Red Seaweed-Derived Compounds as a Potential New Approach for Acne Vulgaris Care
Abstract
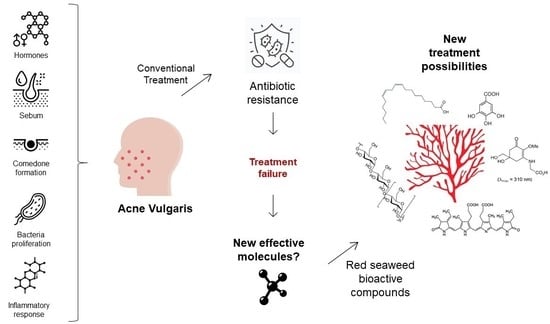
:1. Introduction
2. Research Methodology
3. Pathophysiological Targets for the Management of Acne Vulgaris
3.1. Hormonal Influence
3.2. Seborrhoea
3.3. Comedogenesis
3.4. Microbial Proliferation
3.5. Inflammatory Response
4. Seaweed Extracts and Compounds to Address AV Disease
4.1. Antibacterials from Red Macroalgae
| Red Macroalgal Species | Compound or Extract (Technique) | Concentration/ Volume Tested | Antibacterial Assay | Outcome | Reference |
|---|---|---|---|---|---|
| Cutibacterium acnes | |||||
| Asparagopsis armata | Supercritical extract | 5 mg·mL−1 | Disc Diffusion | Zone of inhibition 17.33 ± 0.58 mm | [88] |
| Supercritical extract | 10 mg·mL−1 | Disc Diffusion | Zone of inhibition 23.00 ± 1.00 mm | [88] | |
| Gracilaria textorii | EtOH (UE) | 0–1.024 μg·mL−1 | MIC | Inhibitory concentration > 1.024 μg·mL−1 | [100] |
| Gracilaria verrucosa | EtOH (UE) | 0–1.024 μg·mL−1 | MIC | Inhibitory concentration > 1.024 μg·mL−1 | [100] |
| Grateloupia angusta | EtOH (UE) | 0–1.024 μg·mL−1 | MIC | Inhibitory concentration > 512 μg·mL−1 | [100] |
| Grateloupia crispata | EtOH (UE) | 0–1.024 μg·mL−1 | MIC | Inhibitory concentration > 512 μg·mL−1 | [100] |
| Grateloupia elliptica | EtOH (UE) | 0–1.024 μg·mL−1 | MIC | Inhibitory concentration > 512 μg·mL−1 | [100] |
| Meristotheca papulosa | EtOH (UE) | 0–1.024 μg·mL−1 | MIC | Inhibitory concentration > 256 μg·mL−1 | [100] |
| Osmundaria serrata | Lanosol ethyl ether | 25 to 0.01 mg·mL−1 | MBC | Bactericidal concentration 0.50 ± 0.29 mg·mL−1 | [23] |
| Lanosol ethyl ether | 25 to 0.01 mg·mL−1 | MIC | Inhibitory concentration 0.08 ± 0.02 mg·mL−1 | [23] | |
| Plocamium telfairiae | EtOH (UE) | 0–1.024 μg·mL−1 | MIC | Inhibitory concentration > 256 μg·mL−1 | [88] |
| Sphaerococcus coronopifolius | 12S-hydroxy-bromosphaerol | 0.1–200 µM | IC50 | Inhibitory concentration 10.88 (7.83–15.12) µM | [106] |
| 12R-hydroxy-bromosphaerol | 0.1–200 µM | IC50 | Inhibitory concentration 8.75 (6.51–11.77) µM | [106] | |
| Bromosphaerol | 0.1–200 µM | IC50 | Inhibitory concentration 14.06 (10.41–19.00) µM | [106] | |
| Symphyocladia latiuscula | MeOH (SLE) | 1 mg·disc−1 | Disc Diffusion | Zone of inhibition 3.5 ± 1.3 mm | [83] |
| MeOH (SLE) | 5 mg·disc−1 | Disc Diffusion | Zone of inhibition 8.8 ± 0.8 mm | [83] | |
| MeOH (SLE) | 19.5 μg–10 mg·mL−1 | MIC | Inhibitory concentration 0.16 mg·mL−1 | [83] | |
| Staphylococcus epidermidis | |||||
| Asparagopsis taxiformis (Falkenbergia-phase) | MeOH (SLE) | 120 µL | Well Diffusion | Zone of inhibition 21 ± 2.31 mm | [107] |
| Bryothamnion seaforthii | Lectin | 125; 250 μg·mL−1 | Microdilution (Growth Inhib) | Inhibitory concentration 10–40% | [108] |
| Chondrus crispus | NA | NA | Piece of algae (3 cm) | Zone of inhibition 6.8 mm | [90] |
| MeOH (SLE) | 100 µ (of 4 mg·mL−1) | Disc Diffusion | Zone of inhibition 21 mm | [90] | |
| Cystoclonium purpureum | NA | NA | Piece of algae (3 cm) | Zone of inhibition 8.2 mm | [90] |
| Gracilaria corticata | MeOH:toluene 3:1 (SLE) | 120 µL | Well Diffusion | Zone of inhibition ± 4 mm | [109] |
| Gracillaria gracilis | Diethyl ether (SLE) | 25 µL | Disc Diffusion | Zone of inhibition 15 mm | [110] |
| Grinnellia americana | NA | NA | Piece of algae (3 cm) | Zone of inhibition 7 mm | [90] |
| Hypnea musciformis | Lectin | 250 μg·mL−1 | Microdilution (Growth Inhib) | Inhibitory concentration 10–40% | [108] |
| Hypnea pannosa | MeOH:toluene 3:1 (SLE) | 120 µL | Well Diffusion | Zone of inhibition ± 7.5 mm | [109] |
| Jania rubens | MeOH (SLE) | 2 mg·disc−1 | Disc Diffusion | Zone of inhibition 6.5 mm | [111] |
| MeOH (SLE) | 4 mg·disc−1 | Disc Diffusion | Zone of inhibition 11 mm | [111] | |
| Chloroform (SLE) | 4 mg·disc−1 | Disc Diffusion | Zone of inhibition 10 mm | [111] | |
| Laurencia majuscula | Elatol | 30 mg·disc−1 | Disc Diffusion | Zone of inhibition 19–24 mm * | [112] |
| Elatol | 1–4 mg·mL−1 | MIC | Inhibitory concentration 2 mg·mL−1 * | [112] | |
| Laurencia obtusa | Essential oil | 0.1 µL | Disc Diffusion | Zone of inhibition 9 mm | [78] |
| Essential oil | 0.2 µL | Disc Diffusion | Zone of inhibition 10 mm | [78] | |
| Essential oil | 0.4 µL | Disc Diffusion | Zone of inhibition 11 mm | [78] | |
| Chloroform (SLE) | 2 mg·disc−1 | Disc Diffusion | Zone of inhibition 6.5 mm | [78] | |
| Hexane (SLE) | 2 mg·disc−1 | Disc Diffusion | Zone of inhibition 6.5 mm | [78] | |
| Laurencia obtusa var. pyramidata | Chloroform (SLE) | 2 mg·disc−1 | Disc Diffusion | Zone of inhibition 6.5 mm | [78] |
| Hexane (SLE) | 2 mg·disc−1 | Disc Diffusion | Zone of inhibition 6.5 mm | [78] | |
| Laurencia sp. | MeOH (SLE) | 120 µL | Well Diffusion | Zone of inhibition 11 ± 2.56 mm | [107] |
| Plocamium angustum | Costatone C | 0.5–129 µg·mL−1 | MIC | Inhibitory concentration 64 µM | [113] |
| Polysiphonia fibrillosa | NA | NA | Piece of algae (3 cm) | Zone of inhibition 7.5 mm | [90] |
| Rhodomela confervoides | 3-bromo-4-[2,3-dibromo-4,5-dihydroxyphenyl] methyl-5-(hydroxymethyl) 1,2-benzenediol | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration 140 μg·mL−1 | [93] |
| 3-bromo-4-[2,3-dibromo-4,5-dihydroxyphenyl] methyl-5-(hydroxymethyl) 1,2-benzenediol | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration >140 μg·mL−1 * | [93] | |
| 3-bromo-4-[2,3-dibromo-4,5-dihydroxyphenyl] methyl-5-(ethoxymethyl) 1,2-benzenediol | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration 140 μg·mL−1 | [93] | |
| 3-bromo-4-[2,3-dibromo-4,5-dihydroxyphenyl] methyl-5-(ethoxymethyl) 1,2-benzenediol | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration 140 μg·mL−1 * | [93] | |
| 3-bromo-4-[2,3-dibromo-4,5-dihydroxyphenyl] methyl-5-(methoxymethyl) 1,2-benzenediol | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration 70 μg·mL−1 | [93] | |
| 3-bromo-4-[2,3-dibromo-4,5-dihydroxyphenyl] methyl-5-(methoxymethyl) 1,2-benzenediol | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration 70 μg·mL−1 * | [93] | |
| 4,40-methylenebis [5,6-dibromo-1,2-benzenediol] | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration 140 μg·mL1 | [93] | |
| 4,40-methylenebis [5,6-dibromo-1,2-benzenediol] | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration 140 μg·mL−1 * | [93] | |
| bis (2,3-dibromo-4,5-dihydroxybenzyl)ether | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration 35 μg·mL−1 | [93] | |
| bis (2,3-dibromo-4,5-dihydroxybenzyl)ether | 35–140 μg·mL−1 | Microdilution (MIC) | Inhibitory concentration 140 μg·mL−1 * | [93] | |
| Sphaerococcus coronopifolius | 12S-hydroxy-bromosphaerol | 0.1–200 µM | Microdilution (IC50) | Inhibitory concentration 10.07 (7.84–12.94) µM | [106] |
| Sphaerococcenol A | 0.1–200 µM | Microdilution (IC50) | Inhibitory concentration 56.58 (41.01–78.06) µM | [106] | |
| 12R-hydroxy-bromosphaerol | 0.1–200 µM | Microdilution (IC50) | Inhibitory concentration 5.61 (4.18–7.53) µM | [106] | |
| Bromosphaerol | 0.1–200 µM | Microdilution (IC50) | Inhibitory concentration 9.05 (7.05–11.63) µM | [106] | |
| Symphyocladia latiuscula | MeOH (SLE) | 19.5 μg·mL−1–10 mg·mL−1 | MIC | Inhibitory concentration 0.63 mg·mL−1 | [83] |
4.2. Anti-Inflammatory Extracts and Compounds from Red Macroalgae
| Red Macroalgal Species | Compound or Extract (Technique) | Concentration Tested | Anti-Inflammatory Assay | Outcome | Reference |
|---|---|---|---|---|---|
| Agardhiella ramosissima | Sulfated polyssacharide | 30 mg·kg−1 | Swiss mice: carrageenan, dextran, serotonin and histamine induced paw oedema; carrageenan induced peritonitis | Reduced neutrophil migration in peritonitis model; Reduced paw oedema | [121] |
| Amansia multifida | Ethanol:water 7:3 (SLE) | 2.5, 5, 10 mg·kg−1 | Swiss mice: carrageenan induced peritonitis; carrageenan induced paw oedema. | Reduced neutrophil migration in peritonitis model; Reduced paw oedema | [162] |
| Lectin | 0.1, 0.3, 1 mg·kg−1 | Swiss mice: carrageenan-induced peritonitis; carrageenan-, compound 48/80-, histamine- and PGE2-induced paw oedema | Inhibition of paw oedema for all stimulators, inhibition of neutrophil migration, increase in GSH levels, inhibition of TNF-α and IL-1β | [163] | |
| Asparagopsis taxiformis | Water (SLE) | n.d. | Enzymatic activity | COX-2 inhibition | [171] |
| Ethanol:water 96:4 (SLE) | 1 mg·mL−1 | Enzymatic activity | COX-2 inhibition | [172] | |
| Bryothamnion triquetrum | Lectin | 1, 5, 10 mg·kg−1 | Swiss mice: carrageenan induced peritonitis; carrageenan and dextran induced paw oedema | Reduction of oedema; Reduction of leukocyte infiltrations. IL-1β and TNF-α inhibition | [169] |
| Chondrus crispus | Lipid extract | 3 μg·mL−1 total fatty acids | LPS-stimulated THP-1 | TLR1, TLR2, TLR4, TLR8, TRAF5, TRAF6, TNFSF18, IL6R, IL23, CCR1, CCR4, CCL17, STAT3, MAP3K1 downregulation | [159] |
