Moderate Heat-Assisted Gene Electrotransfer as a Potential Delivery Approach for Protein Replacement Therapy through the Skin
Abstract
:1. Introduction
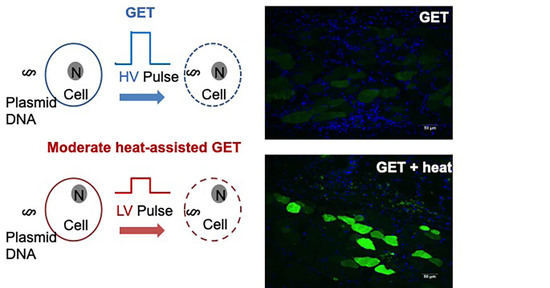
- Although increased temperature can enhance delivery when used together with GET, it is also important to moderate the applied heat so as not to cause tissue damage. It is well established that thermal injury is determined by temperature and duration [46] such that as the temperature is increased, the less time it takes to cause a burn or tissue damage. We previously established that the optimal intradermal temperature for enhancing GET was 43 °C [43,47]. It has also been accepted that it takes several minutes to cause a burn at 43 °C. A protocol was developed that demonstrated a 30 s preheating duration was sufficient for sustained temperature during the proceeding pulsing protocol. This entire process took less than a minute. Furthermore, the synergy between moderate heating and electroporation allows for the reduction of both the necessary applied voltage and pulse number [48]. These reductions have the potential to create an application that is less painful for the recipient and easier to apply for the clinician. Minimizing discomfort is an important consideration for translation of cutaneous deliveries where multiple applications may be necessary, such as protein replacement therapy.
2. Materials and Methods
2.1. Animals
2.2. Plasmids
2.3. Infrared Laser Heat Application
2.4. Measuring Intradermal Skin Temperature
2.5. Electrode Design
2.6. Reporter Gene Delivery
2.7. In Vivo Bioluminescent Imaging and Kinetic Expression Analysis
2.8. Immunofluorescence Staining and Distribution Expression Analysis
2.9. Factor IX Gene Delivery
2.10. Factor IX Protein Expression Analysis
2.11. Statistical Analysis
3. Results
3.1. Moderate Heat Applied by Infrared Laser Yields Fast and Uniform Heating
3.2. Moderate Heat-Assisted GET Yields Sustained Expression Levels Compared to Unheated Counterpart
3.3. Gene Expression Following Moderate Heat-Assisted GET Extended to the Dermis and Underlying Muscle
3.4. Moderate Heating Mitigates a Reduction in Pulse Number and Skin Damage Caused by High Voltage GET
3.5. Factor IX Is Expressed Systemically Following Moderate Heat-Assisted GET to the Skin
3.6. Multiple Site Application Enhances Factor IX Protein Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Animal # | Group | d2 | d7 | d14 | d21 | d35 | d63 | d100 |
|---|---|---|---|---|---|---|---|---|
| 19-0557 | IM-100 V/cm | 2.41 | 2.04 | 0 | 3.98 | 9.27 | 6.59 | 5391 |
| 19-0558 | IM-100 V/cm | 3.28 | 1.28 | 4.4 | 3.47 | 8.53 | 6.34 | 2.45 |
| 19-0559 | IM-100 V/cm | 1.15 | 1.66 | 1.71 | 9.48 | 8.91 | 7.46 | 4.47 |
| 19-0560 | IM-100 V/cm | 2.41 | 1.91 | 9.5 | 4.65 | 7.01 | 8.89 | 5.67 |
| 19-0561 | 45 V-72p | 0 | 5.77 | 9.04 | 1.05 | 0 | 0 | 0 |
| 19-0562 | 45 V-72p | 1.6 | 2.23 | 5.63 | 0 | 0 | 0.38 | 0 |
| 19-0563 | 45 V-72p | 0.89 | 2.91 | 0.22 | 0 | 0 | 0.78 | 0 |
| 19-0564 | 45 V-72p | 1.72 | 3.28 | 6.82 | 0 | 0 | 1.51 | 0 |
| 19-0565 | 45 V-36p + 9 fiber heat | 6.85 | 4.29 | 0.85 | 7.79 | 0 | 0 | 0 |
| 19-0566 | 45 V-36p + 9 fiber heat | 2.04 | 2.47 | 9.65 | 7.74 | 0 | 0 | 0 |
| 19-0567 | 45 V-36p + 9 fiber heat | 1.53 | 3.28 | 22.34 | 7.87 | 0 | 1.62 | 0 |
| 19-0568 | 45 V-36p + 9 fiber heat | 3.65 | 2.29 | 6.1 | 5.79 | 0 | 1.19 | 0 |
| 19-0569 | 35 V-72p + 9 fiber heat | 3.03 | 3.21 | 9.6 | 0 | 0 | 1.14 | 0 |
| 19-0570 | 35 V-72p + 9 fiber heat | 1.66 | 2.46 | 0.11 | 0 | 0 | 0.97 | 0 |
