Hyaluronic Acid Hydrogels for Controlled Pulmonary Drug Delivery—A Particle Engineering Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
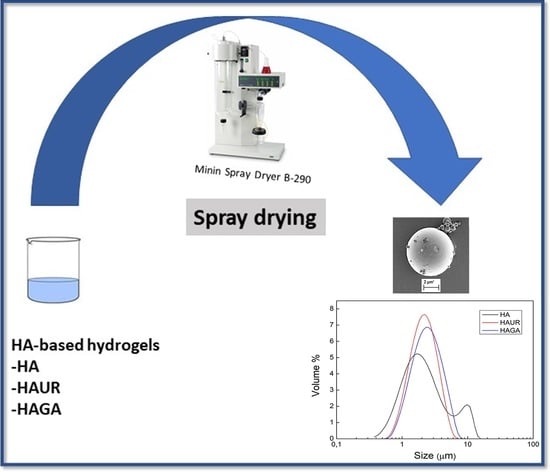
2.2. Formulation of Hyaluronic Acid-Based Hydrogels and Hydrogel Microparticles
2.3. Characterization
2.3.1. Swelling of Hydrogel Films
2.3.2. Particle Size Analysis
2.3.3. Zeta Potential
2.3.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Thermogravimetric Analysis (TGA)
2.3.7. Differential Scanning Calorimetry (DSC)
2.3.8. Swelling of Microparticles
2.3.9. In-Vitro Biodegradation of Microparticles
2.3.10. Aerodynamic Properties
2.3.11. Water-Sorption Measurement
3. Results and Discussion
3.1. Swelling and Integrity of Hydrogel Films as Indicators of Crosslinking
3.2. Process Conditions and Particle Size Analysis
3.3. Zeta Potential
3.4. Fourier Transform Infrared (FTIR)
3.5. Scanning Electron Microscopy (SEM)
3.6. Thermogravimetric Analysis (TGA)
3.7. Differential Scanning Calorimetry (DSC)
3.8. Swelling of Microparticles
3.9. Biodegradation
3.10. Aerodynamic Properties
3.11. Water-Sorption Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Z.; Ni, R.; Zhou, J.; Mao, S. Recent Advances in Controlled Pulmonary Drug Delivery. Drug Discov. Today 2015, 20, 380–389. [Google Scholar]
- Loira-Pastoriza, C.; Todoroff, J.; Vanbever, R. Delivery Strategies for Sustained Drug Release in the Lungs. Adv. Drug Deliv. Rev. 2014, 75, 81–91. [Google Scholar] [CrossRef]
- Sheth, P.; Myrdal, P.B. Polymers for pulmonary drug delivery. In Controlled Pulmonary Drug Delivery, 2nd ed.; Smyth, H.D., Hickey, A.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 265–282. [Google Scholar]
- Patil, J.S.; Sarasija, S. Pulmonary Drug Delivery Strategies: A Concise, Systematic Review. Lung India 2012, 29, 44–49. [Google Scholar] [PubMed]
- Huh, Y.; Cho, H.; Yoon, I.; Choi, M.; Kim, J.S.; Oh, E.; Chung, S.; Shim, C.; Kim, D. Preparation and Evaluation of Spray-Dried Hyaluronic Acid Microspheres for Intranasal Delivery of Fexofenadine Hydrochloride. Eur. J. Pharm. Sci. 2010, 40, 9–15. [Google Scholar]
- Marriott, C.; Frijlink, H.W. Lactose as a Carrier for Inhalation Products: Breathing New Life into an Old Carrier. Adv. Drug Deliv. Rev. 2012, 64, 217–219. [Google Scholar] [CrossRef]
- Ju, D.; Ping, D.; Smyth, H.D.C. Hydrogels for Controlled Pulmonary Delivery. Ther. Deliv. 2013, 4, 1293–1305. [Google Scholar]
- Buhecha, M.D.; Lansley, A.B.; Somavarapu, S.; Pannala, A.S. Development and Characterization of PLA Nanoparticles for Pulmonary Drug Delivery: Co-Encapsulation of Theophylline and Budesonide, a Hydrophilic and Lipophilic Drug. J. Drug Deliv. Sci. Technol. 2019, 53, 101128. [Google Scholar]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and Hybrid Alginate-Hyaluronic Acid Aerogel Microspheres as Potential Carrier for Pulmonary Drug Delivery. J. Supercrit. Fluids 2019, 150, 49–55. [Google Scholar] [CrossRef]
- Fernández-Paz, E.; Feijoo-Siota, L.; Gaspar, M.M.; Csaba, N.; Remuñán-López, C. Microencapsulated Chitosan-Based Nanocapsules: A New Platform for Pulmonary Gene Delivery. Pharmaceutics 2021, 13, 1377. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Smyth, H.D. Controlled Release Pulmonary Administration of Curcumin using Swellable Biocompatible Microparticles. Mol. Pharm. 2012, 9, 269–280. [Google Scholar] [PubMed]
