today 2021, 26, 931–950. [Google Scholar] [CrossRef] [PubMed]
- Del Curto, M.D.; Maroni, A.; Foppoli, A.; Zema, L.; Gazzaniga, A.; Sangalli, M.E. Preparation and evaluation of an oral delivery system for time-dependent colon release of insulin and selected protease inhibitor and absorption enhancer com-pounds. J. Pharm. Sci. 2009, 98, 4661–4669. [Google Scholar] [CrossRef]
- Chey, W.D.; Sayuk, G.S.; Bartolini, W.; Reasner, D.S.; Fox, S.M.; Bochenek, W.; Boinpally, R.; Shea, E.; Tripp, K.; Borgstein, N. Randomized Trial of 2 Delayed-Release Formulations of Linaclotide in Patients With Irritable Bowel Syndrome With Con-stipation. Am. J. Gastroenterol. 2021, 116, 354–361. [Google Scholar] [CrossRef]
- Wilson, C.G. The transit of dosage forms through the colon. Int. J. Pharm. 2010, 395, 17–25. [Google Scholar] [CrossRef]
- Ye, B.; van Langenberg, D.R. Mesalazine preparations for the treatment of ulcerative colitis: Are all created equal? World J. Gastrointest. Pharmacol. Ther. 2015, 6, 137–144. [Google Scholar] [CrossRef]
- Fotaki, N.; Vertzoni, M. Biorelevant dissolution methods and their applications in in vitro in vivo correlations for oral formulations. Open Drug Deliv. J. 2010, 4, 2–13. [Google Scholar] [CrossRef]
- Löbenberg, R.; Krämer, J.; Shah, V.P.; Amidon, G.L.; Dressman, J.B. Dissolution testing as a prognostic tool for oral drug absorption: Dissolution behavior of glibenclamide. Pharm. Res. 2000, 17, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Jantratid, E.; De Maio, V.; Ronda, E.; Mattavelli, V.; Vertzoni, M.; Dressman, J.B. Application of biorelevant dissolution tests to the prediction of in vivo performance of diclofenac sodium from an oral modified-release pellet dosage form. Eur. J. Pharm. Sci. 2009, 37, 434–441. [Google Scholar] [CrossRef]
- Lemmens, G.; Brouwers, J.; Snoeys, J.; Augustijns, P.; Vanuytsel, T. Insight into the colonic disposition of celecoxib in humans. Eur. J. Pharm. Sci. 2020, 145, 105242. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, G.; Brouwers, J.; Snoeys, J.; Augustijns, P.; Vanuytsel, T. Insight into the Colonic Disposition of Sulindac in Hu-mans. J. Pharm. Sci. 2021, 110, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Fotaki, N.; Aivaliotis, A.; Butler, J.; Dressman, J.; Fischbach, M.; Hempenstall, J.; Klein, S.; Reppas, C. A comparative study of different release apparatus in generating in vitro–in vivo correlations for extended release formulations. Eur. J. Pharm. Biopharm. 2009, 73, 115–120. [Google Scholar] [CrossRef]
- Pasta, S.; Gentile, G.; Raffa, G.M.; Scardulla, F.; Bellavia, D.; Luca, A.; Pilato, M.; Scardulla, C. Three-dimensional parametric modeling of bicuspid aortopathy and comparison with computational flow predictions. Artif. Organs 2017, 41, E92–E102. [Google Scholar] [CrossRef]
- Sulaiman, S.; Marciani, L. MRI of the Colon in the Pharmaceutical Field: The Future before us. Pharmaceutics 2019, 11, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgaka, D.; Butler, J.; Kesisoglou, F.; Reppas, C.; Vertzoni, M. Evaluation of Dissolution in the Lower Intestine and Its Impact on the Absorption Process of High Dose Low Solubility Drugs. Mol. Pharm. 2017, 14, 4181–4191. [Google Scholar] [CrossRef]
- Vertzoni, M.; Diakidou, A.; Chatzilias, M.; Söderlind, E.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Biorelevant Media to Simulate Fluids in the Ascending Colon of Humans and Their Usefulness in Predicting Intracolonic Drug Solubility. Pharm. Res. 2010, 27, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.N.; Zhou, D.; Bui, K.H. Development of physiologically based pharmacokinetic model to evaluate the relative systemic exposure to quetiapine after administration of IR and XR formulations to adults, children and adolescents. Biopharm. Drug Dispos. 2014, 35, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Schiller, C.; Frohlich, C.-P.; Giessmann, T.; Siegmund, W.; Monnikes, H.; Hosten, N.; Weitschies, W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2005, 22, 971–979. [Google Scholar] [CrossRef]