| Chondrus verrucosus | Polyssacharides | 100, 200, 400 µg·mL−1 | A23187-stimulated RBL-2H3 cells | Degranulation of basophils inhibition | [114] |
| Coelarthrum muelleri | MeOH (SLE) | n.d. | Carrageenan-induced rat paw oedema | Reduction of oedema | [164] |
| Delesseria sanguinea | Sulfated Polysaccharides | n.d. | Enzymatic activity | Elastase inhibition | [173] |
| Dichotomaria obtusata | MeOH (SLE) | 0.0005–2 mg·ear−1 12.5–100 mg·kg−1 | Cenpalab mice: croton oil induced ear oedema | Reduced ear oedema | [174] |
| Water (SLE) | 12.5, 25 and 50 mg·kg−1 | Cenpalab mice: TPA-induced ear oedema | Reduction of oedema | [175] | |
| Digenia simplex | Polysaccharide | 10, 30 and 60 mg·kg−1 | Swiss mice: carrageenan-induced peritonitis; carrageenan-dextran-, serotonin-, histamine- and bradykinin-induced paw oedema | Reduction of oedema; Reduction of leukocyte infiltrations; Inhibition of IL-1β and TNF-α | [116] |
| Eucheuma cottonii | MeOH:Water 1:1 (SLE) | 150, 300 mg·kg−1 | Sprague-Dawley rats: ovalbumin induced asthma | Reduced lung inflammation and blood cells migration and positively modulated several inflammatory markers | [165] |
| MeOH:Water 1:1 (SLE) | 150, 300 mg·kg−1 | Sprague-Dawley rats: SRBC induced paw oedema | Pro-inflammatory at 150 mg·kg−1 Anti-inflammatory at 300 mg·kg−1 | [165] | |
| Eucheuma denticulatum | Ethanol (SLE) and SPE fractions | 1–100 µg·mL−1 | TNF-γ- and LPS-stimulated RAW264.7 | Non-inflammatory morphology conserved; NO, TNF-α, IL-1β, IL-6 and MCP-1 inhibition | [176] |
| Gelidium amansii | Cellulose microfibril | n.d. | HaCaT | JNK1/2 and p38 inhibition | [119] |
| Cellulose nanocrystal | n.d. | UVB-stimulated HaCaT | AP-1, COX-2, c-Jun translocation inhibition; phosphorilation of ERK1/2/B-Raf, JNK1/2/MKK4/7, Akt and EGFR inhibition | [120] | |
| Cellulose nanocrystal | 40 and 200 mg·kg−1 | UVB-stimulated mice | Epidermal thickening and COX-2 inhibition | [120] | |
| Hot water (SLE) partitioned with Ethanol | n.d. | LPS-stimulated RAW264.7 | Reduced TNF-α, IL-1β and IL-6 | [177] | |
| Gelidium crinale | Sulfated galactan | 0.01, 0.1 and 1 mg·kg−1 | Wistar rats: several stimulatory agents of paw oedema | Reduced paw oedema | [126] |
| Gelidium pacificum | Sulfated Polysaccharides | 0–300 µg·mL−1 | LPS-stimulated THP-1 | NO, TLR4, MyD88 and TRAF6 inhibition | [127] |
| Gelidium sesquipedale | Ethanol (SLE) | 1 mg·mL−1 | Enzymatic activity | COX-2 inhibition | [178] |
| Gloiopeltis furcata | Ethyl acetate (SLE) | 50 µg·mL−1 | LPS-stimulated RAW264.7 | NO, PGE2, IL-6, TNF-α inhibition | [161] |
| Gracilaria birdiae | Sulfated Polysaccharide | 5, 10, 20 mg·kg−1 | Wistar rats: carrageenan-induced peritonitis; carrageenan- and dextran-induced paw oedema | Reduced paw oedema and leukocyte migration | [122] |
| Gracilaria caudata | Sulfated Polysaccharides | 2.5, 5 and 10 mg·kg−1 | Swiss mice: carrageenan, dextran, bradykinin and histamine paw oedema; carrageenan induced peritonitis | Reduction of oedema (some inducers). Reduction of leukocyte infiltrations. IL-1β and TNF-α inhibition | [123] |
| Gracilaria changii | MeOH (SLE) | 10 µg·mL−1 | PMA-differentiated U937 | Inhibition of TNF-α and IL-6 | [179] |
| Gracilaria cornea | Sulfated Polysaccharides | 3, 9, 27 mg·kg−1 | Wistar rats: carrageenan-induced peritonitis; carrageenan and dextran induced paw oedema | Leukocyte infiltration and oedema reduction. | [124] |
| Gracilaria lemaneiformis | Agaro-oligosaccharides | 12.5, 25, 50 µg·mL−1 | LPS-stimulated RAW264.7 | NO, PGE2, COX-2, TNF-α, IL-1β and IL-6 inhibition | [117] |
| Agaro-oligosaccharides | 12.5, 25, 50 µg·mL−1 | LPS-stimulated zebrafish embryo | NO and ROS inhibition | [117] | |
| Gracilaria opuntia | Sulfated galactan | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [129] |
| 2-acetoxy-2-(5-acetoxy-4-methyl-2-oxotetrahydro-2H-pyran-4-yl)ethyl 4-(3-methoxy-2-(methoxymethyl)-7-methyl-3,4,4a,7,8,8a-hexahydro-2H-chromen-4-yloxy)-5-methylheptanoate | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [144] | |
| 3-(2-ethyl-6-((3Z,7Z)-1,2,5,6-tetrahydroazocin-5-yl)hexyl) morpholin-6-one | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [146] | |
| 2-(3-ethyl-9-(2-methoxyethoxy)-1-oxo-2,3,4,9-tetrahydro-1H-xanthen-2-yl) ethyl-5-hydroxy-9-methoxy-7,8-dimethyl-8-(5-methylfuran-2-yl) nona-3,6-dienoate | n.d. | Enzymatic activity | Inhibition of 5-LOX | [145] | |
| Gracilaria salicornia | Ethyl acetate:MeOH 1:1 (SLE) | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [143] |
| Methyl-16(13–>14)-abeo-7-labdene-(12-oxo) carboxylate | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [143] | |
| 4′-[10′-[7-hydroxy-2,8-dimethyl-6-(pentyloxy)-2H-chromen-2-yl]ethyl]-3′,4′-dimethyl-cyclohexanone | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [180] | |
| 3′-[10′-(8-hydroxy-5-methoxy-2,6,7-trimethyl-2H-chromen-2-yl)ethyl]-3′-methyl-2′-methylene cyclohexyl butyrate | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [180] | |
| Salicornolides A-C | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [142] | |
| Gracilaria sp. | Lipid extract | 100 µg·mL−1 | LPS-stimulated RAW264.7 | NO inhibition | [181] |
| Ethanol (SLE) | 5 and 10% (w/w) of cream | UVB-irradiated mice | Reduction of epidermal erosion and thickening induced by UVB radiation | [182] | |
| Gracilaria verrucosa | (5Z,13E)-(8R,12R,15S)-15-Hydroxy-9-oxoprosta-5,13-dienoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] |
| Methyl-(5Z,13E)-(8R,12R,15S)-15-hydroxy-9-oxoprosta-5,13-dienoate | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| (E)-(8R,12R,15S)-15-Hydroxy-9-oxoprost-13-enoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| (Z)-(8R,12S)-9,15-Dioxoprost-5-enoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| (2R,3S)-2-Formamido-1,3-dihydroxyoctadecane | 20 µg·mL−1 | LPS-stimulated RAW264.7 | IL-6 reduction | [139] | |
| (E)-9-Oxohexadec-10-enoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| 10-Oxohexadecanoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| (E)-(S)-10-Hydroxyhexadec-8-enoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| (E)-10-Oxooctadec-8-enoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| (E)-9-Oxooctadec-10-enoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| (E)-(R)-10-Hydroxyoctadec-8-enoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| 10-Oxooctadecanoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| 11-Oxooctadecanoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| 12-Oxooctadecanoic acid | 20 µg·mL−1 | LPS-stimulated RAW264.7 | NO, TNF-α, IL-6 reduction | [139] | |
| Grateloupia elliptica | Ethyl acetate (SLE) | 50 µg·mL−1 | LPS-stimulated RAW264.7 | NO, PGE2, IL-6, TNF-α inhibition | [161] |
| Grateloupia lanceolata | Ethanol:water 7:3 (SLE) | 0–100 µg·mL−1 | LPS-stimulated RAW264.7 | NO, IL-1Β, p38 MAPK/ERK/JNK and NF-κB inhibition | [154] |
| Grateloupia turuturu | Lipids | 12.5–250 µg·mL−1 | Enzymatic activity | COX-2 inhibition | [183] |
| Hydroethanolic and water SLEs | 0.02–0.2 mg·mL−1 | LPS-stimulated RAW264.7 | NO inhibition | [184] | |
| Hypnea cervicornis | Agglutinin | 0.3–3 mg·kg−1 | Wistar rats: Zymosan-induced arthritis | Reduced leukocyte influx. iNOS and TNF-α inhibition. | [168] |
| Agglutinin | 0.1–10 mg·kg−1 | Wistar rats: carrageenan-, ovalbumin- and PGE2-induced inflammation | Inhibition of neutrophil migration; increase in NO | [185] | |
| Hypnea musciformis | Sulfated polysaccharide | 10 mg·kg−1 | Swiss mice: carrageenan-induced peritonitis; carrageenan- and dextran-induced paw oedema | Reduced leukocyte influx; Reduced paw oedema; IL-1β inhibition | [125] |
| Kappaphycus alvarezii | Sulfated galactan | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [129] |
| (3S, 4R, 5S, 6Z)-3-((R)-hexan-2′-yl)-3,4,5,8-tetrahydro-4-methyl-2H-oxocin-5-yl acetate | n.d. | Enzymatic activity | Inhibition of 5-LOX | [186] | |
| 2-ethyl-6-(4-methoxy-2-((2-oxotetrahydro-2H-pyran-4-yl)methyl)butoxy)-6-oxohexyl 5-ethyloct-4-enoate | n.d. | Enzymatic activity | Inhibition of 5-LOX | [187] | |
| 4-(2-chloroethyl)-5-7-(methoxymethyl) undec-3-enyl) cyclooct-4-enone | n.d. | Enzymatic activity | Inhibition of COX-2 and 5-LOX | [188] | |
| Laurencia glandulifera | Neorogioltriol | 0.5–1 mg·kg−1 | Rats: carrageenan induced paw oedema | Reduction of paw oedema | [141] |
| Neorogioltriol | 12.5–62.5 µM | LPS-stimulated RAW264.7 | NO, COX-2, TNF-α and NF- kB inhibition | [141] | |
| Laurencia okamurae | Ethyl acetate (SLE) | 25 µg·mL−1 | LPS-stimulated RAW264.7 | NO, PGE2, IL-6, TNF-α inhibition | [161] |
| Laurencia snackeyi | 5β-hydroxypalisadin B | 0.25, 0.1 and 1 µg·mL−1 | LPS-induced zebrafish embryo | NO and ROS inhibition; Improved survival, heart rate and yolk sac oedema size | [189] |