- Martinelli, F.; Balducci, A.G.; Kumar, A.; Sonvico, F.; Forbes, B.; Bettini, R.; Buttini, F. Engineered Sodium Hyaluronate Respirable Dry Powders for Pulmonary Drug Delivery. Int. J. Pharm. 2017, 517, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallacara, A.; Busato, L.; Pozzoli, M.; Ghadiri, M.; Ong, H.X.; Young, P.M.; Manfredini, S.; Traini, D. Combination of Urea-Crosslinked Hyaluronic Acid and Sodium Ascorbyl Phosphate for the Treatment of Inflammatory Lung Diseases: An in Vitro Study. Eur. J. Pharm. Sci. 2018, 120, 96–106. [Google Scholar] [CrossRef]
- Fallacara, A.; Busato, L.; Pozzoli, M.; Ghadiri, M.; Ong, H.X.; Young, P.M.; Manfredini, S.; Traini, D. In Vitro Characterization of Physico-Chemical Properties, Cytotoxicity, Bioactivity of Urea-Crosslinked Hyaluronic Acid and Sodium Ascorbyl Phosphate Nasal Powder Formulation. Int. J. Pharm. 2019, 558, 341–350. [Google Scholar]
- Collins, M.N.; Birkinshaw, C. Hyaluronic Acid Based Scaffolds for Tissue engineering—A Review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Menegatti, E.; Cortesi, R. Hyaluronan-Based Microspheres as Tools for Drug Delivery: A Comparative Study. Int. J. Pharm. 2005, 288, 35–49. [Google Scholar] [CrossRef]
- Liao, Y.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical Characterization and Drug Delivery. Drug Deliv. 2005, 12, 327–342. [Google Scholar]
- Piao, M.G.; Kim, J.H.; Kim, J.O.; Lyoo, W.S.; Lee, M.H.; Yong, C.S.; Choi, H.G. Enhanced Oral Bioavailability of Piroxicam in Rats by Hyaluronate Microspheres. Drug Dev. Ind. Pharm. 2007, 33, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Makhlof, A.; Werle, M.; Tozuka, Y.; Takeuchi, H. Nanoparticles of Glycol Chitosan and its Thiolated Derivative significantly Improved the Pulmonary Delivery of Calcitonin. Int. J. Pharm. 2010, 397, 92–95. [Google Scholar]
- Morimoto, K.; Metsugi, K.; Katsumata, H.; Iwanaga, K.; Kakemi, M. Effects of Low-Viscosity Sodium Hyaluronate Preparation on the Pulmonary Absorption of Rh-Insulin in Rats. Drug Dev. Ind. Pharm. 2001, 27, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Rouse, J.J.; Whateley, T.L.; Thomas, M.; Eccleston, G.M. Controlled Drug Delivery to the Lung: Influence of Hyaluronic Acid Solution Conformation on its Adsorption to Hydrophobic Drug Particles. Int. J. Pharm. 2007, 330, 175–182. [Google Scholar]
- Surendrakumar, K.; Martyn, G.P.; Hodgers, E.C.M.; Jansen, M.; Blair, J.A. Sustained Release of Insulin from Sodium Hyaluronate Based Dry Powder Formulations After Pulmonary Delivery to Beagle Dogs. J. Control. Release 2003, 91, 385–394. [Google Scholar] [CrossRef]
- Liu, T.; Han, M.; Tian, F.; Cun, D.; Rantanen, J.; Yang, M. Budesonide Nanocrystal-Loaded Hyaluronic Acid Microparticles for Inhalation: In Vitro and in Vivo Evaluation. Carbohydr. Polym. 2018, 181, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.H.L.; Tong, H.H.Y.; Chattopadhyay, T.P.; Shekunov, B.Y. Particle Engineering for Pulmonary Drug Delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [PubMed]
- Seville, P.C.; Li, H.L.; Tristan, P. Spray-Dried Powders for Pulmonary Drug Delivery. Crit. Rev. Ther. Drug Carrier Syst. 2007, 24, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Fourie, P.B.; Germishuizen, W.A.; Wong, Y.; Edwards, D.A. Spray Drying TB Vaccines for Pulmonary Administration. Expert Opin. Biol. Ther. 2008, 8, 857–863. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray Drying of Pharmaceuticals and Biopharmaceuticals: Critical Parameters and Experimental Process Optimization Approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Kenji, T.; Ikada, Y. Crosslinking of Hyaluronic Acid with Glutaraldehyde. J. Polym. Sci. A Polym. Chem. 1997, 35, 3553–3559. [Google Scholar]
