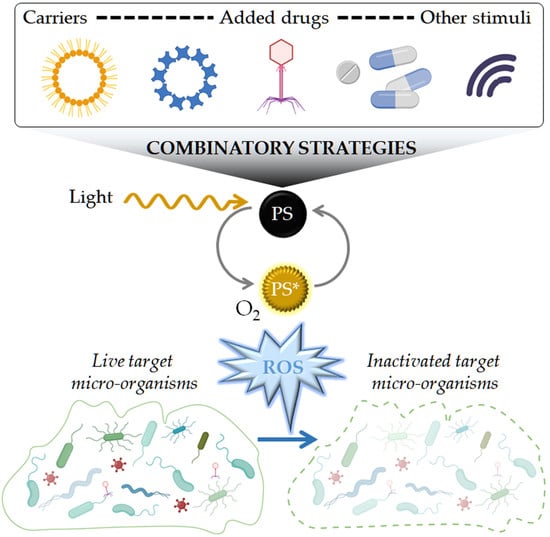
Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies
Abstract
:1. Introduction
2. PDT: General Presentation and Features
2.1. Photochemical Pathways and Reactive Oxygen Species Production
2.2. Biological Effects of aPDT: Potential Targets and Related Mechanisms
2.3. Important Parameters and Requirements for an “Ideal” aPDT
3. Positioning of aPDT in Current Human Healthcare Treatments
3.1. Curative Preclinical aPDT
3.1.1. Treatment of Bacterial Infections
3.1.2. Treatment of Fungal Infections
3.1.3. Treatment of Viral Infections
3.1.4. Treatment of Parasite Infections
3.1.5. Treatment of Polymicrobial Infections
3.2. Current Clinical aPDT Practices
3.3. Toward Preventive/Prophylactic Treatments
4. State of the Art with Recent Photo(nano)System Developments
4.1. Single PSs
4.1.1. Organic PSs and Their Derivatives
4.1.2. Coordination and Organometallic Complexes-Based PSs
4.2. Multicomponent PSs and Nanoscale Implementation: Extension to Nanoedifices with PSs
- (i)
- Role(s) and nature of the nanocomponent(s) in the PS nanosystems: the two criteria typically considered for the discrimination of nano-PSs are the role and nature of the nano building block(s) involved. With regards to the role of the latter, we can conventionally discern on the one hand the “PS nanocarriers” (e.g., polymersomes or Au NPs) in which the nanomoiety acts as a delivery system for singular PS molecules (e.g., MB) while either complementing, facilitating, or enhancing the aPDT activity (depending on the nature and eventual intrinsic properties of the nanovector), and on the other hand, the “PS active” nanoagents with the nanocomponent endorsing the role of PS. Among the examples, some versatile nanotemplates may ultimately display a dual role, i.e., “active PS” and “PS conveyor” (e.g., ZnO NPs), while distinct nanomoieties might be simultaneously required for the design of utterly sophisticated hybrid nano-PSs (e.g., Au@AgNP@SiO2@PS) [117,118]. In addition to the chemical composition, the nature of the nano building block(s) will also be defined by the fundamental characteristics of nano-objects, such as size, shape, topology, and crystal structure, which will all ineluctably contribute to tailor the biological behavior of the nanomaterials and the interactions with the targets (e.g., with the membranes of the bacteria) [119]. Moreover, for the same nanocore, the nature and role of the eventual surfactant(s) involved (e.g., silica coating or poly(ethylene glycol) (PEG) coating for metal NPs) can drastically alter the overall behaviors of the nanosystems.