| Lithothamnion muelleri | Whole seaweed | 1% (w/w) in diet | GVHD mice model | Reduced IFN-γ, TNF-α, CCL2, CCL3, CCL5. | [190] |
| Melanothamnus afaqhusainii | MeOH (SLE) | n.d. | Carrageenan- induced rat paw oedema | Reduction of oedema | [164] |
| Palmaria palmata | Ethyl acetate LLE of MeOH:Chloroform (SLE) | n.d. | LPS-stimulated RAW264.7 | NO and iNOS inhibition | [191] |
| Thermolysin-digested water extract | 100–1000 µg·mL−1 | LPS-stimulated RAW264.7 and carrageenan-induced paw oedema | Reduction of NO, TNF-α and IL-6; Reduction of paw oedema | [157] | |
| Phenolic Extract (LLE of MeOH SLE) | 25, 50 and 100 µg·mL−1 | LPS-stimulated primary human neutrophils | Reduction of ROS, NO, MPO, IL-8, IL-1β, IL-6 and TNF-α; Downregulation of TLR4 | [158] | |
| Lipid extract | 3 μg·mL−1 total fatty acids | LPS-stimulated THP-1 | TLR1, TLR2, TLR4, TLR8, TRAF5, TRAF6, TNFSF18, IL6R, IL23, CCR1, CCR4, CCL17, STAT3, MAP3K1 downregulation; IL-6 and IL-8 inhibition | [159] | |
| Polysiphonia morrowii | Bis (3-bromo-4,5-dihydroxybenzyl) ether | 0.1, 1, 2 µM | LPS-stimulated RAW264.7 | NO, iNOS, COX-2, PGE2, TNF-α, IL-6 and IL-1β inhibition | [192] |
| 3-bromo-5-(ethoxymethyl)-1,2-benzenediol | 12.5–50 µM | LPS-stimulated RAW264.7 and Zebrafish embryos | NO, ROS, iNOS, COX-2 and NF-κB inhibition | [155] | |
| Porphyra columbina | Protein fraction | n.d. | Several cell lines | IL-10 elicitation; pro-inflammatory cytokines inhibition | [193] |
| Porphyra dentata | MeOH (SLE) | 25, 50, 100, 200 µg·mL−1 | LPS-stimulated RAW264.7 | NO reduction | [150] |
| MeOH (SLE) | 50, 100, 200 µg·mL−1 | LPS-stimulated RAW264.7 | iNOS inhibition | [150] | |
| MeOH (SLE) | 200 µg·mL−1 | LPS-stimulated RAW264.7 | NF-κB inhibition | [150] | |
| Catechol | 6 µg·mLl−1 | LPS-stimulated RAW264.7 | NO reduction | [150] | |
| Catechol | 1–11 µg·mL−1 | LPS-stimulated RAW264.7 | iNOS inhibition | [150] | |
| Catechol | 11 µg·mL−1 | LPS-stimulated RAW264.7 | NF-κB inhibition | [150] | |
| Rutin | 250 µg·mL−1 | LPS-stimulated RAW264.7 | NO reduction | [150] | |
| Rutin | 80–250 µg·mL−1 | LPS-stimulated RAW264.7 | iNOS inhibition | [150] | |
| Rutin | 250 µg·mL−1 | LPS-stimulated RAW264.7 | NF-κB inhibition | [150] | |
| Hesperidin | 250 µg·mL−1 | LPS-stimulated RAW264.7 | NO reduction | [150] | |
| Porphyra dioica | Lipid extract | 3 μg·mL−1 total fatty acids | LPS-stimulated THP-1 | TLR1, TLR2, TLR4, TLR8, TRAF5, TRAF6, TNFSF18, IL6R, IL23, CCR1, CCR4, CCL17, STAT3, MAP3K1 downregulation | [159] |
| Porphyra sp. | Shinorine | 12.5–200 µg·mL−1 | LPS-stimulated THP-1 and THP-1-Blue | NF-κB increase and IDO-1 suppression | [151] |
| Porphyra-334 | 12.5–200 µg·mL−1 | LPS-stimulated THP-1 and THP-1-Blue | NF-κB inhibition and IDO-1 suppression | [151] | |
| Porphyra tenera | Several enzymatic extracts | 62.5, 125 and 250 μg·mL−1 | LPS-stimulated RAW264.7 | NO reduction | [194] |
| Porphyra umbilicalis | Hydroethanolic and water SLEs | 0.005–0.02 mg·mL−1 | LPS-stimulated RAW264.7 | NO reduction | [184] |
| Porphyra vietnamensis | MeOH:Water 4:1 (Soxhlet) | 200 mg·kg−1 | Wistar rats: Carrageenan-induced paw oedema | Reduction of paw oedema | [115] |
| Precipitated polysaccharide | 250 mg·kg−1 | Wistar rats: Carrageenan-induced paw oedema | Reduction of paw oedema | [115] | |
| Porphyra yezoensis | Aqueous protein extract (SLE) | 25, 50, 100 µg·mL−1 | HK2 | MAPK and NF-κB downregulation | [152] |
| MAAs (EtOH, SLE) | 5, 10, 20 µg·mL−1 | Male ICR mice (skin) | IL-1β, IL-6, IL-10, NF-κB expression reduction | [153] | |
| Porphyridium cruentum | Sulfoglycolipid fraction | n.d. | Activated peritoneal mono nuclear cells from Wistar rats | Inhibition of Superoxide generation | [195] |
| Porphyridium sp. | Polysaccharide | 50–500 µg·mL−1 | HCAEC induced with angiotensin II | Inhibition of adhesion molecules and NF-κB expression; increase in aantioxidant system activity | [118] |
| Polysaccharide | 0.005–1% w/v | fMLP-stimulted PMNs | Inhibition of PMN chemotaxis | [196] | |
| Pterocladiella capillacea | Ethanol (SLE) | 1 mg·mL−1 | Enzymatic activity | COX-2 inhibition | [178] |
| Lectin | 8.1 mg·kg−1 | Wistar rats: carrageenan-induced paw oedema and peritonitis | Reduced paw oedema and leukocyte migration. | [170] | |
| Pyropia yezoensis | MeOH (SLE) | 40, 200, 1000 µg·mL−1 | HaCaT induced with IFN-ϒ | TARC and MDC expression inhibition | [156] |
| MeOH (SLE) | 40, 200, 1000 µg·mL−1 | HaCaT induced with TNF-α | TARC and MDC expression inhibition | [156] | |
| MeOH (SLE) | 40, 200, 1000 µg·mL−1 | HaCaT induced with IFN-ϒ | ERK, JNK, p38 inhibition | [156] | |
| MeOH (SLE) | 40 µg·mL−1 | HaCaT induced with TNF-α | ERK inhibition | [156] | |
| MeOH (SLE) | 1000 µg·mL−1 | HaCaT induced with TNF-α | JNK and p38 inhibition | [156] | |
| MeOH (SLE) | 40, 200, 1000 µg·mL−1 | HaCaT induced with IFN-ϒ | NF-κB and IkB-α inhibition | [156] | |
| Peptide PPY1 | 250–1000 ng·mL−1 | LPS-stimulated RAW264.7 | NO, ROS, iNOS, COX-2, IL-1β and TNF-α inhibition; p38 and MAPK downregulation | [160] | |
| Porphyran | 0–100 µg·mL−1 | C57BL/6 mice derived, LPS-stimulated BDMCs | Supression of CCR7, IL-6, IL-12 and TNF-α expression | [134] | |
| Porphyran | 0–100 mg·kg−1 | LPS-stimulated C57BL/6 mice | Supression of Th1 and Tc1 cells differentiation | [134] | |
| Sarcodia ceylanica | Ethyl acetate LLE of Ethanol (95% v/v) SLE | 10, 20, 50 µg·mL−1 | LPS-stimulated RAW264.7 | iNOS and COX-2 inhibition | [166] |
| Ethyl acetate LLE of Ethanol (95% v/v) SLE | 20, 50 mg·kg−1 | Wistar rats: carrageenan-induced paw oedema | Reduced paw oedema | [166] | |
| Solieria filiformis | Lectin | 1, 3, 9 mg·kg−1 | Wistar rats: carrageenan induced peritonitis; carrageenan, dextran, bradykinin, histamin and serotonin induced paw oedema. | Reduced neutrophil migration in peritonitis model; Reduced paw oedema | [167] |
| Lectin | 10 µg·mL−1 | BALB/c mice splenocytes | IL-6 and IL-10 production (Th2 immune-response stimulators) | [197] | |
| Sulfated polysaccharide | 0.03, 0.3, 3 mg·kg−1 | Wistar rats: formalin induced TMJ inflammation | Reduced IL-1β and TNF-α | [128] | |
| Solieria robusta | MeOH (SLE) | n.d. | Carrageenan- induced rat paw oedema | Reduction of oedema | [164] |
| Tichocarpus crinitus | Kappa/beta Carrageenan | n.d. | Human blood cells | Increase in IL-10 | [137] |
| Vidalia obtusaloba | Vidalols A and B | n.d. | PMA-induced mouse ear oedema; enzymatic activity | Reduction of oedema and inhibition of Phospholipase A2 | [198] |
4.3. Extracts and Compounds from Red Macroalgae Targeting Other Mechanisms of AV
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adamski, Z.; Gornowicz-Porowska, J.; Sobkowska, D.; Kaszuba, K.; Czajkowski, R. Acne–Therapeutic challenges to the cooperation between a dermatologist and a cosmetologist. Adv. Dermatol. Allergol. 2021, 38, 21–31. [Google Scholar] [CrossRef]
- Patel, D.J.; Bhatia, N. Oral Antibiotics for Acne. Am. J. Clin. Dermatol. 2021, 22, 193–204. [Google Scholar] [CrossRef]
- Tan, J.; Bhate, K. A global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172, 3–12. [Google Scholar] [CrossRef]
- Aydemir, E.H. Acne vulgaris. Türk Pediatri Arşivi 2017, 49, 13–16. [Google Scholar] [CrossRef]
- Kim, S.; Park, T.H.; Kim, W.I.; Park, S.; Kim, J.H.; Cho, M.K. The effects of green tea on acne vulgaris: A systematic review and meta-analysis of randomized clinical trials. Phytotherapy Res. 2021, 35, 374–383. [Google Scholar] [CrossRef]
- Arora, M.K.; Yadav, A.; Saini, V. Role of hormones in acne vulgaris. Clin. Biochem. 2011, 44, 1035–1040. [Google Scholar] [CrossRef]
- Greydanus, D.E.; Azmeh, R.; Cabral, M.D.; Dickson, C.A.; Patel, D.R. Acne in the first three decades of life: An update of a disorder with profound implications for all decades of life. Dis. Mon. 2021, 67, 101103. [Google Scholar] [CrossRef]
- Adler, B.L.; Kornmehl, H.; Armstrong, A.W. Antibiotic Resistance in Acne Treatment. JAMA Dermatol. 2017, 153, 810–811. [Google Scholar] [CrossRef]
- Karadag, A.S.; Kayıran, M.A.; Wu, C.; Chen, W.; Parish, L.C. Antibiotic resistance in acne: Changes, consequences and concerns. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; del Rosso, J.Q.; Baum, E.W.; Mcguigan, K.A. The Use of Isotretinoin in the Treatment of Acne Vulgaris Clinical Considerations and Future Directions. J. Clin. Aesthetic Dermatol. 2014, 7, S3. [Google Scholar]
