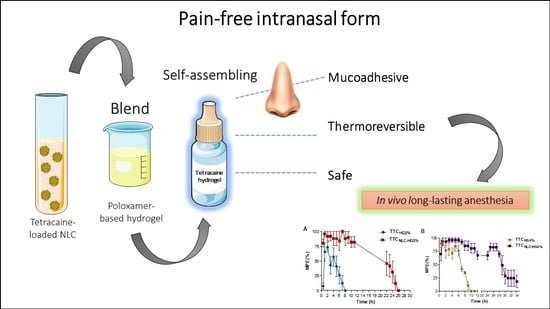
Mucoadhesive, Thermoreversible Hydrogel, Containing Tetracaine-Loaded Nanostructured Lipid Carriers for Topical, Intranasal Needle-Free Anesthesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Procedures
2.3. Preparation of the Formulation
2.4. Characterization of the Formulations
2.4.1. Rheological Analysis
2.4.2. Texture Profile Analysis (TPA)
2.4.3. In Vitro Mucoadhesive and Permeation Studies
Preparation of Porcine Nasal Mucosa
In Vitro Evaluation of Mucoadhesive Strength
In Vitro Permeation Studies
2.4.4. Cell Culture and In Vitro Cytotoxicity Assays
2.4.5. In Vivo Antinociceptive Assays
2.4.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of TTCNLC as the Lipidic Component of the Hybrid Hydrogel
3.2. Preparation of Hybrid Hydrogels
3.3. Rheological Analyses
3.4. Texture Profile Analysis (TPA)
3.5. In Vitro Evaluation of Mucoadhesive Strength
3.6. In Vitro Permeation Studies
3.7. Cell Viability Assays
3.8. Antinociceptive Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capetillo, J.; Drum, M.; Reader, A.; Fowler, S.; Nusstein, J.; Beck, M. Anesthetic Efficacy of Intranasal 3% Tetracaine plus 0.05% Oxymetazoline (Kovanaze) in Maxillary Teeth. J. Endod. 2019, 45, 257–262. [Google Scholar] [CrossRef]
- Kumar, A.; Raj, J.D. Knowledge, attitude, and practices regarding the use of nasal spray anesthesia by dental practitioners. Drug Invent. Today 2018, 10, 2023–2028. [Google Scholar]
- Hersh, E.V.; Saraghi, M.; Moore, P.A. Intranasal tetracaine and oxymetazoline: A newly approved drug formulation that provides maxillary dental anesthesia without needles. Curr. Med. Res. Opin. 2016, 32, 1919–1925. [Google Scholar] [CrossRef]
- Ciancio, S.G.; Hutcheson, M.C.; Ayoub, F.; Pantera, E.A., Jr.; Pantera, C.T.; Garlapo, D.A.; Sobieraj, B.D.; Almubarak, S.A. Safety and efficacy of a novel nasal spray for maxillary dental anesthesia. J. Dent. Res. 2013, 92 (Suppl. S7), 43S–48S. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Barbi, M.S.; Sarmento, V.H.; Chiavacci, L.A.; Netto, F.M.; Gremiao, M.P. Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. J. Pharm. Pharmacol. 2010, 62, 430–439. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Campos, M.L.; Peccinini, R.G.; Gremião, M.P.D. Nasal administration of liquid crystal precursor mucoadhesive vehicle as an alternative antiretroviral therapy. Eur. J. Pharm. Biopharm. 2013, 84, 219–227. [Google Scholar] [CrossRef]
- Matarazzo, A.P.; Elisei, L.M.S.; Carvalho, F.C.; Bonfílio, R.; Ruela, A.L.M.; Galdino, G.; Pereira, G.R. Mucoadhesive nanostructured lipid carriers as a cannabidiol nasal delivery system for the treatment of neuropathic pain. Eur. J. Pharm. Sci. 2021, 159, 105698. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, M.; Zhu, X.; Shan, W.; Huang, Y. Modification Strategies of Lipid-Based Nanocarriers for Mucosal Drug Delivery. Curr. Pharm. Des. 2015, 21, 5198–5211. [Google Scholar] [CrossRef]
- Gadhave, D.; Choudhury, H.; Kokare, C. Neutropenia and leukopenia protective intranasal olanzapine-loaded lipid-based nanocarriers engineered for brain delivery. Appl. Nanosci. 2019, 9, 151–168. [Google Scholar] [CrossRef]