| 19-0571 | 35 V-72p + 9 fiber heat | 1.28 | 3.34 | 3.16 | 0 | 1.12 | 0 | 0 |
| 19-0572 | 35 V-72p + 9 fiber heat | 1.02 | 3.22 | 5.68 | 0 | 0 | 0.04 | 0 |
| Animal # | Group | d2 | d7 | d14 | d21 | d35 | d63 | d100 |
|---|---|---|---|---|---|---|---|---|
| 19-1544 | 2 sites | 0.42 | 0.85 | 3.99 | 4.79 | 6.21 | 0.38 | 0 |
| 19-1545 | 2 sites | 0 | 1.77 | 4.21 | 7.46 | 8.8 | 0.82 | 1.64 |
| 19-1546 | 2 sites | 0 | 5.74 | 6.8 | 9.06 | 4.83 | 1 | 0.45 |
| 19-1547 | 2 sites | 0 | 6.67 | 13.92 | 9.98 | 7.71 | 3.83 | 2.06 |
| 19-1548 | 3 sites | 0 | 1.41 | 3.27 | 7.46 | 8.46 | 4.84 | 3.4 |
| 19-1549 | 3 sites | 2.17 | 3.2 | 4.77 | 8.41 | 9.5 | 7.98 | 3.37 |
| 19-1550 | 3 sites | 0.54 | 5.99 | 4.78 | 8.39 | 10.29 | 6.12 | 2.97 |
| 19-1551 | 3 sites | 0 | 5.61 | 8.87 | 9.17 | 3.97 | 2.33 | 2.04 |
| 19-1552 | 4 sites | 0 | 1.7 | 5.33 | 2.94 | 6.34 | 4.09 | 8.04 |
| 19-1553 | 4 sites | 0 | 3.11 | 5.34 | 7.87 | 2.29 | 2.36 | 3.42 |
| 19-1554 | 4 sites | 0 | 4.7 | 5.9 | 10.27 | 8.19 | 5.47 | 4.21 |
| 19-1555 | 4 sites | 0 | 7.1 | 6.99 | 10.79 | 8.43 | 6.45 | 6.09 |
References
- Daud, A.I.; DeConti, R.C.; Andrews, S.; Urbas, P.; Riker, A.I.; Sondak, V.K.; Munster, P.N.; Sullivan, D.M.; Ugen, K.E.; Messina, J.L.; et al. Phase I Trial of Interleukin-12 Plasmid Electroporation in Patients with Metastatic Melanoma. J. Clin. Oncol. 2008, 26, 5896–5903. [Google Scholar] [CrossRef] [Green Version]
- Algazi, A.; Bhatia, S.; Agarwala, S.; Molina, M.; Lewis, K.; Faries, M.; Fong, L.; Levine, L.P.; Franco, M.; Oglesby, A.; et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann. Oncol. 2020, 31, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greaney, S.K.; Algazi, A.P.; Tsai, K.K.; Takamura, K.T.; Chen, L.; Twitty, C.G.; Zhang, L.; Paciorek, A.; Pierce, R.H.; Le, M.H.; et al. Intratumoral Plasmid IL12 Electroporation Therapy in Patients with Advanced Melanoma Induces Systemic and Intratumoral T-cell Responses. Cancer Immunol. Res. 2020, 8, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety efficacy and immunognecity of VGX-3100 a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomized, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [Green Version]
- El-Kamary, S.S.; Billington, M.; Deitz, S.; Colby, E.; Rhinehart, H.; Wu, Y.; Blackwelder, W.; Edelman, R.; Lee, A.; King, A. Safety and tolerability of the easy vax clinical epidermal electroporation system in healthy adults. Mol. Ther. 2012, 20, 214–220. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; de la Fuenta, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar] [CrossRef]
- Low, L.; Mander, A.M.; McCann, K.; Dearnaley, D.; Tjelle, T.; Mathiesen, I.; Stevenson, F.; Ottensmeier, C.H. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum. Gene Ther. 2009, 20, 1269–1278. [Google Scholar] [CrossRef]
- Tebas, P.; Yang, S.; Boyer, J.D.; Reuschel, E.L.; Patel, A.; Christensen-Quick, A.; Andrade, V.M.; Morrow, M.P.; Kraynuak, K.; Agnes, J.; et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, phase 1 clinical trial. EClin. Med. 2021, 100689. [Google Scholar] [CrossRef]
- Young, J.L.; Dean, D.A. Electroporation-mediated gene delivery. Adv. Genet. 2015, 89, 49–88. [Google Scholar]
- Shinkuma, S. Advances in gene therapy and their application to skin diseases: A review. J. Dermatol. Sci. 2021, 103, 2–9. [Google Scholar] [CrossRef]
- Maruyama, H.; Ataka, K.; Higuchi, N.; Sakamoto, F.; Gejyo, F.; Miyazaki, J. Skin targeted gene transfer using in vivo electroporation. Gene Ther. 2001, 8, 1808–1812. [Google Scholar] [CrossRef] [Green Version]