- Tan, A.; Schlosser, B.; Paller, A. A review of diagnosis and treatment of acne in adult female patients. Int. J. Women’s Dermatol. 2018, 4, 56–71. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Pereira, A.; Fraga-Corral, M.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Carpena, M.; Prieto, M.; Simal-Gandara, J. The Use of Invasive Algae Species as a Source of Secondary Metabolites and Biological Activities: Spain as Case-Study. Mar. Drugs 2021, 19, 178. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2011; Volume 65, pp. 115–217. [Google Scholar]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-Like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2019, 25, 5512–5527. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Di Tomo, P.; Canali, R.; Ciavardelli, D.; Di Silvestre, S.; De Marco, A.; Giardinelli, A.; Pipino, C.; Di Pietro, N.; Virgili, F.; Pandolfi, A. β-Carotene and lycopene affect endothelial response to TNF-α reducing nitro-oxidative stress and interaction with monocytes. Mol. Nutr. Food Res. 2012, 56, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Biochem. 2011, 151, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Etahiri, S.; Bultel-Poncé, V.; Caux, C.; Guyot, M. New bromoditerpenes from the red alga Sphaerococcus coronopifolius. J. Nat. Prod. 2001, 64, 1024–1027. [Google Scholar] [CrossRef]
- Darias, J.; Rovirosa, J.; Martin, A.S.; Díaz, A.-R.; Dorta, A.E.; Cueto, M. Furoplocamioids A–C, Novel Polyhalogenated Furanoid Monoterpenes from Plocamium cartilagineum. J. Nat. Prod. 2001, 64, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.; Meyer, J. Isolation and antimicrobial activity of a lanosol derivative from Osmundaria serrata (Rhodophyta) and a visual exploration of its biofilm covering. S. Afr. J. Bot. 2006, 72, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Kavita, K.; Singh, V.K.; Jha, B. 24-Branched Δ5 sterols from Laurencia papillosa red seaweed with antibacterial activity against human pathogenic bacteria. Microbiol. Res. 2014, 169, 301–306. [Google Scholar] [CrossRef]
- Amorim, R.D.N.D.S.; Rodrigues, J.A.G.; Holanda, M.; Quinderé, A.L.G.; de Paula, R.; Melo, V.; Benevides, N.M.B. Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed Gracilaria ornata. Braz. Arch. Biol. Technol. 2012, 55, 171–181. [Google Scholar] [CrossRef]
- Stabili, L.; Acquaviva, M.; Biandolino, F.; Cavallo, R.; De Pascali, S.; Fanizzi, F.; Narracci, M.; Petrocelli, A.; Cecere, E. The lipidic extract of the seaweed Gracilariopsis longissima (Rhodophyta, Gracilariales): A potential resource for biotechnological purposes? New Biotechnol. 2012, 29, 443–450. [Google Scholar] [CrossRef]
- Thiboutot, D.; Dréno, B.; Sanders, V.; Rueda, M.J.; Gollnick, H. Changes in the management of acne: 2009–2019. J. Am. Acad. Dermatol. 2020, 82, 1268–1269. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, I.; Nakase, K. Recent advances in understanding and managing acne. F1000Research 2020, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. Endocrinology and immunology of acne: Two sides of the same coin. Exp. Dermatol. 2020, 29, 840–859. [Google Scholar] [CrossRef]
- Dawson, A.L.; Dellavalle, R. Acne vulgaris. BMJ 2013, 346, f2634. [Google Scholar] [CrossRef]
- Araviiskaia, E.; Dréno, B. The role of topical dermocosmetics in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.; Latief, I.; Hassan, I. Update on etiopathogenesis and treatment of Acne. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Beylot, C.; Auffret, N.; Poli, F.; Claudel, J.-P.; Leccia, M.; Del Giudice, P.; Dreno, B. Propionibacterium acnes: An update on its role in the pathogenesis of acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 271–278. [Google Scholar] [CrossRef]
- Dréno, B.; Pécastaings, S.; Corvec, S.; Veraldi, S.; Khammari, A.; Roques, C. Cutibacterium acnes(Propionibacterium acnes) and acne vulgaris: A brief look at the latest updates. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gollnick, H.P.M. From new findings in acne pathogenesis to new approaches in treatment. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Knutsen-Larson, S.; Dawson, A.L.; Dunnick, C.A.; Dellavalle, R. Acne Vulgaris: Pathogenesis, Treatment, and Needs Assessment. Dermatol. Clin. 2012, 30, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, C.; Chen, Z.; Zhou, C.; Gan, Y.; Jia, Y. A review of the role of sebum in the mechanism of acne pathogenesis. J. Cosmet. Dermatol. 2017, 16, 168–173. [Google Scholar] [CrossRef]
- Tuchayi, S.M.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.P.D. Acne vulgaris. Nat. Rev. Dis. Prim. 2015, 1, 1–20. [Google Scholar] [CrossRef]
- Cappel, M. Correlation Between Serum Levels of Insulin-like Growth Factor 1, Dehydroepiandrosterone Sulfate, and Dihydrotestosterone and Acne Lesion Counts in Adult Women. Arch. Dermatol. 2005, 141, 333–338. [Google Scholar] [CrossRef]
- Rocha, M.A.; Bagatin, E. Adult-onset acne: Prevalence, impact, and management challenges. Clin. Cosmet. Investig. Dermatol. 2018, 11, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Belgorosky, A.; Guercio, G.; Pepe, C.; Saraco, N.; Rivarola, M. Genetic and Clinical Spectrum of Aromatase Deficiency in Infancy, Childhood and Adolescence. Horm. Res. Paediatr. 2009, 72, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C.C. An update on the role of the sebaceous gland in the pathogenesis of acne. Dermato. Endocrinol. 2011, 3, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Bohl, C.E.; Dalton, J.T. Chemistry and Structural Biology of Androgen Receptor. Chem. Rev. 2005, 105, 3352–3370. [Google Scholar] [CrossRef] [Green Version]
- Balachandrudu, B.; Niveditadevi, V.; Rani, T.P. Hormonal Pathogenesis of Acne–Simplified. Int. J. Sci. Study 2015, 3, 183–185. [Google Scholar] [CrossRef]
- Hu, T.; Wei, Z.; Ju, Q.; Chen, W. Sex hormones and acne: State of the art. J. der Dtsch. Dermatol. Ges. 2021, 19, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Szöllősi, A.G.; Oláh, A.; Bíró, T.; Tóth, B.I. Recent advances in the endocrinology of the sebaceous gland. Dermato-Endocrinology 2017, 9, e1361576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnik, B.C. Acne vulgaris: The metabolic syndrome of the pilosebaceous follicle. Clin. Dermatol. 2018, 36, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Bagatin, E.; De Freitas, T.H.P.; Rivitti-Machado, M.C.; Ribeiro, B.M.; Nunes, S.; Da Rocha, M.A.D. Adult female acne: A guide to clinical practice. An. Bras. de Dermatol. 2019, 94, 62–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, T.; Ahmed, M.; Preece, M.; Hindmarsh, P.; Dunger, D. The relationship between Insulin-like Growth Factor 1, sex steroids and timing of the pubertal growth spurt. Clin. Endocrinol. 2015, 82, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Regidor, P.-A. The clinical relevance of progestogens in hormonal contraception: Present status and future developments. Oncotarget 2018, 9, 34628–34638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammes, S.R.; Levin, E.R. Impact of estrogens in males and androgens in females. J. Clin. Investig. 2019, 129, 1818–1826. [Google Scholar] [CrossRef] [Green Version]
- Lephart, E.D.; Naftolin, F. Menopause and the Skin: Old Favorites and New Innovations in Cosmeceuticals for Estrogen-Deficient Skin. Dermatol. Ther. 2021, 11, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Watanabe, S. 17β-estradiol Inhibits the Production of Interferon-induced Protein of 10kDa by Human Keratinocytes. J. Investig. Dermatol. 2003, 120, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivers, K.-Y.; Amador, N.; Abrams, L.; Hunter, D.; Jenab, S.; Quiñones-Jenab, V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic–pituitary–adrenal axis activity. Cytokine 2015, 72, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, A.J.; Guyre, P.M.; Pioli, P.A. Estradiol Suppresses NF-κB Activation through Coordinated Regulation of let-7a and miR-125b in Primary Human Macrophages. J. Immunol. 2010, 184, 5029–5037. [Google Scholar] [CrossRef]
- Rao, A.; Douglas, S.; Hall, J. Endocrine Disrupting Chemicals, Hormone Receptors, and Acne Vulgaris: A Connecting Hypothesis. Cells 2021, 10, 1439. [Google Scholar] [CrossRef]
- Straub, R.H. The Complex Role of Estrogens in Inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanghetti, E.A. The Role of Inflammation in the Pathology of Acne. J. Clin. Aesthetic Dermatol. 2013, 6, 27–35. [Google Scholar]
- Clayton, R.; Göbel, K.; Niessen, C.; Paus, R.; Van Steensel, M.; Lim, X. Homeostasis of the sebaceous gland and mechanisms of acne pathogenesis. Br. J. Dermatol. 2019, 181, 677–690. [Google Scholar] [CrossRef]
- Chen, F.; Hu, X.; Dong, K. Consistency changes of potential lipid markers in acne patients of different ages and their role in acne pathogenesis. J. Cosmet. Dermatol. 2021, 20, 2031–2035. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond acne: Current aspects of sebaceous gland biology and function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef]
- Zouboulis, C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 527–532. [Google Scholar] [CrossRef]
- Bakry, O.A.; El Farargy, S.M.; Kady, N.N.E.D.E.; Abu Dawy, H.F. Immunohistochemical Expression of Cyclo-oxygenase 2 and Liver X Receptor-α in Acne Vulgaris. J. Clin. Diagn. Res. 2017, 11, WC01–WC07. [Google Scholar] [CrossRef] [PubMed]