- Citernesi, U.R.; Beretta, L.; Citernesi, L. Cross-Linked Hyaluronic Acid, Process for the Preparation Thereof and use Thereof in the Aesthetic Field. U.S. Patent WO/2015/007773 A1, 22 January 2015. [Google Scholar]
- Delgado, Á.V.; González-Caballero, F.; Hunter, R.; Koopal, L.; Lyklema, J. Measurement and Interpretation of Electrokinetic Phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef]
- Franek, F.; Fransson, R.; Thörn, H.; Bäckman, P.; Andersson, P.U.; Tehler, U. Ranking in Vitro Dissolution of Inhaled Micronized Drug Powders Including a Candidate Drug with Two Different Particle Sizes. Mol. Pharm. 2018, 15, 5319–5326. [Google Scholar]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar]
- Umashankar, P.R.; Mohanan, P.V.; Kumari, T.V. Glutaraldehyde Treatment Elicits Toxic Response Compared to Decellularization in Bovine Pericardium. Toxicol. Int. 2012, 19, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Han, M.; Liu, T.; Cun, D.; Fang, L.; Yang, M. Inhaled Hyaluronic Acid Microparticles Extended Pulmonary Retention and Suppressed Systemic Exposure of a Short-Acting Bronchodilator. Carbohydr. Polym. 2017, 172, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Killeen, P.R. An Alternative to Null-Hypothesis Significance Tests. Psychol. Sci. 2005, 16, 345–353. [Google Scholar]
- Fallacara, A.; Busato, L.; Pozzoli, M.; Ghadiri, M.; Ong, H.X.; Young, P.M.; Manfredini, S.; Traini, D. Co-Spray-Dried Urea Cross-Linked Hyaluronic Acid and Sodium Ascorbyl Phosphate as Novel Inhalable Dry Powder Formulation. J. Pharm. Sci. 2019, 108, 2964–2971. [Google Scholar] [CrossRef]
- Dao, T.H.; Tran, T.T.; Nguyen, V.R.; Pham, T.N.M.; Vu, C.M.; Pham, T.D. Removal of Antibiotic from Aqueous Solution using Synthesized TiO2 Nanoparticles: Characteristics and Mechanisms. Environ. Earth Sci. 2018, 77, 359. [Google Scholar] [CrossRef]
- Mishra, R.; Militky, J. Nanocomposites. In Nanotechnology in Textiles; Mishra, R., Militky, J., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 263–310. [Google Scholar]
- Poinard, B.; Kamaluddin, S.; Tan, A.Q.Q.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Coating Enhances Mucopenetration and Cell Uptake of Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 4777–4789. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in Mucoadhesive Drug-Delivery Systems: A Brief Note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Russo, E.; Selmin, F.; Baldassari, S.; Gennari, C.G.M.; Caviglioli, G.; Cilurzo, F.; Minghetti, P.; Parodi, B. A Focus on Mucoadhesive Polymers and their Application in Buccal Dosage Forms. J. Drug Deliv. Sci. Technol. 2016, 32, 113–125. [Google Scholar]
- Sandri, G.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Zerrouk, N.; Caramella, C. Mucoadhesive and Penetration Enhancement Properties of Three Grades of Hyaluronic Acid using Porcine Buccal and Vaginal Tissue, Caco-2 Cell Lines, and Rat Jejunum. J. Pharm. Pharmacol. 2010, 56, 1083–1090. [Google Scholar] [CrossRef]