- Bellefroid, C.; Lechanteur, A.; Evrard, B.; Piel, G. Lipid nanocarriers for the treatment of skin diseases: Current state-of-the-art. Eur. J. Pharma. Biopharma 2019, 137, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Gorell, E.; Nguyen, N.; Lane, A.; Siprashvili, Z. Gene therapy for skin disease. CSPH Perspect. Med. 2014, 4, a015149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasquet, L.; Chabot, S.; Bellard, E.; Markelc, B.; Rols, M.-P.; Reynes, J.-P.; Tiraby, G.; Couillaud, F.; Teissie, J.; Golzio, M. Safe and efficient novel approach for non-invasive gene electrotransfer to skin. Sci. Rep. 2018, 8, 16833. [Google Scholar] [CrossRef] [PubMed]
- Kos, S.; Blagus, T.; Cemazar, M.; Tratar, U.L.; Stimac, M.; Prosen, L.; Dolinsek, T.; Kamensek, U.; Kranjc, S.; Steinstraesser, L.; et al. Electrotransfer parameters as a tool for controlled and targeted gene expression in the skin. Mol. Ther. Nucleic Acids. 2016, 5, e356. [Google Scholar] [CrossRef] [Green Version]
- Amante, D.H.; Smith, T.R.F.; Kiosses, B.B.; Sardesai, N.Y.; Humeau, L.M.P.F.; Broderick, K.E. Direct transfection of dendritic cells in the epidermis after plasmid delivery enhanced by surface electroporation. Hum. Gene Ther. Methods 2014, 25, 315–316. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, B.; Cruz, Y.L.; Baldwin, M.; Coppola, D.; Heller, R. Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid FGF-2 delivered by noninvasive electroporation. Gene Ther. 2010, 17, 763–769. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, M.; Byrnes, C.; Sun, L.; Marti, G.; Bonde, P.; Duncan, M.; Harmon, J.W. Wound healing enhancement: Electroporation to address a classic problem of military medicine. World J. Surg. 2005, 29 (Suppl. 1), S55–S59. [Google Scholar] [CrossRef]
- Marti, G.; Ferguson, M.; Wang, J.; Byrnes, C.; Dieb, R.; Qaiser, R.; Bonde, P.; Duncan, M.D.; Harmon, J.W. Electroporative transfection with KGF-1 DNA improves wound healing in a diabetic mouse model. Gene Ther. 2004, 11, 1780–1785. [Google Scholar] [CrossRef]
- Donate, A.; Coppola, D.; Cruz, Y.; Heller, R. Evaluation of a novel non-penetrating electrode for use in DNA vaccination. PLoS ONE. 2011, 6, e19181. [Google Scholar] [CrossRef] [Green Version]
- Medi, B.M.; Hoselton, S.; Marepalli, R.B.; Singh, J. Skin targeted DNA vaccine delivery using electroporation in rabbits. I: Efficacy. Int. J. Pharm. 2005, 294, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Roos, A.K.; Moreno, S.; Leder, C.; Pavlenko, M.; King, A.; Pisa, P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol. Ther. 2006, 13, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Hooper, J.W.; Golden, J.W.; Ferro, A.M.; King, A.D. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine 2007, 25, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Brave, A.; Nystrom, S.; Roos, A.K.; Applequist, S.E. Plasmid DNA vaccination using skin electroporation promotes poly-functional CD4 T-cell responses. Immunol. Cell Biol. 2010, 89, 492–496. [Google Scholar] [CrossRef]
- Hirao, L.A.; Draghia-Akli, R.; Prigge, J.T.; Yang, M.; Satishchandran, A.; Wu, L.; Hammarlund, E.; Khan, A.S.; Babas, T.; Rhodes, L.; et al. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 2011, 203, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Son, W.R.; Choi, J.Y.; Yu, C.H.; Hur, G.H.; Jeong, S.T.; Shin, Y.K.; Hong, S.Y.; Shin, S. Immunogenicity and biodistribution of anthrax DNA vaccine delivered by intradermal electroproation. Curr. Drug Deliv. 2020, 17, 414–421. [Google Scholar] [CrossRef]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Preat, V.; Vandermeulen, G. Clinical potential of electroporation for gene therapy and DNA vaccine delviery. Expert Opin. Drug Deliv. 2016, 13, 295–310. [Google Scholar] [CrossRef]