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am. J. Clin. Dermatol. 2020, 21, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two Major Sentinels of Skin Microbiota and the Influence of Cosmetics. Microorg. 2020, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Claudel, J.-P.; Auffret, N.; Leccia, M.-T.; Poli, F.; Corvec, S.; Dréno, B. Staphylococcus epidermidis: A Potential New Player in the Physiopathology of Acne? Dermatology 2019, 235, 287–294. [Google Scholar] [CrossRef]
- Mustarichie, R.; Sulistyaningsih, S.; Runadi, D. Antibacterial Activity Test of Extracts and Fractions of Cassava Leaves (Manihot esculenta Crantz) against Clinical Isolates of Staphylococcus epidermidis and Propionibacterium acnes Causing Acne. Int. J. Microbiol. 2020, 2020, 1975904. [Google Scholar] [CrossRef] [Green Version]
- Mayslich, C.; Grange, P.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Leyden, J. How does our increased understanding of the role of inflammation and innate immunity in acne impact treatment approaches? J. Dermatol. Treat. 2016, 27, 1. [Google Scholar] [CrossRef]
- Harvey, A.; Huynh, T.T. Inflammation and acne: Putting the pieces together. J. drugs Dermatol. JDD 2014, 13, 459–463. [Google Scholar]
- Abdi, F.; Kashani, H.H.; Naeini, F.F.; Narimani, T.; Khorvash, F. Staphylococcus aureus in acne pathogenesis: A case-control study. N. Am. J. Med Sci. 2012, 4, 573–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Saif, S.S.A.-L.; Abdel-Raouf, N.; El-Wazanani, H.A.; Aref, I.A. Antibacterial substances from marine algae isolated from Jeddah coast of Red sea, Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Águila-Ramírez, R.N.; Arenas-gonzález, A.; Hernández-guerrero, C.J.; González-acosta, B.; Borges-souza, J.M.; Véron, B.; Pope, J.; Hellio, C. Antimicrobial and Antifouling Activities Achieved by Extracts of Seaweeds from Gulf of California, Mexico. Actividades Antimicrobiana y Anti-Incrustante Obtenidas de Los Extractos de Algas Marinas Del Golfo de California, México. Hidrobiologica 2012, 22, 8–15. [Google Scholar]
- Bianco, É.M.; De Oliveira, S.Q.; Rigotto, C.; Tonini, M.L.; Guimarães, T.D.R.; Bittencourt, F.; Gouvêa, L.P.; Aresi, C.; De Almeida, M.T.R.; Moritz, M.I.G.; et al. Anti-Infective Potential of Marine Invertebrates and Seaweeds from the Brazilian Coast. Molecules 2013, 18, 5761–5778. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, I.; Cotas, J.; Rodrigues, A.; Ferreira, D.; Osório, N.; Pereira, L. Extraction and Analysis of Compounds with Antibacterial Potential from the Red Alga Grateloupia turuturu. J. Mar. Sci. Eng. 2019, 7, 220. [Google Scholar] [CrossRef] [Green Version]
- Félix, C.; Félix, R.; Carmona, A.; Januário, A.; Dias, P.; Vicente, T.; Silva, J.; Alves, C.; Pedrosa, R.; Novais, S.; et al. Cosmeceutical Potential of Grateloupia turuturu: Using Low-Cost Extraction Methodologies to Obtain Added-Value Extracts. Appl. Sci. 2021, 11, 1650. [Google Scholar] [CrossRef]
- Demirel, Z.; Yilmaz-Koz, F.F.; Karabay-Yavasoglu, N.U.; Ozdemir, G.; Sukatar, A. Antimicrobial and Antioxidant Activities of Solvent Extracts and the Essential Oil Composition of Laurencia Obtusa and Laurencia Obtusa Var. Pyramidata. Rom. Biotechnol. Lett. 2011, 16, 5927–5936. [Google Scholar]
- Ertürk, Ö.; Tas, B. Antibacterial and Antifungal Effects of Some Marine Algae. Kafkas Univ. Vet. Fak. Derg. 2011, 17, 121–124. [Google Scholar]
- Ghania, A.; Nabila, B.-B.; Larbi, B.; Elisabeth, M.; Philippe, G.; Mariem, B.; Khadidja, K.-K.; Wacila, B.-R.; Fawzia, A.-B. Antimicrobial and antiparasitic activities of three algae from the northwest coast of Algeria. Nat. Prod. Res. 2017, 33, 742–745. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.M.; Alotaibi, B.S.; El-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- Choi, J.-S.; Bae, H.-J.; Kim, S.-J.; Choi, I.S. In vitro antibacterial and anti-inflammatory properties of seaweed extracts against acne inducing bacteria, Propionibacterium acnes. J. Environ. Biol. 2011, 32, 313–318. [Google Scholar] [PubMed]
- Gribble, G.W. The natural production of organobromine compounds. Environ. Sci. Pollut. Res. 2000, 7, 37–49. [Google Scholar] [CrossRef]
- Burreson, B.J.; Moore, R.E.; Roller, P.P. Volatile halogen compounds in the alga Asparagopsis taxiformis (Rhodophyta). J. Agric. Food Chem. 1976, 24, 856–861. [Google Scholar] [CrossRef]
- Suzuki, M.; Takahashi, Y.; Nakano, S.; Abe, T.; Masuda, M.; Ohnishi, T.; Noya, Y.; Seki, K.-I. An experimental approach to study the biosynthesis of brominated metabolites by the red algal genus Laurencia. Phytochemistry 2009, 70, 1410–1415. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Heo, S.H.; Lee, J.; Park, S.I.; Kim, M.; Shin, M.S. Antimicrobial, Antioxidative, Elastase and Tyrosinase Inhibitory Effect of Supercritical and Hydrothermal Halopteris scoparia Extract. Turk. J. Comput. Math. Educ. (TURCOMAT) 2021, 12, 407–413. [Google Scholar] [CrossRef]
- Blum, R.A.; Rodvold, K.A. Recognition and importance of Staphylococcus epidermidis infections. Clin. Pharm. 1987, 6, 464–475. [Google Scholar] [PubMed]
- Lustigman, B.; Brown, C. Antibiotic production by marine algae isolated from the New York/New Jersey coast. Bull. Environ. Contam. Toxicol. 1991, 46, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.; Curtis, G.; Brkljača, R.; Urban, S. Bromophenolics from the Red Alga Polysiphonia decipiens. Mar. Drugs 2019, 17, 497. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, S.; Kamenarska, Z.; Bankova, V.; Stefanov, K.; Dimitrova-Konaklieva, S.; Najdenski, H.; Tzevtkova, I.; Popov, S. Chemical Composition and Biological Activities of the Black Sea Algae Polysiphonia denudata (Dillw.) Kutz. and Polysiphonia denudata f. fragilis (Sperk) Woronich. Z. Nat. C 2001, 56, 1008–1014. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Katsui, N.; Suzuki, Y.; Kitamura, S.; Irie, T. 5,6-dibromoprotocatechualdehyde and 2,3-dibromo-4,5-dihydroxybenzyl methyl ether. Tetrahedron 1967, 23, 1185–1188. [Google Scholar] [CrossRef]
- Harizani, M.; Ioannou, E.; Roussis, V. The Laurencia Paradox: An Endless Source of Chemodiversity. In Progress in the Chemistry of Organic Natural Products 112; Springer Science and Business Media: Cham, Switzerland, 2016; Volume 102, pp. 91–252. [Google Scholar]
- Al-Massarani, S. Phytochemical and Biological Properties of Sesquiterpene Constituents From the Marine Red Seaweed Laurencia: A Review. Nat. Prod. Chem. Res. 2014, 2. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Kawamoto, T.; Miwa, H.; Suzuki, M. Potent Antibacterial Activity of Halogenated Compounds against Antibiotic-Resistant Bacteria. Planta Medica 2004, 70, 1087–1090. [Google Scholar] [CrossRef] [Green Version]
- Félix, R.; Carmona, A.M.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Industry-Friendly Hydroethanolic Extraction Protocols for Grateloupia turuturu UV-Shielding and Antioxidant Compounds. Appl. Sci. 2020, 10, 5304. [Google Scholar] [CrossRef]
- De Alencar, D.B.; De Carvalho, F.C.T.; Rebouças, R.H.; Dos Santos, D.R.; Pires-Cavalcante, K.M.D.S.; De Lima, R.L.; Baracho, B.M.; Bezerra, R.M.; Viana, F.A.; Vieira, R.H.S.D.F.; et al. Bioactive extracts of red seaweeds Pterocladiella capillacea and Osmundaria obtusiloba (Floridophyceae: Rhodophyta) with antioxidant and bacterial agglutination potential. Asian Pac. J. Trop. Med. 2016, 9, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Yim, M.; Lee, J.M.; Lee, D.; Kim, M. In Vitro Antimicrobial Activities of Edible Seaweeds Extracts Against Cuti-bacterium Acnes. Korean J. Fish. Aquat. Sci. 2021, 54, 111–117. [Google Scholar]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Baek, K.-H. Chemical Composition and Antioxidant and Antibacterial Activities of an Essential Oil Extracted from an Edible Seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef] [Green Version]
- Gressler, V.; Stein, É.M.; Dorr, F.; Fujii, M.; Colepicolo, P.; Pinto, E. Sesquiterpenes from the essential oil of Laurencia dendroidea (Ceramiales, Rhodophyta): Isolation, biological activities and distribution among seaweeds. Rev. Bras. Farm. 2011, 21, 248–254. [Google Scholar] [CrossRef]
- Nafis, A.; Khalloufi, F.E.; Aknaf, A.; Oudra, B.; Marraiki, N.; Al-Rashed, S.; Elgorban, A.M.; Syed, A.; Hassani, L.; Custódio, L. In Vitro Antimicrobial and Synergistic Effect of Essential Oil from the Red Macroalgae Centroceras Clavulatum (C. Agardh) Montagne with Conventional Antibiotics. Asian Pac. J. Trop. Biomed. 2021, 11, 414–420. [Google Scholar]
- Pedrosa, R.; Gaudêncio, S.P.; Vasconcelos, V. XVI International Symposium on Marine Natural Products|XI European Conference on Marine Natural Products. Mar. Drugs. 2020, 18, 40. [Google Scholar] [CrossRef] [Green Version]