- Pritchard, S.E.; Paul, J.; Major, G.; Marciani, L.; Gowland, P.A.; Spiller, R.C.; Hoad, C.L. Assessment of motion of colonic contents in the human colon using MRI tagging. Neurogastroenterol. Motil. 2017, 29, e13091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsume, Y.; Patel, S.; Fotaki, N.; Bergström, C.; Amidon, G.L.; Brasseur, J.G.; Mudie, D.M.; Sun, D.; Bermejo, M.; Gao, P.; et al. In Vivo Predictive Dissolution and Simulation Workshop Report: Facilitating the Development of Oral Drug Formulation and the Prediction of Oral Bioperformance. AAPS J. 2018, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Baker, J.R.; Fioritto, A.F.; Wang, Y.; Luo, R.; Li, S.; Wen, B.; Bly, M.; Tsume, Y.; Koenigsknecht, M.J.; et al. Measurement of in vivo Gastrointestinal Release and Dissolution of Three Locally Acting Mesalamine Formulations in Regions of the Human Gastrointestinal Tract. Mol. Pharm. 2017, 14, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.R.; Edginton, A.N. Physiologically based pharmacokinetic modeling and simulation in pediatric drug devel-opment. CPT Pharmacomet. Syst. Pharmacol. 2014, 3, e150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinarov, Z.; Abrahamsson, B.; Artursson, P.; Batchelor, H.; Berben, P.; Bernkop-Schnürch, A.; Butler, J.; Ceulemans, J.; Davies, N.; Dupont, D.; et al. Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network. Adv. Drug Deliv. Rev. 2021, 171, 289–331. [Google Scholar] [CrossRef] [PubMed]
- Stillhart, C.; Vučićević, K.; Augustijns, P.; Basit, A.W.; Batchelor, H.; Flanagan, T.R.; Gesquiere, I.; Greupink, R.; Keszthelyi, D.; Koskinen, M.; et al. Impact of gastrointestinal physiology on drug absorption in special populations––An UNGAP review. Eur. J. Pharm. Sci. 2020, 147, 105280. [Google Scholar] [CrossRef]
- Vinarov, Z.; Abdallah, M.; Agundez, J.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.; Grimm, M.; et al. Impact of gastroin-testinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Duan, J.; Kesisoglou, F.; Novakovic, J.; Amidon, G.; Jamei, M.; Lukacova, V.; Eissing, T.; Tsakalozou, E.; Zhao, L.; et al. Mechanistic Oral Absorption Modeling and Simulation for Formulation Development and Bioequivalence Evaluation: Report of an FDA Public Workshop. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 492–495. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, J.-M.; Bouzom, F.; Hugues, C.; Ungell, A.-L. Oral drug absorption in pediatrics: The intestinal wall, its developmental changes and current tools for predictions. Biopharm. Drug Dispos. 2017, 38, 209–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Zheng, Q.-S.; Li, G.-F. Similarities and Differences in Gastrointestinal Physiology Between Neonates and Adults: A Physiologically Based Pharmacokinetic Modeling Perspective. AAPS J. 2014, 16, 1162–1166. [Google Scholar] [CrossRef] [Green Version]
- Mudie, D.M.; Murray, K.; Hoad, C.L.; Pritchard, S.E.; Garnett, M.C.; Amidon, G.L.; Gowland, P.A.; Spiller, R.C.; Amidon, G.E.; Marciani, L. Quantification of Gastrointestinal Liquid Volumes and Distribution Following a 240 mL Dose of Water in the Fasted State. Mol. Pharm. 2014, 11, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, S.E.; Marciani, L.; Garsed, K.C.; Hoad, C.; Thongborisute, W.; Roberts, E.; Gowland, P.; Spiller, R.C. Fasting and postprandial volumes of the undisturbed colon: Normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol. Motil. 2014, 26, 124–130. [Google Scholar] [CrossRef]
- The Horos Project. Horos is a free and open source code software (FOSS) program that is distributed free of charge under the LGPL license at Horosproject.org and sponsored by Nimble Co LLC d/b/a Purview in Annapolis, MD, USA. Available online: https://horosproject.org/faqs/ (accessed on 5 September 2021).