- Williams, M.; Ewing, D.; Blevins, M.; Sun, P.; Sundaram, A.K.; Raviprakash, K.S.; Porter, K.R.; Sanders, J.W. Enjanced immunogenecity and protective efficacy of a tetravalent dengue DNA vaccine using electroporation and intradermal delivery. Vaccine 2019, 37, 4444–4453. [Google Scholar] [CrossRef]
- Maricq, H.R.; Darke, C.S.; Archibald, R.M.; Leroy, E.C. In vivo observations of skin capillaries in workers exposed to vinyl chloride. An English-American comparison. Br. J. Ind. Med. 1978, 35, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gothelf, A.; Eriksen, J.; Hojman, P.; Gehl, J. Duration and level of transgene expression after gene electrotransfer to skin in mice. Gene Ther. 2010, 17, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Khavari, P.A.; Rollman, O.; Vahlquist, A. Cutaneous gene transfer for skin and systemic diseases. J. Intern. Med. 2002, 252, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.G. Strategies for long-term gene expression in the skin to treat metabolic disorders. Expert Opin. Biol. Ther. 2004, 4, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, B.; Heller, L.C.; Cruz, Y.L.; Guo, S.; Donate, A.; Heller, R. Evaluation of delivery conditions for cutaneous plasmid electrotransfer using a multielectrode array. Gene Ther. 2011, 18, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Donate, A.; Basu, G.; Lundberg, C.; Heller, L.; Heller, R. Electro-gene transfer to skin using a noninvasive multielectrode array. J. Control. Release 2011, 151, 256–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, L.C.; Jaroszeski, M.J.; Coppola, D.; McCrae, A.N.; Hickey, J.; Heller, R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007, 14, 275–280. [Google Scholar] [CrossRef]
- Heller, R.; Cruz, Y.; Heller, L.C.; Gilbert, R.A.; Jaroszeski, M.J. Electrically mediated delivery of plasmid DNA to the skin, using a multielectrode array. Hum. Gene Ther. 2010, 21, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Broderick, K.E.; Shen, X.; Soderholm, J.; Lin, F.; McCoy, J.; Khan, A.S.; Morrow, M.P.; Patel, A.; Kobinger, G.P.; Kemmerrer, S.; et al. Protoype development and preclinical immunogenicity analysis of a novel minimally invasive electroproation device. Gene Ther. 2011, 18, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraro, B.; Cruz, Y.L.; Coppola, D.; Heller, R. Intradermal Delivery of Plasmid VEGF165 by Electroporation Promotes Wound Healing. Mol. Ther. 2009, 17, 651–657. [Google Scholar] [CrossRef]
- McCoy, J.R.; Mendoza, J.M.; Spik, K.W.; Badger, C.; Gomez, A.F.; Schmaljohn, C.S.; Sardesai, N.Y.; Broderick, K.E. A multi-head intradermal electroproation device allows for tailored and increased dose DNA vaccine to the skin. Hum. Vaccin. Immunother. 2015, 3, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Kos, S.; Vanvarenberg, K.; Dolinsek, T.; Cemazar, M.; Jelenc, J.; Preat, V.; Sersa, G.; Vandermeulen, G. Gene electrotransfer into skin using noninvasive multi-electrode array for vaccination and wound healing. Bioelectrochemistry 2017, 114, 33–41. [Google Scholar] [CrossRef]