- Ribeiro, L.N.M.; Franz-Montan, M.; Breitkreitz, M.C.; Alcântara, A.C.; Castro, S.R.; Guilherme, V.A.; Barbosa, R.M.; de Paula, E. Nanostructured lipid carriers as robust systems for topical lidocaine-prilocaine release in dentistry. Eur. J. Pharm. Sci. 2016, 93, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.S.; Ribeiro, L.N.M.; Breitkreitz, M.C.; Guilherme, V.A.; da Silva, G.H.R.; Mitsutake, H.; Alcântara, A.C.S.; Yokaichiya, F.; Franco, M.K.K.D.; Clemens, D.; et al. A pre-formulation study of tetracaine loaded in optimized nanostructured lipid carriers. Sci. Rep. 2021, in press. [Google Scholar]
- Russo, J.; Fiegel, J.; Brogden, N.K. Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia. Pharmaceutics 2020, 12, 1214. [Google Scholar] [CrossRef]
- Salmazi, R.; Calixto, G.; Bernegossi, J.; dos Santos Ramos, M.A.; Bauab, T.M.; Chorilli, M. A curcumin-loaded liquid crystal precursor mucoadhesive system for the treatment of vaginal candidiasis. Int. J. Nanomed. 2015, 10, 4815. [Google Scholar]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal applications of poloxamer 407-based hydrogels: An overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelke, S.; Pathan, I.; Shinde, G.; Agrawal, G.; Damale, M.; Chouthe, R.; Panzade, P.; Kulkarni, D. Poloxamer-Based In Situ Nasal Gel of Naratriptan Hydrochloride Deformable Vesicles for Brain Targeting. BioNanoScience 2020, 10, 633–648. [Google Scholar] [CrossRef]
- Xia, Y.; Li, L.; Huang, X.; Wang, Z.; Zhang, H.; Gao, J.; Du, Y.; Chen, W.; Zheng, A. Performance and toxicity of different absorption enhancers used in the preparation of Poloxamer thermosensitive in situ gels for ketamine nasal administration. Drug Dev. Ind. Pharm. 2020, 46, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; de Paula, E.; Rossi, D.A.; Martins, F.A.; de Melo, R.T.; Monteiro, G.P.; Breitkreitz, M.C.; Goulart, L.R.; Fonseca, B.B. Nanocarriers from natural lipids with in vitro activity against Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2021, 10, 571040. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, C.M.; de Oliveira, C.C.; Gambero, A.; Rocha, T.; Cereda, C.M.S.; de Araújo, D.R.; Tofoli, G.R. Evaluation of budesonide–hydroxypropyl-β-cyclodextrin inclusion complex in thermoreversible gels for ulcerative colitis. Dig. Dis. Sci. 2020, 65, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.C.M.; Akkari, A.C.S.; Ferreira, I.R.S.; Maruyama, C.R.; Pascoli, M.; Guilherme, V.A.; de Paula, E.; Fraceto, L.F.; de Lima, R.; da Silva Melo, P. Poloxamer-based binary hydrogels for delivering tramadol hydrochloride: Sol-gel transition studies, dissolution-release kinetics, in vitro toxicity, and pharmacological evaluation. Int. J. Nanomed. 2015, 10, 2391. [Google Scholar]
- Vigato, A.A.; Querobino, S.M.; de Faria, N.C.; de Freitas, A.C.P.; Leonardi, G.R.; de Paula, E.; Cereda, C.M.S.; Tofoli, G.R.; de Araujo, D.R. Synthesis and characterization of nanostructured lipid-poloxamer organogels for enhanced skin local anesthesia. Eur. J. Pharm. Sci. 2019, 128, 270–278. [Google Scholar] [CrossRef]
- Lindemann, J.; Leiacker, R.; Rettinger, G.; Keck, T. Nasal mucosal temperature during respiration. Clin. Otolaryngol. Allied Sci. 2002, 27, 135–139. [Google Scholar] [CrossRef]
- Muniz, B.V.; Baratelli, D.; Di Carla, S.; Serpe, L.; da Silva, C.B.; Guilherme, V.A.; Ribeiro, L.N.M.; Cereda, C.M.S.; de Paula, E.; Volpato, M.C. Hybrid hydrogel composed of polymeric nanocapsules co-loading lidocaine and prilocaine for topical intraoral anesthesia. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Osth, K.; Paulsson, M.; Bjork, E.; Edsman, K. Evaluation of drug release from gels on pig nasal mucosa in a horizontal Ussing chamber. J. Control. Release 2002, 83, 377–388. [Google Scholar] [CrossRef]