- Hoad, C.L.; Marciani, L.; Foley, S.; Totman, J.J.; Wright, J.; Bush, D.; Cox, E.; Campbell, E.; Spiller, R.C.; Gowland, P.A. Non-invasive quantification of small bowel water content by MRI: A validation study. Phys. Med. Biol. 2007, 52, 6909–6922. [Google Scholar] [CrossRef]
- Laqua, R. Global Tresholding v1.0 OsiriX Plugin [Software]. Available online: https://osirixpluginbasics.wordpress.com/2012/10/30/plugin-global-thresholding/ (accessed on 26 March 2020).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.; Koziolek, M.; Saleh, M.; Schneider, F.; Garbacz, G.; Kühn, J.-P.; Weitschies, W. Gastric Emptying and Small Bowel Water Content after Administration of Grapefruit Juice Compared to Water and Isocaloric Solutions of Glucose and Fructose: A Four-Way Crossover MRI Pilot Study in Healthy Subjects. Mol. Pharm. 2018, 15, 548–559. [Google Scholar] [CrossRef]
- Sharif, H.; Devadason, D.; Abrehart, N.; Stevenson, R.; Marciani, L. Imaging Measurement of Whole Gut Transit Time in Paediatric and Adult Functional Gastrointestinal Disorders: A Systematic Review and Narrative Synthesis. Diagnostics 2019, 9, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IBM. Released 2020. In IBM SPSS Statistics for Windows, Version 27.0; IBM: Armonk, NY, USA, 2020. [Google Scholar]
- Guimarães, M.; Statelova, M.; Holm, R.; Reppas, C.; Symilllides, M.; Vertzoni, M.; Fotaki, N. Biopharmaceutical considera-tions in paediatrics with a view to the evaluation of orally administered drug products—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 603–642. [Google Scholar] [CrossRef] [Green Version]
- Committee for Medicinal Products for Human Use (CHMP). Reflection Paper: Formulations of Choice for the Paediatric Population; EMEA: London, UK, 2006; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-formulations-choice-paediatric-population_en.pdf (accessed on 5 September 2021).
- Sharif, H.; Hoad, C.; Abrehart, N.; Gowland, P.; Spiller, R.; Kirkham, S.; Loganathan, S.; Papadopoulos, M.; Benninga, M.; Devadason, D.; et al. Colonic Volume Changes in Paediatric Constipation Compared to Normal Values Measured Using MRI. Diagnostics 2021, 11, 974. [Google Scholar] [CrossRef]
- Nilsson, M.; Sandberg, T.H.; Poulsen, J.L.; Gram, M.; Frøkjaer, J.B.; Østergaard, L.R.; Krogh, K.; Brock, C.; Drewes, A. Quantification and variability in colonic volume with a novel magnetic resonance imaging method. Neurogastroenterol. Motil. 2015, 27, 1755–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirjalili, S.A.; Tarr, G.; Stringer, M.D. The length of the large intestine in children determined by computed tomography scan. Clin. Anat. 2017, 30, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J.; Christensen, L.A.; Ingeman-Nielsen, M.; Jacobsen, B.A.; Abildgaard, K.; Rasmussen, H.H.; Rasmussen, S.N. Measurement of Gastrointestinal pH and Regional Transit Times in Normal Children. J. Pediatr. Gastroenterol. Nutr. 1990, 11, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Broesder, A.; Woerdenbag, H.J.; Prins, G.H.; Nguyen, D.N.; Frijlink, H.W.; Hinrichs, W.L. pH-dependent ileocolonic drug delivery, part I: In vitro and clinical evaluation of novel systems. Drug Discov. Today 2020, 25, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A.; Lam, C.; Rehman, S.; Marciani, L.; Costigan, C.; Hoad, C.L.; Lingaya, M.R.; Banwait, R.; Bawden, S.J.; Gowland, P.A.; et al. Corticotropin-releasing factor increases ascending colon volume after a fructose test meal in healthy humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 1318–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson-Smith, V.; Menys, A.; Bradley, C.; Corsetti, M.; Marciani, L.; Atkinson, D.; Coupland, C.; Taylor, S.A.; Gowland, P.; Spiller, R.; et al. The MRI colonic function test: Reproducibility of the Macrogol stimulus challenge. Neurogastroenterol. Motil. 2020, 32, e13942. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Lee, S.; Kim, J.H. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Elec-tronic Medical Records. J. Korean Med. Sci. 2018, 33, e213. [Google Scholar] [CrossRef] [PubMed]