- Lin, F.; Shen, X.; McCoy, J.R.; Mendoza, J.M.; Yan, J.; Kemmerrer, S.V.; Khan, A.S.; Weiner, D.B.; Broderick, K.E.; Sardesai, N.Y. A novel prototype device for electroproation-enhanced DNA vaccine delivery simultaneously to both skin and muscle. Vaccine 2011, 29, 6771–6780. [Google Scholar] [CrossRef]
- Fan, W.; Evans, R.M. Turning up the heat on membrane fluidity. Cell 2015, 161, 962–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donate, A.; Burcus, N.; Schoenbach, K.; Heller, R. Application of increased temperature from an exogenous source to enhance gene electrotransfer. Bioelectrochemistry 2014, 103, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.A.; Davalos, R.V.; Miklavcic, D. A numerical investigation of the electric and thermal cell kill distributions in electroporation-based therapies in tissue. PLoS ONE 2014, 9, e103083. [Google Scholar] [CrossRef]
- Bulysheva, A.; Hornef, J.; Edelblute, C.; Jiang, C.; Schoenbach, K.; Lundberg, C.G.; Malik, M.A.; Heller, R. Coalesced thermal and electrotransfer mediated delivery of plasmid DNA to the skin. Bioelectrochemistry 2019, 125, 127–133. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Donate, A.; Bulysheva, A.; Edelblute, C.; Jung, D.; Malik, M.A.; Guo, S.; Burcus, N.; Schoenbach, K.; Heller, R. Thermal Assisted In Vivo Gene Electrotransfer. Curr. Gene Ther. 2016, 16, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Edelblute, C.M.; Hornef, J.; Burcus, N.I.; Norman, T.; Beebe, S.J.; Schoenbach, K.; Heller, R.; Jiang, C.; Guo, S. Controllable Moderate Heating Enhances the Therapeutic Efficacy of Irreversible Electroporation for Pancreatic Cancer. Sci. Rep. 2017, 7, 11767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGraw, R.A.; Davis, L.M.; Lundblad, R.L.; Stafford, D.W.; Roberts, H.R. Structure and function of factor IX: Defects in haemophilia B. Clin. Haematol. 1985, 14, 359–383. [Google Scholar] [CrossRef]
- Peyvandi, F.; Garagiola, I.; Young, G. The past and future of haemophilia: Diagnosis, treatments, and its complications. Lancet 2016, 388, 187–197. [Google Scholar] [CrossRef]
- Viala, N.O.; Larsen, S.R.; Rasko, R.E. Gene therapy for hemophilia: Clinical trials and technical tribulations. Semin. Thromb. Hemost. 2009, 35, 81–92. [Google Scholar] [CrossRef]
- High, K.A. Gene therapy for haemophilia: A long and winding road. J. Thromb. Haemost. 2011, 9, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.S. Advancements in Non Viral Gene Transfer for Hemophilia. J. Genet. Syndr. Gene Ther. 2011, S1, 002. [Google Scholar]
- Batty, P.; Lillicrap, D. Advances and Challenges for Hemophilia Gene Therapy. Hum. Mol. Genet. 2019, 28, R95–R101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyvandi, F.; Garagiola, I. Clinical advances in gene therapy updates on clinical trials of gene therapy in haemophilia. Haemophilia 2019, 25, 738–746. [Google Scholar] [CrossRef]
- Guo, S.; Israel, A.L.; Basu, G.; Donate, A.; Heller, R. Topical Gene Electrotransfer to the Epidermis of Hairless Guinea Pig by Non-Invasive Multielectrode Array. PLoS ONE 2013, 8, e73423. [Google Scholar]
- Bulysheva, A.; Heller, L.; Francis, M.; Varghese, F.; Boye, C.; Heller, R. Monopolar gene electrotransfer enhances plasmid DNA to the skin. Bioelectrochemistry 2021, 140, 107814. [Google Scholar] [CrossRef]
- Papaioannou, T.G.; Karamanou, M.; Protogerou, A.D.; Tousoulis, D. Heat therapy: An ancient concept re-examined in the era of advanced biomedical technologies. J. Physiol. 2016, 594, 7141–7142. [Google Scholar] [CrossRef]
- Karamanou, M.; Antoniou, L.C.; Androutsos, G.; Lykouras, E. Julius Wagner-Jauregg (1857–1940): Introducing fever therapy in the treatment of neurosyphilis. Psychiatriki 2013, 24, 208–212. [Google Scholar]