- Antunes Viegas, D.; Rodrigues, M.; Francisco, J.; Falcao, A.; Alves, G.; Santos, A.O. Development and application of an ex vivo fosphenytoin nasal bioconversion/permeability evaluation method. Eur. J. Pharm. Sci. 2016, 89, 61–72. [Google Scholar] [CrossRef]
- Pérez-González, G.L.; Villarreal-Gómez, L.J.; Serrano-Medina, A.; Torres-Martínez, E.J. Mucoadhesive electrospun nanofibers for drug delivery systems: Applications of polymers and the parameters’ roles. Int. J. Nanomed. 2019, 15, 5271–5285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, A.; Stolnik, S.; Illum, L. Nose-to-brain delivery: Investigation of the transport of nanoparticles with different surface characteristics and sizes in excised porcine olfactory epithelium. Mol. Pharm. 2015, 12, 2755–2766. [Google Scholar] [CrossRef]
- European Food Safety Authority. Guidance on Dermal Absorption. EFSA J. 2012, 10, 2665. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Jara, J.A.; Rojas, D.; Castro-Castillo, V.; Fuentes-Retamal, S.; Sandoval-Acuña, C.; Parra, E.; Pavani, M.; Maya, J.D.; Ferreira, J.; Catalán, M. Novel benzoate-lipophilic cations selectively induce cell death in human colorectal cancer cell lines. Toxicol. Vitr. 2020, 65, 104814. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Rutter, N. Local anaesthetic effect of topical amethocaine gel in neonates: Randomised controlled trial. Arch. Dis. Child.-Fetal Neonatal Ed. 2000, 82, F42–F45. [Google Scholar] [CrossRef]
- Jain, A.; Rutter, N.; Ratnayaka, M. Topical amethocaine gel for pain relief of heel prick blood sampling: A randomised double blind controlled trial. Arch. Dis. Child.-Fetal Neonatal Ed. 2001, 84, F56–F59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, J.; Alves, G.; Oliveira, P.; Fortuna, A.; Falcão, A. Intranasal delivery of ciprofloxacin to rats: A topical approach using a thermoreversible in situ gel. Eur. J. Pharm. Sci. 2017, 97, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Mariano, K.C.F.; do Nascimento, M.H.M.; Querobino, S.M.; Campos, E.V.R.; de Oliveira, J.L.; Yokaichiya, F.; Franco, M.K.; Alberto-Silva, C.; de Paula, E.; Lombello, C.B. Influence of chitosan-tripolyphosphate nanoparticles on thermosensitive polymeric hydrogels: Structural organization, drug release mechanisms and cytotoxicity. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 592–603. [Google Scholar] [CrossRef]
- Grillo, R.; Dias, F.V.; Querobino, S.M.; Alberto-Silva, C.; Fraceto, L.F.; de Paula, E.; de Araujo, D.R. Influence of hybrid polymeric nanoparticle/thermosensitive hydrogels systems on formulation tracking and in vitro artificial membrane permeation: A promising system for skin drug-delivery. Colloids Surf. B Biointerfaces 2019, 174, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Soni, G.; Yadav, K.S. High encapsulation efficiency of poloxamer-based injectable thermoresponsive hydrogels of etoposide. Pharm. Dev. Technol. 2014, 19, 651–661. [Google Scholar] [CrossRef]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D.; Shengule, S. Formulation and evaluation of thermoreversible mucoadhesive in-situ gel for intranasal delivery of naratriptan hydrochloride. J. Drug Deliv. Sci. Technol. 2015, 29, 238–244. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Victorelli, F.D.; Dovigo, L.N.; Chorilli, M. Polyethyleneimine and Chitosan Polymer-Based Mucoadhesive Liquid Crystalline Systems Intended for Buccal Drug Delivery. AAPS PharmSciTech 2018, 19, 820–836. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: Mechanical, rheological and sol-gel transition analysis. Carbohydr. Polym. 2020, 240, 116268. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Woolfson, A.D.; Djokic, J. Texture profile analysis of bioadhesive polymeric semisolids: Mechanical characterization and investigation of interactions between formulation components. J. Appl. Polym. Sci. 1996, 61, 2229–2234. [Google Scholar] [CrossRef]
- Ferreira, S.B.D.S.; Da Silva, J.B.; Borghi-Pangoni, F.B.; Junqueira, M.V.; Bruschi, M.L. Linear correlation between rheological, mechanical and mucoadhesive properties of polycarbophil polymer blends for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 68, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Karavana, S.Y. A new in-situ gel formulation of itraconazole for vaginal administration. Pharmacol. Pharm. 2012, 3, 417. [Google Scholar] [CrossRef] [Green Version]
- de Francisco, L.M.B.; Pinto, D.; Rosseto, H.C.; de Toledo, L.d.A.S.; Dos Santos, R.S.; Costa, P.J.C.d.; Oliveira, M.B.P.; Sarmento, B.; Rodrigues, F.; Bruschi, M.L. Design and characterization of an organogel system containing ascorbic acid microparticles produced with propolis by-product. Pharm. Dev. Technol. 2020, 25, 54–67. [Google Scholar] [CrossRef]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Guneri, T. Rheological and mechanical properties of poloxamer mixtures as a mucoadhesive gel base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Bruschi, M.L.; Evangelista, R.C.; Gremião, M.P.D. Mucoadhesive drug delivery systems. Braz. J. Pharm. Sci. 2010, 46, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Sudheer, P. Mucoadhesive Polymers: A Review. J. Pharm. Res. 2018, 17, 47–55. [Google Scholar]
- Ribeiro, L.N.M.; Alcantara, A.C.; Franz-Montan, M.; Couto, V.M.; Nista, S.V.; de Paula, E. Nanostructured organic-organic bio-hybrid delivery systems. In Biomedical Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 341–374. [Google Scholar]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.D.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Bassi da Silva, J.; Ferreira, S.B.d.S.; de Freitas, O.; Bruschi, M.L. A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Dev. Ind. Pharm. 2017, 43, 1053–1070. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Ferreira, S.B.D.S.; Da Silva, J.B.; Junqueira, M.V.; Borghi-Pangoni, F.B.; Gomes, R.G.; Bruschi, M.L. The importance of the relationship between mechanical analyses and rheometry of mucoadhesive thermoresponsive polymeric materials for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 74, 142–153. [Google Scholar] [CrossRef]
- Puglia, C.; Blasi, P.; Rizza, L.; Schoubben, A.; Bonina, F.; Rossi, C.; Ricci, M. Lipid nanoparticles for prolonged topical delivery: An in vitro and in vivo investigation. Int. J. Pharm. 2008, 357, 295–304. [Google Scholar] [CrossRef]
- Puglia, C.; Sarpietro, M.G.; Bonina, F.; Castelli, F.; Zammataro, M.; Chiechio, S. Development, characterization, and in vitro and in vivo evaluation of benzocaine-and lidocaine-loaded nanostructrured lipid carriers. J. Pharm. Sci. 2011, 100, 1892–1899. [Google Scholar] [CrossRef]
- Mena, M.Á.; Perucho, J.; Rubio, I.; de Yébenes, J.G. Studies in animal models of the effects of anesthetics on behavior, biochemistry, and neuronal cell death. J. Alzheimer’s Dis. 2010, 22, S43–S48. [Google Scholar] [CrossRef] [PubMed]