- Kiran, G.S.; Manilal, A.; Sujith, S.; Selvin, J.; Shakir, C.; Lipton, A.P. Antimicrobial potential of marine organisms collected from the southwest coast of India against multiresistant human and shrimp pathogens. Sci. Mar. 2010, 74, 287–296. [Google Scholar] [CrossRef]
- Vasconcelos, M.A.; Arruda, F.V.S.; Carneiro, V.A.; Silva, H.C.; Nascimento, K.S.; Sampaio, A.H.; Cavada, B.; Teixeira, E.H.; Henriques, M.; Pereira, M.O. Effect of Algae and Plant Lectins on Planktonic Growth and Biofilm Formation in Clinically Relevant Bacteria and Yeasts. BioMed Res. Int. 2014, 2014, 365272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmughapriya, S.; Manilal, A.; Sujith, S.; Selvin, J.; Kiran, G.S.; Natarajaseenivasan, K. Antimicrobial activity of seaweeds extracts against multiresistant pathogens. Ann. Microbiol. 2008, 58, 535–541. [Google Scholar] [CrossRef]
- Tuney, I.; Cadirci, B.H.; Unal, D.; Sukatar, A. Locational and Organic Solvent Variation in Antimicrobial Activities of Crude Extracts of Marine Algae from the Coast of Izmir (Turkey). Fresenius Environ. Bull. 2007, 16, 428–434. [Google Scholar]
- Karabay-Yavasoglu, N.U.; Sukatar, A.; Ozdemir, G.; Horzum, Z. Antimicrobial activity of volatile components and various extracts of the red algaJania rubens. Phytotherapy Res. 2007, 21, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Vairappan, C.S. Potent antibacterial activity of halogenated metabolites from Malaysian red algae, Laurencia majuscula (Rhodomelaceae, Ceramiales). Biomol. Eng. 2003, 20, 255–259. [Google Scholar] [CrossRef]
- Bracegirdle, J.; Sohail, Z.; Fairhurst, M.J.; Gerth, M.L.; Zuccarello, G.C.; Hashmi, M.A.; Keyzers, R.A. Costatone C—A New Halogenated Monoterpene from the New Zealand Red Alga Plocamium angustum. Mar. Drugs 2019, 17, 418. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Yamauchi, A.; Nakano, T.; Yamaguchi, T.; Ochiai, Y. The composition and anti-inflammatory effect of polysaccharides from the red alga Chondrus verrucosus. Fish. Sci. 2019, 85, 859–865. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, K.; Sharma, A.; Nagpal, K.; Bers, T. Anti-inflammatory, Analgesic and Antiulcer properties of Porphyra vietnamensis. Avicenna J. Phytomedicine 2015, 5, 69–77. [Google Scholar]
- Pereira, J.G.; Mesquita, J.X.; Aragão, K.S.; Franco, Á.X.; Souza, M.H.; Brito, T.V.; Dias, J.M.; Silva, R.O.; Medeiros, J.-V.R.; Oliveira, J.S.; et al. Polysaccharides isolated from Digenea simplex inhibit inflammatory and nociceptive responses. Carbohydr. Polym. 2014, 108, 17–25. [Google Scholar] [CrossRef]
- Zou, Y.; Fu, X.; Liu, N.; Duan, D.; Wang, X.; Xu, J.; Gao, X. The synergistic anti-inflammatory activities of agaro-oligosaccharides with different degrees of polymerization. Environ. Boil. Fishes 2019, 31, 2547–2558. [Google Scholar] [CrossRef]
- Hamias, R.; Wolak, T.; Huleihel, M.; Paran, E.; Levy-Ontman, O. Red alga polysaccharides attenuate angiotensin II-induced inflammation in coronary endothelial cells. Biochem. Biophys. Res. Commun. 2018, 500, 944–951. [Google Scholar] [CrossRef]
- Jang, J.H.; So, B.R.; Yeo, H.J.; Kang, H.J.; Kim, M.J.; Lee, J.J.; Jung, S.K.; Jung, Y.H. Preparation of cellulose microfibril (CMF) from Gelidium amansii and feasibility of CMF as a cosmetic ingredient. Carbohydr. Polym. 2021, 257, 117569. [Google Scholar] [CrossRef] [PubMed]
- So, B.R.; Yeo, H.J.; Lee, J.J.; Jung, Y.H.; Jung, S.K. Cellulose nanocrystal preparation from Gelidium amansii and analysis of its anti-inflammatory effect on the skin in vitro and in vivo. Carbohydr. Polym. 2021, 254, 117315. [Google Scholar] [CrossRef]
- Batista, J.A.; Dias, E.G.; Brito, T.V.; Prudêncio, R.S.; Silva, R.O.; Ribeiro, R.A.; Souza, M.H.L.; de Paula, R.C.; Feitosa, J.P.; Chaves, L.S.; et al. Polysaccharide isolated from Agardhiella ramosissima: Chemical structure and anti-inflammation activity. Carbohydr. Polym. 2014, 99, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Vanderlei, E.D.S.O.; de Araújo, I.W.F.; Quinderé, A.L.G.; Fontes, B.P.; Eloy, Y.R.G.; Rodrigues, J.A.G.; e Silva, A.A.R.; Chaves, H.V.; Jorge, R.J.B.; de Menezes, D.B.; et al. The involvement of the HO-1 pathway in the anti-inflammatory action of a sulfated polysaccharide isolated from the red seaweed Gracilaria birdiae. Inflamm. Res. 2011, 60, 1121–1130. [Google Scholar] [CrossRef]
- Chaves, L.D.S.; Nicolau, L.; Silva, R.O.; Barros, F.C.N.; Freitas, A.L.P.; Aragão, K.S.; Ribeiro, R.D.A.; Souza, M.H.L.P.; Barbosa, A.L.R.; Medeiros, J. Antiinflammatory and antinociceptive effects in mice of a sulfated polysaccharide fraction extracted from the marine red algaeGracilaria caudata. Immunopharmacol. Immunotoxicol. 2012, 35, 93–100. [Google Scholar] [CrossRef]
- Coura, C.O.; De Araújo, I.W.F.; Vanderlei, E.S.O.; Rodrigues, J.A.G.; Quinderé, A.L.G.; Fontes, B.P.; De Queiroz, I.N.L.; De Menezes, D.B.; Bezerra, M.M.; E Silva, A.A.R.; et al. Antinociceptive and Anti-Inflammatory Activities of Sulphated Polysaccharides from the Red Seaweed Gracilaria cornea. Basic Clin. Pharmacol. Toxicol. 2011, 110, 335–341. [Google Scholar] [CrossRef]
- de Brito, T.V.; Prudêncio, R.D.S.; Sales, A.B.; Júnior, F.D.C.V.; Candeira, S.J.N.; Franco, Á.X.; Aragão, K.S.; Ribeiro, R.D.A.; de Souza, M.H.L.P.; Chaves, L.D.S.; et al. Anti-inflammatory effect of a sulphated polysaccharide fraction extracted from the red algae Hypnea musciformis via the suppression of neutrophil migration by the nitric oxide signalling pathway. J. Pharm. Pharmacol. 2013, 65, 724–733. [Google Scholar] [CrossRef]
- De Sousa, A.A.; Benevides, N.M.B.; Pires, A.D.F.; Fiuza, F.; Queiroz, M.G.; Morais, T.M.; Pereira, M.G.; Assreuy, A.M. A report of a galactan from marine algaGelidium crinalewith in vivo anti-inflammatory and antinociceptive effects. Fundam. Clin. Pharmacol. 2011, 27, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wu, J.; Wang, S.; Shu, H.; Zhang, M.; Liu, K.; Liu, K. Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int. J. Biol. Macromol. 2019, 129, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Araújo, I.W.F.; Chaves, H.V.; Pachêco, J.M.; Val, D.R.; Vieira, L.V.; Santos, R.; Freitas, R.S.; Rivanor, R.L.; Monteiro, V.S.; Clemente-Napimoga, J.; et al. Role of central opioid on the antinociceptive effect of sulfated polysaccharide from the red seaweed Solieria filiformis in induced temporomandibular joint pain. Int. Immunopharmacol. 2017, 44, 160–167. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Antidiabetic and Anti-inflammatory Potential of Sulphated Polygalactans from Red Seaweeds Kappaphycus Alvarezii and Gracilaria Opuntia. Int. J. Food Prop. 2017, 20, 1326–1337. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.H.; Beury, D.W.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Sup-pression in the Tumor Microenvironment. In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 128, pp. 95–139. ISBN 9780128023167. [Google Scholar]
- Yang, Y.; Wei, Z.; Teichmann, A.T.; Wieland, F.H.; Wang, A.; Lei, X.; Zhu, Y.; Yin, J.; Fan, T.; Zhou, L.; et al. Development of a novel nitric oxide (NO) production inhibitor with potential therapeutic effect on chronic inflammation. Eur. J. Med. Chem. 2020, 193, 112216. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Wang, Y.; Hwang, J.-Y.; Park, H.-B.; Yadav, D.; Oda, T.; Jin, J.-O. Porphyran isolated from Pyropia yezoensis inhibits lipopolysaccharide-induced activation of dendritic cells in mice. Carbohydr. Polym. 2020, 229, 115457. [Google Scholar] [CrossRef]
- Myers, M.J.; Deaver, C.M.; Lewandowski, A.J. Molecular mechanism of action responsible for carrageenan-induced inflammatory response. Mol. Immunol. 2019, 109, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, B.; Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Yermak, I.M.; Sokolova, E.; Davydova, V.N.; Solov’Eva, T.F.; Aminin, D.L.; Reunov, A.V.; Lapshina, L.A. Influence of red algal polysaccharides on biological activities and supramolecular structure of bacterial lipopolysaccharide. Environ. Boil. Fishes 2016, 28, 619–627. [Google Scholar] [CrossRef]
- Rocha, C.; Pacheco, D.; Cotas, J.; Marques, J.; Pereira, L.; Gonçalves, A. Seaweeds as Valuable Sources of Essential Fatty Acids for Human Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Shinde, P.B.; Lee, Y.M.; Hong, J.; Kim, D.K.; Jung, J.H. Anti-inflammatory Constituents of the Red Alga Gracilaria verrucosa and Their Synthetic Analogues. J. Nat. Prod. 2008, 71, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Łukasz; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef] [PubMed]
- Chatter, R.; Ben Othman, R.; Rabhi, S.; Kladi, M.; Tarhouni, S.; Vagias, C.; Roussis, V.; Guizani-Tabbane, L.; Kharrat, R. In Vivo and in Vitro Anti-Inflammatory Activity of Neorogioltriol, a New Diterpene Extracted from the Red Algae Laurencia glandulifera. Mar. Drugs 2011, 9, 1293–1306. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, K.; Antony, T. Salicornolides A-C from Gracilaria salicornia attenuate pro-inflammatory 5-lipoxygense: Prospective natural anti-inflammatory leads. Phytochemistry 2020, 172, 112259. [Google Scholar] [CrossRef]