- Antunes, J.C.; Oliveira, J.M.; Reis, R.; Soria, J.M.; Gómez-Ribelles, J.; Mano, J. Novel Poly (L-lactic Acid)/Hyaluronic Acid Macroporous Hybrid Scaffolds: Characterization and Assessment of Cytotoxicity. J. Biomed. Mater. Res. A 2010, 94, 856–869. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X. Synthesis and Characterization of a Novel Hyaluronic Acid Hydrogel. J. Biomater. Sci. Polym. Ed. 2006, 17, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Shahin, H.I.; Vinjamuri, B.P.; Mahmoud, A.A.; Shamma, R.N.; Mansour, S.M.; Ammar, H.O.; Ghorab, M.M.; Chougule, M.B.; Chablani, L. Design and Evaluation of Novel Inhalable Sildenafil Citrate Spray-Dried Microparticles for Pulmonary Arterial Hypertension. J. Control. Release 2019, 302, 126–139. [Google Scholar] [CrossRef]
- Carrigy, N.B.; Ordoubadi, M.; Liu, Y.; Melhem, O.; Barona, D.; Wang, H.; Milburn, L.; Ruzycki, C.A.; Finlay, W.H.; Vehring, R. Amorphous Pullulan Trehalose Microparticle Platform for Respiratory Delivery. Int. J. Pharm. 2019, 563, 156–168. [Google Scholar]
- Rabbani, N.R.; Seville, P.C. The Influence of Formulation Components on the Aerosolisation Properties of Spray-Dried Powders. J. Control. Release 2005, 110, 130–140. [Google Scholar] [CrossRef]
- Réeff, J.; Gaignaux, A.; Goole, J.; Vriese, C.D.; Amighi, K. New Sustained-Release Intraarticular Gel Formulations Based on Monolein for Local Treatment of Arthritic Diseases. Drug Dev. Ind. Pharm. 2013, 39, 1731–1741. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B. Surface and Thermal Properties of Collagen/Hyaluronic Acid Blends Containing Chitosan. Int. J. Biol. Macromol. 2016, 92, 371–376. [Google Scholar] [PubMed]
- Fallacara, A.; Marchetti, F.; Pozzoli, M.; Citernesi, U.R.; Manfredini, S.; Vertuani, S. Formulation and Characterization of Native and Crosslinked Hyaluronic Acid Microspheres for Dermal Delivery of Sodium Ascorbyl Phosphate: A Comparative Study. Pharmaceutics 2018, 10, 254. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.N.; Birkinshaw, C. Physical Properties of Crosslinked Hyaluronic Acid Hydrogels. J. Mater. Sci. Mater. Med. 2008, 19, 3335–3343. [Google Scholar] [CrossRef]
- Collins, M.; Birkinshaw, C. Comparison of the Effectiveness of Four Different Crosslinking Agents with Hyaluronic Acid Hydrogel Films for Tissue-culture Applications. J. Appl. Polym. Sci. 2007, 104, 3183–3191. [Google Scholar]
- Kafedjiiski, K.; Jetti, R.K.R.; Föger, F.; Hoyer, H.; Werle, M.; Hoffer, M.; Bernkop-Schnürch, A. Synthesis and in Vitro Evaluation of Thiolated Hyaluronic Acid for Mucoadhesive Drug Delivery. Int. J. Pharm. 2007, 343, 48–58. [Google Scholar] [CrossRef]
- Makino, K.; Yamamoto, N.; Higuchi, K.; Harada, N.; Ohshima, H.; Terada, H. Phagocytic Uptake of Polystyrene Microspheres by Alveolar Macrophages: Effects of the Size and Surface Properties of the Microspheres. Colloids Surf. B Biointerfaces 2003, 27, 33–39. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; McGill, S.; Smyth, H.D. Swellable Microparticles as Carriers for Sustained Pulmonary Drug Delivery. J. Pharm. Sci. 2010, 99, 2343–2356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T. Antimicrobial Polypeptides in Host Defense of the Respiratory Tract. J. Clin. Investig. 2002, 109, 693–697. [Google Scholar] [CrossRef]
- Nsereko, S.; Amiji, M. Localized Delivery of Paclitaxel in Solid Tumors from Biodegradable Chitin Microparticle Formulations. Biomaterials 2002, 23, 2723–2731. [Google Scholar]