- Hillman, S.K.; Delforge, G. The use of physical agents in rehabilitation of athletic injuries. Clin. Sports Med. 1985, 4, 431–438. [Google Scholar] [CrossRef]
- Brosseau, L.; Yonge, K.A.; Robinson, V.; Marchand, S.; Judd, M.; Wells, G.; Tugwell, P. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst. Rev. 2003. [Google Scholar] [CrossRef]
- Nyland, J.; Nolan, M.F. Therapeutic modality: Rehabilitation of the injured athlete. Clin. Sports Med. 2004, 23, 299–313. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta. Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.P.; Plourde, B.; Vallez, L.; Stark, J.; Diller, K.R. Estimating the time and temperature relationship for causation of deep-partial thickness skin burns. Burns 2015, 41, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Kanduser, M.; Sentjurc, M.; Miklavcic, D. The temperature effect during pulse application on cell membrane fluidity and permeabilization. Bioelectrochemistry 2008, 74, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Rossmanna, C.; Haemmerich, D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit. Rev. Biomed. Eng. 2014, 42, 467–492. [Google Scholar] [CrossRef] [Green Version]
- Edelblute, C.M.; Guo, S.; Hornef, J.; Yang, E.; Jiang, C.; Schoenbach, K.; Heller, R. Moderate Heat Application Enhances the Efficacy of Nanosecond Pulse Stimulation for the Treatment of Squamous Cell Carcinoma. Technol. Cancer Res. Treat. 2018, 17, 1533033818802305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornef, J.; Edelblute, C.M.; Schoenbach, K.H.; Heller, R.; Guo, S.; Jiang, C. Thermal Analysis of Infrared Irradiation-Assisted Nanosecond-Pulsed Tumor Ablation. Sci. Rep. 2020, 10, 5122. [Google Scholar] [CrossRef] [Green Version]
- Ng, E.Y.; Chua, L.T. Prediction of skin burn injury. Part 2: Parametric and sensitivity analysis. Proc. Inst. Mech. Eng. H 2002, 216, 171–183. [Google Scholar] [CrossRef]
- Ye, H.; De, S. Thermal injury of skin and subcutaneous tissues: A review of experimental approaches and numerical models. Burns 2017, 43, 909–932. [Google Scholar] [CrossRef]
- Monahan, P.E. Gene therapy in an era of emerging treatment options for hemophilia B. J. Thromb. Haemost. 2015, 13, S151–S160. [Google Scholar] [CrossRef] [Green Version]
- Nathwani, A.C.; Davidoff, A.M.; Tuddenham, E.G.D. Gene Therapy for Hemophilia. Hematol. Oncol. Clin. North Am. 2017, 31, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Perrin, G.Q.; Herzog, R.W.; Markusic, D.M. Update on clinical gene therapy for hemophilia. Blood 2019, 133, 407–414. [Google Scholar] [CrossRef] [Green Version]
- George, L.A.; Sullivan, S.K.; Giermasz, A.; Rasko, J.E.J.; Samelson-Jones, B.J.; Ducore, J.; Cuker, A.; Sullivan, L.M.; Majumdar, S.; Teitel, J.; et al. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017, 377, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C.; Reiss, U.M.; Tuddenham, E.G.D.; Rosales, C.; Chowdary, P.; McIntosh, J.; Della Peruta, M.; Lheriteau, E.; Patel, N.; Raj, D.; et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014, 371, 1994–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, E.; Zhang, Z.; Woodlis, J.; Breau, R.; Suen, J.; Li, S. Intramuscular electroporation delivery of IL-12 gene for treatment of squamous cell carcinoma located at distant site. Cancer Gene. Ther. 2001, 8, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Heller, L.C.; Ugen, K.; Heller, R. Electroporation for targeted gene transfer. Expert Opin. Drug Deliv. 2005, 2, 255–268. [Google Scholar] [CrossRef]