- Antony†, T.; Chakraborty†, K. First report of antioxidant abeo-labdane type diterpenoid from intertidal red seaweed Gracilaria salicornia with 5-lipoxygenase inhibitory potential. Nat. Prod. Res. 2018, 34, 1409–1416. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Highly oxygenated antioxidative 2H-chromen derivative from the red seaweed Gracilaria opuntia with pro-inflammatory cyclooxygenase and lipoxygenase inhibitory properties. Nat. Prod. Res. 2017, 32, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Makkar, F.; Chakraborty, K. Novel furanyl derivatives from the red seaweed Gracilaria opuntia with pharmacological activities using different in vitro models. Med. Chem. Res. 2018, 27, 1245–1259. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Previously undescribed antioxidative azocinyl morpholinone alkaloid from red seaweed Gracilaria opuntia with anti-cyclooxygenase and lipoxygenase properties. Nat. Prod. Res. 2017, 32, 1150–1160. [Google Scholar] [CrossRef]
- Zhang, B.; Choi, Y.M.; Lee, J.; An, I.S.; Li, L.; He, C.; Dong, Y.; Bae, S.; Meng, H. Toll-like receptor 2 plays a critical role in pathogenesis of acne vulgaris. Biomed. Dermatol. 2019, 3, 4. [Google Scholar] [CrossRef]
- Kim, J.M.; Choo, J.E.; Lee, H.J.; Kim, K.N.; Chang, S.E. Epidermal Growth Factor Attenuated the Expression of Inflammatory Cytokines in Human Epidermal Keratinocyte Exposed to Propionibacterium acnes. Ann. Dermatol. 2018, 30, 54. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Cho, S.; Chung, J.H.; Hammerberg, C.; Fisher, G.J.; Voorhees, J.J. Inflammation and Extracellular Matrix Degradation Mediated by Activated Transcription Factors Nuclear Factor-κB and Activator Protein-1 in Inflammatory Acne Lesions in Vivo. Am. J. Pathol. 2005, 166, 1691–1699. [Google Scholar] [CrossRef]
- Kazłowska, K.; Hsu, T.; Hou, C.-C.; Yang, W.-C.; Tsai, G.-J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef]
- Becker, K.; Hartmann, A.; Ganzera, M.; Fuchs, D.; Gostner, J.M. Immunomodulatory Effects of the Mycosporine-Like Amino Acids Shinorine and Porphyra-334. Mar. Drugs 2016, 14, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.-H.; Kwon, M.-J.; Jung, J.-H.; Nam, T.-J. Protein extracted from Porphyra yezoensis prevents cisplatin-induced nephrotoxicity by downregulating the MAPK and NF-κB pathways. Int. J. Mol. Med. 2017, 41, 511–520. [Google Scholar] [CrossRef]
- Ying, R.; Zhang, Z.; Zhu, H.; Li, B.; Hou, H. The Protective Effect of Mycosporine-Like Amino Acids (MAAs) from Porphyra yezoensis in a Mouse Model of UV Irradiation-Induced Photoaging. Mar. Drugs 2019, 17, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.H.; Kim, M.E.; Lee, J.S. Inhibitory Effects of Extract from G. lanceolata on LPS-Induced Production of Nitric Oxide and IL-1β via Down-regulation of MAPK in Macrophages. Appl. Biochem. Biotechnol. 2014, 175, 657–665. [Google Scholar] [CrossRef]
- Ko, E.-Y.; Heo, S.-J.; Cho, S.-H.; Lee, W.; Kim, S.-Y.; Yang, H.-W.; Ahn, G.; Cha, S.-H.; Kwon, S.-H.; Jeong, M.S.; et al. 3-Bromo-5-(ethoxymethyl)-1,2-benzenediol inhibits LPS-induced pro-inflammatory responses by preventing ROS production and downregulating NF-κB in vitro and in a zebrafish model. Int. Immunopharmacol. 2019, 67, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Lee, W.-H.; Jeong, J.; Park, M.; Ko, J.-Y.; Kwon, O.W.; Lee, J.; Kim, Y.-J. Pyropia yezoensis Extract Suppresses IFN-Gamma- and TNF-Alpha-Induced Proinflammatory Chemokine Production in HaCaT Cells via the Down-Regulation of NF-κB. Nutrients 2020, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017, 100, 514–521. [Google Scholar] [CrossRef]
- Millan-Linares, M.C.; Martín, N.M.R.; Rodriguez, N.M.; Toscano, R.; Claro, C.; Bermudez, B.; Pedroche, J.; Millan, F.; La Paz, S.M.-D. Nutraceutical Extract from Dulse (Palmaria palmata L.) Inhibits Primary Human Neutrophil Activation. Mar. Drugs 2019, 17, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.; Stanton, C. The Anti-Inflammatory Effect of Algae-Derived Lipid Extracts on Lipopolysaccharide (LPS)-Stimulated Human THP-1 Macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-A.; Kim, I.-H.; Nam, T.-J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.-J.; Moon, J.-Y.; Kim, M.-J.; Kim, D.S.; Kim, C.-S.; Lee, W.J.; Lee, N.H.; Hyun, C.-G. Inhibitory effect of Jeju endemic seaweeds on the production of pro-inflammatory mediators in mouse macrophage cell line RAW 264.7. J. Zhejiang Univ. Sci. B 2010, 11, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Aragao, G.F.; Nonato, D.T.T.; Da Ponta, E.L.; Sales, J.R.; Alencar, D.B.; Sampaio, S.S.; Chaves, E.M.C.; Assreuy, A.M.S. Protective effects of ethanolic extract from the red algae Amansia multifida on experimental inflammation, nociception and seizure experimental models. Acta Sci. Biol. Sci. 2016, 38, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, J.X.; de Brito, T.V.; Fontenelle, T.P.C.; Damasceno, R.O.S.; de Souza, M.H.L.P.; Lopes, J.L.D.S.; Beltramini, L.M.; Barbosa, A.L.D.R.; Freitas, A.L.P. Lectin from red algae Amansia multifida Lamouroux: Extraction, characterization and anti-inflammatory activity. Int. J. Biol. Macromol. 2021, 170, 532–539. [Google Scholar] [CrossRef]
- Yasmeen, A.; Ibrahim, M.; ul Hasan, M.M.; Jilani, T.; Shafique, S.; Rasheed, M. Phycochemical Analyses and Pharmacological Activities of Seven Macroalgae of Arabian Sea (Northern Coast Line). Pak. J. Pharm. Sci. 2021, 34, 963–969. [Google Scholar] [CrossRef]
- Abu Bakar, N.A.; Anyanji, V.U.; Mustapha, N.M.; Lim, S.-L.; Mohamed, S. Seaweed (Eucheuma cottonii) reduced inflammation, mucin synthesis, eosinophil infiltration and MMP-9 expressions in asthma-induced rats compared to Loratadine. J. Funct. Foods 2015, 19, 710–722. [Google Scholar] [CrossRef]
- Shih, C.-C.; Hwang, H.-R.; Chang, C.-I.; Su, H.-M.; Chen, P.-C.; Kuo, H.-M.; Li, P.-J.; Tsui, K.-H.; Lin, Y.-C.; Huang, S.-Y.; et al. Anti-Inflammatory and Antinociceptive Effects of Ethyl Acetate Fraction of an Edible Red Macroalgae Sarcodia ceylanica. Int. J. Mol. Sci. 2017, 18, 2437. [Google Scholar] [CrossRef] [Green Version]
- Abreu, T.M.; Ribeiro, N.A.; Chaves, H.V.; Jorge, R.J.B.; Bezerra, M.M.; Monteiro, H.S.A.; Vasconcelos, I.M.; Mota, É.F.; Benevides, N.M.B. Antinociceptive and Anti-inflammatory Activities of the Lectin from Marine Red Alga Solieria filiformis. Planta Medica 2016, 82, 596–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bringel, P.H.D.S.F.; Marques, G.F.O.; Martins, M.G.D.Q.; Da Silva, M.T.L.; Nobre, C.A.S.; Nascimento, K.S.D.; Cavada, B.S.; Castro, R.R.; Assreuy, A.M.S. The Lectin Isolated from the Alga Hypnea cervicornis Promotes Antinociception in Rats Subjected to Zymosan-Induced Arthritis: Involvement of cGMP Signalization and Cytokine Expression. Inflammation 2020, 43, 1446–1454. [Google Scholar] [CrossRef]
- Fontenelle, T.P.C.; Lima, G.C.; Mesquita, J.X.; Lopes, J.; de Brito, T.V.; Júnior, F.D.C.V.; Sales, A.B.; Aragão, K.S.; Souza, M.H.L.P.; Barbosa, A.L.D.R.; et al. Lectin obtained from the red seaweed Bryothamnion triquetrum: Secondary structure and anti-inflammatory activity in mice. Int. J. Biol. Macromol. 2018, 112, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.C.M.; Lima, V.; Holanda, M.; Pinheiro, P.G.; Rodrigues, J.A.G.; Lima, M.E.P.; Benevides, N.M.B. Antinociceptive and Anti-inflammatory Activities of Lectin from Marine Red Alga Pterocladiella capillacea. Biol. Pharm. Bull. 2010, 33, 830–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julião, D.R.; Afonso, C.; Gomes-Bispo, A.; Bandarra, N.M.; Cardoso, C. The effect of drying on undervalued brown and red seaweed species: Bioactivity alterations. Phycol. Res. 2021, 69, 246–257. [Google Scholar] [CrossRef]
- Regal, A.L.; Alves, V.D.; Gomes, R.; Matos, J.; Bandarra, N.M.; Afonso, C.; Cardoso, C. Drying process, storage conditions, and time alter the biochemical composition and bioactivity of the anti-greenhouse seaweed Asparagopsis taxiformis. Eur. Food Res. Technol. 2020, 246, 781–793. [Google Scholar] [CrossRef]
- Grünewald, N.; Groth, I.; Alban, S. Evaluation of Seasonal Variations of the Structure and Anti-inflammatory Activity of Sulfated Polysaccharides Extracted from the Red Alga Delesseria sanguinea (Hudson) Lamouroux (Ceramiales, Delesseriaceae). Biomacromolecules 2009, 10, 1155–1162. [Google Scholar] [CrossRef]
- Delgado, N.G.; Vázquez, A.I.F.; Sánchez, H.C.; Del Valle, R.M.S.; Gómez, Y.S.; Alfonso, A.M.S. Anti-inflammatory and antinociceptive activities of methanolic extract from red seaweed Dichotomaria obtusata. Braz. J. Pharm. Sci. 2013, 49, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, A.I.; Sánchez, C.M.; Delgado, G.; María, A.; Alfonso, S.; Ortega, Y.; Sánchez, H. Anti-Inflammatory and Analgesic Activities of Red Seaweed Dichotomaria Obtusata. Artic. Braz. J. Pharm. Sci. 2011, 47, 111–118. [Google Scholar]