- Eedara, B.B.; Tucker, I.G.; Das, S.C. In Vitro Dissolution Testing of Respirable Size Anti-Tubercular Drug Particles using a Small Volume Dissolution Apparatus. Int. J. Pharm. 2019, 559, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Takami, T.; Murakami, Y. Porous PLGA Microparticles Formed by “one-Step” Emulsification for Pulmonary Drug Delivery: The Surface Morphology and the Aerodynamic Properties. Colloids Surf. B Biointerfaces 2017, 159, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Saigal, A.; Ng, W.K.; Tan, R.B.; Chan, S.Y. Development of Controlled Release Inhalable Polymeric Microspheres for Treatment of Pulmonary Hypertension. Int. J. Pharm. 2013, 450, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Lin, Y.W.; Kwok, P.C.L.; Niemelä, V.; Crapper, J.; Chan, H.K. Measuring Bipolar Charge and Mass Distributions of Powder Aerosols by a Novel Tool (BOLAR). Mol. Pharm. 2015, 12, 3433–3440. [Google Scholar]
- Nokhodchi, A.; Larhrib, H. Overcoming the Undesirable Properties of Dry-Powder Inhalers with Novel Engineered Mannitol Particles. Ther. Deliv. 2013, 4, 879–882. [Google Scholar] [CrossRef]
- Mangal, S.; Nie, H.; Xu, R.; Guo, R.; Cavallaro, A.; Zemlyanov, D.; Zhou, Q.T. Physico-Chemical Properties, Aerosolization and Dissolution of Co-Spray Dried Azithromycin Particles with L-Leucine for Inhalation. Pharm. Res. 2018, 35, 28. [Google Scholar] [PubMed] [Green Version]
- Sing, K.; Everett, D.; Haul, R.; Moscou, L.; Pierotti, R.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: London, UK, 1999. [Google Scholar]
- Thommes, M.; Köhn, R.; Fröba, M. Sorption and Pore Condensation Behavior of Pure Fluids in Mesoporous MCM-48 Silica, MCM-41 Silica, SBA-15 Silica and Controlled-Pore Glass at Temperatures Above and Below the Bulk Triple Point. Appl. Surf. Sci. 2002, 196, 239–249. [Google Scholar] [CrossRef]











| Sample | Inlet Temperature (°C) | Atomizing Air-Flow (L/h) | Drying Air-Flow (L/h) | Feeding Rate (mL/min) | Yield% | Mean (µm) | SD a (µm) | Median (µm) | Span |
|---|---|---|---|---|---|---|---|---|---|
| HA1 | 100 | 375 | 35 | 1 | 28.5 | 3.2 | 3.0 | 2.1 | 3.5 |
| HA2 | 110 | 375 | 35 | 1 | 28.1 | 2.9 | 2.7 | 2.0 | 2.8 |
| HA3 b | 120 | 375 | 35 | 1 | 22.7 | 3.0 | 2.6 | 2.1 | 2.2 |
| HA4 | 120 | 473 | 35 | 1 | 28.7 | 2.5 | 2.5 | 1.8 | 2.8 |
| HAUR | 120 | 375 | 35 | 1 | 37.2 | 2.4 | 1.1 | 2.2 | 1.2 |
| HAGA | 120 | 375 | 35 | 1 | 43.4 | 2.3 | 1.1 | 2.2 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikjoo, D.; van der Zwaan, I.; Brülls, M.; Tehler, U.; Frenning, G. Hyaluronic Acid Hydrogels for Controlled Pulmonary Drug Delivery—A Particle Engineering Approach. Pharmaceutics 2021, 13, 1878. https://doi.org/10.3390/pharmaceutics13111878
Nikjoo D, van der Zwaan I, Brülls M, Tehler U, Frenning G. Hyaluronic Acid Hydrogels for Controlled Pulmonary Drug Delivery—A Particle Engineering Approach. Pharmaceutics. 2021; 13(11):1878. https://doi.org/10.3390/pharmaceutics13111878
Chicago/Turabian StyleNikjoo, Dariush, Irès van der Zwaan, Mikael Brülls, Ulrika Tehler, and Göran Frenning. 2021. "Hyaluronic Acid Hydrogels for Controlled Pulmonary Drug Delivery—A Particle Engineering Approach" Pharmaceutics 13, no. 11: 1878. https://doi.org/10.3390/pharmaceutics13111878
APA StyleNikjoo, D., van der Zwaan, I., Brülls, M., Tehler, U., & Frenning, G. (2021). Hyaluronic Acid Hydrogels for Controlled Pulmonary Drug Delivery—A Particle Engineering Approach. Pharmaceutics, 13(11), 1878. https://doi.org/10.3390/pharmaceutics13111878