- Rohrer, T.R.; Horikawa, R.; Kappelgaard, A.M. Growth hormone delivery devices: Current features and potential for enhanced treatment adherence. Expert Opin. Drug Deliv. 2017, 14, 1253–1264. [Google Scholar] [CrossRef]
- Bettan, M.; Emmanuel, F.; Darteil, R.; Caillaud, J.M.; Soubrier, F.; Delaere, P.; Branelec, D.; Mahfoudi, A.; Duverger, N.; Scherman, D. High-level protein secretion into blood circulation after electric pulse-mediated gene transfer into skeletal muscle. Mol. Ther. 2000, 3, 204–210. [Google Scholar] [CrossRef]
- Edelblute, C.; Mangiamele, C.; Heller, R. Moderate Heat-Assisted Gene Electrotransfer for Cutaneous Delivery of a DNA Vaccine Against Hepatitis B Virus. Hum. Gene Ther. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Song, Y.; Hemmady, K.; Puri, A.; Banga, A.K. Transdermal delivery of human growth hormone via laser-generated micropores. Drug Deliv. Transl. Res. 2018, 8, 450–460. [Google Scholar] [CrossRef]









| Group | pDNA | Heat | GET | Voltage | Pulse Number | N |
|---|---|---|---|---|---|---|
| IO | Yes | No | No | NA | NA | 6 |
| IO + Heat | Yes | Yes | No | NA | NA | 6 |
| 35 V 72p | Yes | No | Yes | 35 | 72 | 6 |
| 35 V 72p + Heat | Yes | Yes | Yes | 35 | 72 | 6 |
| 45 V 36p | Yes | No | Yes | 45 | 36 | 6 |
| 45 V 36p + Heat | Yes | Yes | Yes | 45 | 36 | 6 |
| 45 V 72p | Yes | No | Yes | 45 | 72 | 6 |
| Group | pDNA | Heat | GET | Voltage | Pulse Number | N |
|---|---|---|---|---|---|---|
| IO | Yes | No | No | NA | NA | 6 |
| IO + Heat | Yes | Yes | No | NA | NA | 6 |
| 45 V 72p | Yes | No | Yes | 45 | 72 | 8 |
| 45 V 36p | Yes | No | Yes | 45 | 36 | 6 |
| 45 V 36p + 9 Fibers | Yes | Yes | Yes | 45 | 36 | 6 |
| 45 V 36p + 6 Fibers | Yes | Yes | Yes | 45 | 36 | 6 |
| 45 V 36p + 3 Fibers | Yes | Yes | Yes | 45 | 72 | 6 |
| Group | phFIX | Heat | Injection Route | Voltage | Pulse Number | N |
|---|---|---|---|---|---|---|
| IM 100 V/cm | Yes | Yes | Muscle | 23 & 50 * | 12 | 5 |
| 45 V 72p | Yes | No | Dermis | 45 | 72 | 5 |
| 45 V 36p + Heat | Yes | Yes | Dermis | 45 | 36 | 5 |
| 35 V 72p + Heat | Yes | Yes | Dermis | 35 | 72 | 5 |
| Group | pDNA | Heat | Injection Route | Voltage | Pulse Number | N |
|---|---|---|---|---|---|---|
| 2 Sites | Yes | Yes | Dermis | 45 | 36 | 5 |
| 3 Sites | Yes | Yes | Dermis | 45 | 36 | 5 |
| 4 Sites | Yes | Yes | Dermis | 45 | 36 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelblute, C.; Mangiamele, C.; Heller, R. Moderate Heat-Assisted Gene Electrotransfer as a Potential Delivery Approach for Protein Replacement Therapy through the Skin. Pharmaceutics 2021, 13, 1908. https://doi.org/10.3390/pharmaceutics13111908
Edelblute C, Mangiamele C, Heller R. Moderate Heat-Assisted Gene Electrotransfer as a Potential Delivery Approach for Protein Replacement Therapy through the Skin. Pharmaceutics. 2021; 13(11):1908. https://doi.org/10.3390/pharmaceutics13111908
Chicago/Turabian StyleEdelblute, Chelsea, Cathryn Mangiamele, and Richard Heller. 2021. "Moderate Heat-Assisted Gene Electrotransfer as a Potential Delivery Approach for Protein Replacement Therapy through the Skin" Pharmaceutics 13, no. 11: 1908. https://doi.org/10.3390/pharmaceutics13111908
APA StyleEdelblute, C., Mangiamele, C., & Heller, R. (2021). Moderate Heat-Assisted Gene Electrotransfer as a Potential Delivery Approach for Protein Replacement Therapy through the Skin. Pharmaceutics, 13(11), 1908. https://doi.org/10.3390/pharmaceutics13111908