- Balasubramaniam, V.; Lee, J.C.; Noh, M.F.M.; Ahmad, S.; Brownlee, I.A.; Ismail, A. Alpha-amylase, antioxidant, and anti-inflammatory activities of Eucheuma denticulatum (N.L. Burman) F.S. Collins and Hervey. Environ. Boil. Fishes 2015, 28, 1965–1974. [Google Scholar] [CrossRef]
- Wang, M.-L.; Hou, Y.-Y.; Chiu, Y.-S.; Chen, Y.-H. Immunomodulatory activities of Gelidium amansii gel extracts on murine RAW 264.7 macrophages. J. Food Drug Anal. 2013, 21, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.; Gomes, A.; Cardoso, C.; Afonso, C.; Campos, A.M.; Gomes, R.; Falé, P.; Delgado, I.; Coelho, I.; Castanheira, I.; et al. Commercial Red Seaweed in Portugal (Gelidium sesquipedale and Pterocladiella capillacea, Florideophyceae): Going beyond a Single-Purpose Product Approach by Valorizing Bioactivity. Thalass. Int. J. Mar. Sci. 2020, 36, 213–224. [Google Scholar] [CrossRef]
- Shu, M.-H.; Appleton, D.; Zandi, K.; Abubakar, S. Anti-inflammatory, gastroprotective and anti-ulcerogenic effects of red algae Gracilaria changii (Gracilariales, Rhodophyta) extract. BMC Complement. Altern. Med. 2013, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Antony, T.; Chakraborty, K. First report of antioxidative 2H-chromenyl derivatives from the intertidal red seaweed Gracilaria salicornia as potential anti-inflammatory agents. Nat. Prod. Res. 2020, 34, 3470–3482. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Moreira, A.S.P.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.T.; Rego, A.M.; Domingues, P.; Calado, R.; et al. Valorization of Lipids from Gracilaria sp. through Lipidomics and Decoding of Antiproliferative and Anti-Inflammatory Activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef] [Green Version]
- Ver, M.M.; Wiraw, I.G.P.; Jawi, I.M.; Sritamin, M.; Dewi, N.N.A.; Mirah, A.A.A. Anti-inflammatory Effect of Red Macroalgae Bulung Sangu (Gracilaria sp.) Extract in UVB-Irradiated Mice. Pak. J. Biol. Sci. 2021, 24, 80–89. [Google Scholar] [CrossRef]
- da Costa, E.; Melo, T.; Reis, M.; Domingues, P.; Calado, R.; Abreu, M.; Domingues, M. Polar Lipids Composition, Antioxidant and Anti-Inflammatory Activities of the Atlantic Red Seaweed Grateloupia turuturu. Mar. Drugs 2021, 19, 414. [Google Scholar] [CrossRef]
- Ferreira, J.; Hartmann, A.; Martins-Gomes, C.; Nunes, F.M.; Souto, E.B.; Santos, D.L.; Abreu, H.; Pereira, R.; Pacheco, M.; Gaivão, I.; et al. Red seaweeds strengthening the nexus between nutrition and health: Phytochemical characterization and bioactive properties of Grateloupia turuturu and Porphyra umbilicalis extracts. Environ. Boil. Fishes 2021, 33, 3365–3381. [Google Scholar] [CrossRef]
- Figueiredo, J.G.; Bitencourt, F.S.; Cunha, T.M.; Luz, P.B.; Nascimento, K.S.; Mota, M.R.; Sampaio, A.H.; Cavada, B.S.; Cunha, F.Q.; Alencar, N.M. Agglutinin isolated from the red marine alga Hypnea cervicornis J. Agardh reduces inflammatory hypernociception: Involvement of nitric oxide. Pharmacol. Biochem. Behav. 2010, 96, 371–377. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Unprecedented antioxidative cyclic ether from the red seaweed Kappaphycus alvarezii with anti-cyclooxygenase and lipoxidase activities. Nat. Prod. Res. 2017, 31, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Makkar, F.; Chakraborty, K. Antioxidant and anti-inflammatory oxygenated meroterpenoids from the thalli of red seaweed Kappaphycus alvarezii. Med. Chem. Res. 2018, 27, 2016–2026. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. First report of dual cyclooxygenase-2 and 5-lipoxygenase inhibitory halogen derivatives from the thallus of intertidal seaweed Kappaphycus alvarezii. Med. Chem. Res. 2018, 27, 2331–2340. [Google Scholar] [CrossRef]
- Wijesinghe, W.; Kim, E.-A.; Kang, M.-C.; Lee, W.-W.; Lee, H.-S.; Vairappan, C.S.; Jeon, Y.-J. Assessment of anti-inflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ. Toxicol. Pharmacol. 2014, 37, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Rezende, B.M.; Bernardes, P.T.T.; Resende, C.B.; Arantes, R.M.E.; Souza, D.G.; Braga, F.C.; Castor, M.G.M.; Teixeira, M.M.; Pinho, V. Lithothamnion muelleri Controls Inflammatory Responses, Target Organ Injury and Lethality Associated with Graft-versus-Host Disease in Mice. Mar. Drugs 2013, 11, 2595–2615. [Google Scholar] [CrossRef] [Green Version]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef]
- Choi, Y.K.; Ye, B.-R.; Kim, E.-A.; Kim, J.; Kim, M.-S.; Lee, W.W.; Ahn, G.-N.; Kang, N.; Jung, W.-K.; Heo, S.-J. Bis (3-bromo-4,5-dihydroxybenzyl) ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177. [Google Scholar] [CrossRef]
- Cian, R.E.; López-Posadas, R.; Drago, S.R.; de Medina, F.S.; Martínez-Augustin, O. Immunomodulatory Properties of the Protein Fraction from Phorphyra columbina. J. Agric. Food Chem. 2012, 60, 8146–8154. [Google Scholar] [CrossRef]
- Senevirathne, M.; Ahn, C.-B.; Je, J.-Y. Enzymatic extracts from edible red algae, Porphyra tenera, and their antioxidant, anti-acetylcholinesterase, and anti-inflammatory activities. Food Sci. Biotechnol. 2010, 19, 1551–1557. [Google Scholar] [CrossRef]
- Bergé, J.P.; Debiton, E.; Dumay, J.; Durand, P.; Barthomeuf, C. In Vitro Anti-inflammatory and Anti-proliferative Activity of Sulfolipids from the Red Alga Porphyridium cruentum. J. Agric. Food Chem. 2002, 50, 6227–6232. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.S.; Muizzuddin, N.; Arad, S.; Marenus, K. Sulfated Polysaccharides from Red Microalgae Have Antiinflammatory Properties In Vitro and In Vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef]
- Abreu, T.M.; Silva, L.M.C.M.; Vanderlei, E.S.O.; De Melo, C.M.L.; Pereira, V.R.A.; Benevides, N.M.B. Cytokine production induced by marine algae lectins in BALB/c mice splenocytes. Protein Pept. Lett. 2012, 19, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Wiemer, D.F.; Idler, D.D.; Fenical, W. Vidalols A and B, new anti-inflammatory bromophenols from the Caribbean marine red algaVidalia obtusaloba. Cell. Mol. Life Sci. 1991, 47, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Gillon, L.-A. WESOURCE—Active Science to Empower Beauty. From the Sea to the Skin—How Algae Adaptations Benefit Skin & Hair? Available online: https://www.flickr.com/photos/fotoosvanrobin/3644416895 (accessed on 27 September 2021).
- Sobhan, M.; Rabiei, M.A.S.; Amerifar, M. Correlation Between Lipid Profile and Acne Vulgaris. Clin. Cosmet. Investig. Dermatol. 2020, 13, 67–71. [Google Scholar] [CrossRef]
- Jiang, H.; Li, C.Y.; Zhou, L.; Lu, B.; Lin, Y.; Huang, X.; Wei, B.; Wang, Q.; Wang, L.; Lu, J.; et al. Acne patients frequently associated with abnormal plasma lipid profile. J. Dermatol. 2015, 42, 296–299. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-C.; Chang, C.-J.; Yang, T.-H.; Chiang, M.-T. Long-term feeding of red algae (Gelidium amansii) ameliorates glucose and lipid metabolism in a high fructose diet-impaired glucose tolerance rat model. J. Food Drug Anal. 2016, 25, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Young, C.N.; Koepke, J.I.; Terlecky, L.J.; Borkin, M.S.; Boyd, S.L.; Terlecky, S.R. Reactive Oxygen Species in Tumor Necrosis Factor-α-Activated Primary Human Keratinocytes: Implications for Psoriasis and Inflammatory Skin Disease. J. Investig. Dermatol. 2008, 128, 2606–2614. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Andresen, B.; Hill, M.; Zhang, J.; Booth, F.; Zhang, C. Role of Reactive Oxygen Species in Tumor Necrosis Factor-alpha Induced Endothelial Dysfunction. Curr. Hypertens. Rev. 2008, 4, 245–255. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januário, A.P.; Félix, R.; Félix, C.; Reboleira, J.; Valentão, P.; Lemos, M.F.L. Red Seaweed-Derived Compounds as a Potential New Approach for Acne Vulgaris Care. Pharmaceutics 2021, 13, 1930. https://doi.org/10.3390/pharmaceutics13111930
Januário AP, Félix R, Félix C, Reboleira J, Valentão P, Lemos MFL. Red Seaweed-Derived Compounds as a Potential New Approach for Acne Vulgaris Care. Pharmaceutics. 2021; 13(11):1930. https://doi.org/10.3390/pharmaceutics13111930
Chicago/Turabian StyleJanuário, Adriana P., Rafael Félix, Carina Félix, João Reboleira, Patrícia Valentão, and Marco F. L. Lemos. 2021. "Red Seaweed-Derived Compounds as a Potential New Approach for Acne Vulgaris Care" Pharmaceutics 13, no. 11: 1930. https://doi.org/10.3390/pharmaceutics13111930
APA StyleJanuário, A. P., Félix, R., Félix, C., Reboleira, J., Valentão, P., & Lemos, M. F. L. (2021). Red Seaweed-Derived Compounds as a Potential New Approach for Acne Vulgaris Care. Pharmaceutics, 13(11), 1930. https://doi.org/10.3390/pharmaceutics13111930