- Neal-Kluever, A.; Fisher, J.; Grylack, L.; Kakiuchi-Kiyota, S.; Halpern, W. Physiology of the Neonatal Gastrointestinal System Relevant to the Disposition of Orally Administered Medications. Drug Metab. Dispos. 2018, 47, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.M.D.M.; Alvarez, I.G.; Revert, A.C.; Álvarez, M.G.; Ruiz, A.N.; Amidon, G.L.; Bermejo, M.; Sanz, M.B. Biopharmaceutical optimization in neglected diseases for paediatric patients by applying the provisional paediatric biopharmaceutical classification system. Br. J. Clin. Pharmacol. 2018, 84, 2231–2241. [Google Scholar] [CrossRef]
- DelMoral-Sanchez, J.-M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Navarro, A.; Bermejo, M. Classification of WHO Es-sential Oral Medicines for Children Applying a Provisional Pediatric Biopharmaceutics Classification System. Pharmaceutics 2019, 11, 567. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, S.V.; Rodriguez, W.; Khan, M.; Polli, J.E. Considerations for a Pediatric Biopharmaceutics Classification System (BCS): Application to Five Drugs. AAPS PharmSciTech 2014, 15, 601–611. [Google Scholar] [CrossRef]
- Maharaj, A.R.; Edginton, A.N. Examining Small Intestinal Transit Time as a Function of Age: Is There Evidence to Support Age-Dependent Differences among Children? Drug Metab. Dispos. 2016, 44, 1080–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Placidi, E.; Marciani, L.; Hoad, C.; Napolitano, A.; Garsed, K.C.; Pritchard, S.E.; Cox, E.; Costigan, C.; Spiller, R.; Gowland, P.A. The effects of loperamide, or loperamide plus simethicone, on the distribution of gut water as assessed by MRI in a mannitol model of secretory diarrhoea. Aliment. Pharmacol. Ther. 2012, 36, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.J.; Vajjah, P.; Abduljalil, K.; Jamei, M.; Rostami-Hodjegan, A.; Tucker, G.T.; Johnson, T.N. Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm. Drug Dispos. 2015, 36, 245–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuhelwa, A.Y.; Foster, D.; Upton, R. A Quantitative Review and Meta-models of the Variability and Factors Affecting Oral Drug Absorption—Part II: Gastrointestinal Transit Time. AAPS J. 2016, 18, 1322–1333. [Google Scholar] [CrossRef]
- Lemmens, G.; Van Camp, A.; Kourula, S.; Vanuytsel, T.; Augustijns, P. Drug Disposition in the Lower Gastrointestinal Tract: Targeting and Monitoring. Pharmaceutics 2021, 13, 161. [Google Scholar] [CrossRef]
- Huang, W.; Lee, S.L.; Yu, L.X. Mechanistic Approaches to Predicting Oral Drug Absorption. AAPS J. 2009, 11, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Pawar, G.; Wu, F.; Zhao, L.; Fang, L.; Burckart, G.J.; Feng, K.; Mousa, Y.M.; Naumann, F.; Batchelor, H.K. Development of a Pediatric Relative Bioavailability/Bioequivalence Database and Identification of Putative Risk Factors Associated with Evaluation of Pediatric Oral Products. AAPS J. 2021, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- DelMoral-Sanchez, J.-M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Navarro-Ruiz, A.; Bermejo, M. Availability of Au-thorizations from EMA and FDA for Age-Appropriate Medicines Contained in the WHO Essential Medicines List for Children 2019. Pharmaceutics 2020, 12, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Study | Feed Status (Intake of Food/Fluid) | Time of Ingestion before MRI Acquisition | Number of Participants | Median (Min-Max) (mL) | Mean (±SD) (mL) |
|---|---|---|---|---|---|