- Perez-Castro, R.; Patel, S.; Garavito-Aguilar, Z.V.; Rosenberg, A.; Recio-Pinto, E.; Zhang, J.; Blanck, T.J.; Xu, F. Cytotoxicity of local anesthetics in human neuronal cells. Anesth. Analg. 2009, 108, 997–1007. [Google Scholar] [CrossRef]
- Albalawi, F.; Lim, J.C.; DiRenzo, K.V.; Hersh, E.V.; Mitchell, C.H. Effects of lidocaine and articaine on neuronal survival and recovery. Anesth. Prog. 2008, 65, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Zhang, Q.; Li, L.; Lai, L.; Zheng, T.; Su, J.; Yang, N.; Li, Y. Cytotoxicity study on SH-SY5Y cells cultured at high glucose levels and treated with bupivacaine. Mol. Med. Rep. 2014, 9, 515–520. [Google Scholar] [CrossRef]
- Wang, S.; Xia, B.; Qiao, Z.; Duan, L.; Wang, G.; Meng, W.; Liu, Z.; Wang, Y.; Zhang, M. Tetramethylpyrazine attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells through regulating apoptosis, autophagy and oxidative damage. Drug Des. Dev. Ther. 2019, 13, 1187. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.N.M.; Franz-Montan, M.; Alcântara, A.C.; Breitkreitz, M.C.; Castro, S.R.; Guilherme, V.A.; Muniz, B.V.; da Silva, G.H.R.; de Paula, E. Hybrid nanofilms as topical anesthetics for pain-free procedures in dentistry. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Bezamat, J.M.; Yokaichiya, F.; Franco, M.K.K.D.; Castro, S.R.; de Paula, E.; Cabeca, L.F. Complexation of the local anesthetic pramoxine with hydroxypropyl-beta-cyclodextrin can improve its bioavailability. J. Drug Deliv. Sci. Technol. 2020, 55, 101475. [Google Scholar] [CrossRef]
- Rojanasakul, Y.; Wang, L.-Y.; Bhat, M.; Glover, D.D.; Malanga, C.J.; Ma, J.K. The transport barrier of epithelia: A comparative study on membrane permeability and charge selectivity in the rabbit. Pharm. Res. 1992, 9, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, V.A.; Ribeiro, L.N.M.; Tofoli, G.R.; Franz-Montan, M.; de Paula, E.; de Jesus, M.B. Current challenges and future of lipid nanoparticles formulations for topical drug application to oral mucosa, skin, and eye. Curr. Pharm. Des. 2017, 23, 6659–6675. [Google Scholar] [CrossRef]
- Nair, M.K.; Chetty, D.J.; Chien, Y.W.; Ho, H. Biomembrane permeation of nicotine: Mechanistic studies with porcine mucosae and skin. J. Pharm. Sci. 1997, 86, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Franz-Montan, M.; Baroni, D.; Brunetto, G.; Sobral, V.R.V.; da Silva, C.M.G.; Venâncio, P.; Zago, P.W.; Cereda, C.M.S.; Volpato, M.C.; de Araújo, D.R. Liposomal lidocaine gel for topical use at the oral mucosa: Characterization, in vitro assays and in vivo anesthetic efficacy in humans. J. Liposome Res. 2015, 25, 11–19. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Cereda, C.M.S.; Gaspari, A.; da Silva, C.M.G.; de Araujo, D.R.; Padula, C.; Santi, P.; Narvaes, E.; Novaes, P.D.; Groppo, F.C. Liposomal-benzocaine gel formulation: Correlation between in vitro assays and in vivo topical anesthesia in volunteers. J. Liposome Res. 2013, 23, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.C.; Kohane, D.S.; Park, Y.J.; Bartlett, R.H.; Langer, R.; Yang, V.C. Injectable microparticle-gel system for prolonged and localized lidocaine release. II. In vivo anesthetic effects. J. Biomed. Mater. Res. Part A 2004, 70, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.N.M.; Rodrigues da Silva, G.H.; Couto, V.M.; Castro, S.R.; Breitkreitz, M.C.; Martinez, C.S.; Igartua, D.E.; Prieto, M.J.; de Paula, E. Functional Hybrid Nanoemulsions for Sumatriptan Intranasal Delivery. Front. Chem. 2020, 8, 589503. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, D.R.; Padula, C.; Cereda, C.M.; Tofoli, G.R.; Brito, R.B., Jr.; de Paula, E.; Nicoli, S.; Santi, P. Bioadhesive films containing benzocaine: Correlation between in vitro permeation and in vivo local anesthetic effect. Pharm. Res. 2010, 27, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Mei, N.; Feng, L.; Jiang, X.G. Hydrophilic nasal gel of lidocaine hydrochloride. 1st Communication: Preparation, formulation optimization and in vitro release study. Arzneimittelforschung 2009, 59, 543–549. [Google Scholar] [PubMed]
- Mathison, S.; Nagilla, R.; Kompella, U.B. Nasal route for direct delivery of solutes to the central nervous system: Fact or fiction? J. Drug Target. 1998, 5, 415–441. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef] [PubMed]





| Formulation | Size (nm) | PDI | ZP (mV) | %EE |
|---|---|---|---|---|
| TTCNLC | 222.2 ± 2.6 | 0.154 ± 0.020 | −30.1 ± 0.3 | 63.7 ± 4.2 |
| Formulation | Cohesiveness (Dimensionless) | Adhesiveness (N) | Elasticity (Dimensionless) |
|---|---|---|---|
| TTCHG4% | 0.920 ± 0.062 | 0.534 ± 0.154 * | 0.987 ± 0.009 |
| TTCNLC-HG4% | 0.916 ± 0.016 | 1.062 ± 0.056 | 0.989 ± 0.006 |
| Formulation | Mucoadhesion Parameters | |
|---|---|---|
| Detachment Force (N) | Mucoadhesion Work (N·mm) | |
| TTCNLC-HG4% | 0.108 ± 0.045 | 0.051 ± 0.007 |
| TTCHG4% | 0.128 ± 0.029 | 0.071 ± 0.015 * |
| LA | Formulation | Jss (µg·cm−2·h−1) | Lag Time (h) | R2 |
|---|---|---|---|---|
| TTC | TTCNLC-HG4% | 150.23 ± 9.76 | 0.463 ± 0.120 | 0.998 ± 0.00135 |
| TTCHG4% | 176.42 ± 29.49 | 0.370 ± 0.154 | 0.995 ± 0.0073 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calixto, G.M.F.; Muniz, B.V.; Castro, S.R.; de Araujo, J.S.M.; de Souza Amorim, K.; Ribeiro, L.N.M.; Ferreira, L.E.N.; de Araújo, D.R.; de Paula, E.; Franz-Montan, M. Mucoadhesive, Thermoreversible Hydrogel, Containing Tetracaine-Loaded Nanostructured Lipid Carriers for Topical, Intranasal Needle-Free Anesthesia. Pharmaceutics 2021, 13, 1760. https://doi.org/10.3390/pharmaceutics13111760
Calixto GMF, Muniz BV, Castro SR, de Araujo JSM, de Souza Amorim K, Ribeiro LNM, Ferreira LEN, de Araújo DR, de Paula E, Franz-Montan M. Mucoadhesive, Thermoreversible Hydrogel, Containing Tetracaine-Loaded Nanostructured Lipid Carriers for Topical, Intranasal Needle-Free Anesthesia. Pharmaceutics. 2021; 13(11):1760. https://doi.org/10.3390/pharmaceutics13111760
Chicago/Turabian StyleCalixto, Giovana Maria Fioramonti, Bruno Vilela Muniz, Simone R. Castro, Jaiza Samara Macena de Araujo, Klinger de Souza Amorim, Lígia N. M. Ribeiro, Luiz Eduardo Nunes Ferreira, Daniele Ribeiro de Araújo, Eneida de Paula, and Michelle Franz-Montan. 2021. "Mucoadhesive, Thermoreversible Hydrogel, Containing Tetracaine-Loaded Nanostructured Lipid Carriers for Topical, Intranasal Needle-Free Anesthesia" Pharmaceutics 13, no. 11: 1760. https://doi.org/10.3390/pharmaceutics13111760
APA StyleCalixto, G. M. F., Muniz, B. V., Castro, S. R., de Araujo, J. S. M., de Souza Amorim, K., Ribeiro, L. N. M., Ferreira, L. E. N., de Araújo, D. R., de Paula, E., & Franz-Montan, M. (2021). Mucoadhesive, Thermoreversible Hydrogel, Containing Tetracaine-Loaded Nanostructured Lipid Carriers for Topical, Intranasal Needle-Free Anesthesia. Pharmaceutics, 13(11), 1760. https://doi.org/10.3390/pharmaceutics13111760