| Schiller 2005 [31] | Fasted | - | 12 | 8 (1–44) | 13 ± 12 |
| Fed (standardised meal) | 1 h | 18 (2–97) | 11 ± 26 | ||
| Pritchard 2017 [32] | Fasted | - | 11 | 2 (0–7) | - |
| Fed (500 mL Moviprep) | 1 h | 140 (104–347) | - | ||
| Murray 2017 [3] | Fasted | - | 12 | N/A (0–11) | 2 ± 1 |
| Fed (240 mL water) | 30 min | N/A (0–49) | 7 ± 4 |
| Site | UHCW | UHCW | BCH | BCH |
|---|---|---|---|---|
| Participants | Fluid-fed | Fluid-fed | Fasted | Fasted |
| 1.5 T MR Imaging Unit: series and manufacturer | Optima MR450w, GE Healthcare, Chicago, IL, USA | Aera, Siemens Healthcare, Erlangen, Germany | Siemens MAGNETOM Avanto 1.5 T MRI System, Siemens Healthcare, Erlangen, Germany | Aera, Siemens Healthcare, Erlangen, Germany |
| MRI coil | 48-channel body coil | body coil | 16-element parallel imaging receiver coil | 16-element parallel imaging receiver coil |
| MRI protocol | Coronal balanced steady-state gradient echo sequence (fast-imaging employing steady-state acquisition, FIESTA) | Coronal balanced steady-state gradient echo sequence (true FISP) | Coronal T2 SPACE sequence | Coronal T2 SPACE sequence |
| Median slice thickness (range) | 4.0 mm (2.998 mm–6 mm) | 6.0 mm (2.998 mm–6 mm) | 0.9 mm (0.09 mm–0.55 mm) | 0.9 mm (0.09 mm–0.55 mm) |
| Echo train length | 1 | 1 | 1 | 1 |
| Median intersection gap | 5.0 mm | 3.0 mm | None | None |
| Matrix | 0.35 × 0.35 mm | 1.0 × 1.0 mm | 0.8 × 0.8 mm | 0.8 × 0.8 mm |
| Field of view | 420 cm2 | 420 cm2 | 250 cm2 | 400 cm2 |
| TR/TE | 5.7/1.9 ms | 652.8/2.1 ms | 1700/98 ms | 2000/241 ms |
| Age Range | Number of Datasets Available | |
|---|---|---|
| Fasted Children | Fluid-Fed Children | |
| <2 years (neonate/infant/toddler) | 9 | 0 |
| 2–5 years (pre-school children) | 12 | 0 |
| 6–11 years (school-age children) | 6 | 2 |
| 12–16 years (adolescents) | 1 | 16 |
| Colon Segment | Total | Ascending | Transverse | Descending |
|---|---|---|---|---|
| Mean ± SD (mL) | 22.48 ± 41.30 | 16.44 ± 27.62 | 3.78 ± 11.49 | 2.27 ± 7.09 |
| Median (mL) | 0.80 | 0.63 | 0.004 | 0.003 |
| Interquartile range (mL) | 19.69 | 18.52 | 0.65 | 0.12 |
| Range (min-max) (mL) | 0–167.47 | 0–102.30 | 0–56.87 | 0–37.94 |
| Mean ± SD number of fluid pockets | 15.5 ± 17.5 | 14.5 ± 16.4 | 1.0 ± 2.5 | 0.05 ± 0.2 |
| Median number of fluid pockets | 12 | 10 | 0 | 0 |
| All Pockets | Pockets Bigger than 1 mL | |||
|---|---|---|---|---|
| Number per Participant | Volume (mL) | Number per Participant | Volume (mL) | |
| Mean ± SD | 15.5 ± 17.5 | 1.52 ± 12.09 | 3.6 ± 2.9 | 14.60 ± 36.29 |
| Median | 12 | 0.04 | 3 | 3.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goelen, J.; Alexander, B.; Wijesinghe, H.E.; Evans, E.; Pawar, G.; Horniblow, R.D.; Batchelor, H.K. Quantification of Fluid Volume and Distribution in the Paediatric Colon via Magnetic Resonance Imaging. Pharmaceutics 2021, 13, 1729. https://doi.org/10.3390/pharmaceutics13101729
Goelen J, Alexander B, Wijesinghe HE, Evans E, Pawar G, Horniblow RD, Batchelor HK. Quantification of Fluid Volume and Distribution in the Paediatric Colon via Magnetic Resonance Imaging. Pharmaceutics. 2021; 13(10):1729. https://doi.org/10.3390/pharmaceutics13101729
Chicago/Turabian StyleGoelen, Jan, Benoni Alexander, Haren Eranga Wijesinghe, Emily Evans, Gopal Pawar, Richard D. Horniblow, and Hannah K. Batchelor. 2021. "Quantification of Fluid Volume and Distribution in the Paediatric Colon via Magnetic Resonance Imaging" Pharmaceutics 13, no. 10: 1729. https://doi.org/10.3390/pharmaceutics13101729
APA StyleGoelen, J., Alexander, B., Wijesinghe, H. E., Evans, E., Pawar, G., Horniblow, R. D., & Batchelor, H. K. (2021). Quantification of Fluid Volume and Distribution in the Paediatric Colon via Magnetic Resonance Imaging. Pharmaceutics, 13(10), 1729. https://doi.org/10.3390/pharmaceutics13101729