- (ii)
- Type of interactions between the nano entity and the PS, and localization of the PS in the nanosystems: other criteria of relevance when describing nano-PSs—specifically PS nanocarriers—reside in the nature of the interactions between the nanocomponent and the PS molecules involved, but also the location of the PSs. Thus, we can distinguish the common cases of PS molecules “embedded” within a nanovector either by physisorption or functionalized (chemisorption), and alternately the nanoplatforms with surfaces decorated with PSs, again, either by physical or chemical adsorption. The differences between the two types of interactions and distinct localizations of the PSs implicitly imply distinctive chemical engineering and related requirements, and may potentially impact the resulting stability of the nanoedifices, but also the aPDT activity. For instance, in the case of PS molecules located inside the edifice and not released, the selected “nanomatrix” should adequately permit the photoactivation process of the internalized PSs, be sufficiently porous/permeable to both triplet and singlet oxygen and eventual ROS generated by the photosensitizers (i.e., efficient internal diffusion of molecular oxygen to react with the PSs then external diffusion of 1O2 to the targets) while also presenting inertness to the latter to not compromise or quench the aPDT activity. Meanwhile, with surfaces of nano-objects decorated with PSs, the PSs may then contribute to some extent as an interface with the biological medium or the target.
- (iii)
- Biological impacts of the PS nanosystems: in addition to the biocompatibility and aPDT efficiency (including the critical concentrations just as the half-maximal effective concentration EC50, minimum inhibitory concentration MIC, or 50% growth inhibition concentration GIC50), the eventual biodegradability, elimination process, or ecotoxicity of the aPDT nanomaterials can markedly vary from one system to another (based on factors such as composition and size/shape), but are rather difficult to evaluate or compare; ergo, these factors are not systematically addressed in the reports.
- (iv)
- Relative sustainability of the nano-PSs for aPDT applications: the reproducibility, eco-friendliness, and cost-effectiveness parameters of the synthetic protocols and production of aPDT nanomaterials, as well as the ease of storage and use, and the stability over time are also ultimately to be evaluated for any system aiming to be viable and reasonably applied; however, similar to (iii), these parameters are complex and so scarcely investigated.
4.2.1. Metal-Based Systems
Metal NPs
Metal Oxides
QDs and Metal Chalcogenide Nanomaterials
Metal–Organic Framework (MOF) Nanoscaffolds, Upconversion Nanomaterials and Other Metal Ion Nanostructures
4.2.2. Silicon-Based NPs
4.2.3. Carbon-Based Nanomaterials
Fullerenes, Carbon Nanotubes (CNTs), and Nanodiamonds
Carbon QDs (CQDs)
Graphene, Graphene QDs (GQDs), and Graphene Oxide (GO) Nanostructures
4.2.4. Lipid-Based Systems
4.2.5. Polymer-Based Systems
Conjugated Polymers as PSs or Polymer-Functionalized PSs
Dendrimers
Polymeric NPs and Nanocomposites
Polymersomes
Polymeric Micelles
Niosomes
Polymeric Fibers
5. Focus on Combinatory aPDT Approaches
5.1. “Basic” aPDT Combinations
5.1.1. Combination of Several PSs
5.1.2. Addition of Inorganic Salts
5.2. Combinations of aPDT with Other Antimicrobial Drugs or Antimicrobial Therapies
5.2.1. Antibiotics
5.2.2. Antifungals
5.2.3. Other Antimicrobial Compounds
5.2.4. Viral NPs and Phagotherapy
5.3. Combinations of aPDT with Other Light-Based Treatments
5.3.1. aPDT and Photothermal Therapy
5.3.2. aPDT and NO Phototherapy
5.3.3. aPDT and Low Laser Therapy
5.4. Coupling of aPDT with Other Physical Treatments
5.4.1. aPDT and Sonodynamic Therapy
5.4.2. aPDT and Electrochemotherapy
5.5. aPDT and Other Antimicrobial-Related Therapies
6. Other aPDT Perspectives: New Strategies to Efficiently Target Bacteria
6.1. Aggregation-Induced Emission (AIE) Luminogens
6.2. Photochemical Internalization (PCI)
6.3. Genetically-Encoded PSs
6.4. pH-Sensitive aPDT
6.5. DNA Origami as PS Carriers
7. Discussion
8. Concluding Remarks
Funding
Conflicts of Interest
Abbreviations
| AIE | aggregation-induced emission |
| ALA | alanine |
| AMP | antimicrobial peptide |
| AMR | antimicrobial resistance |
| aPDT | antimicrobial PDT |
| ConA | concanavalin A |
| CNTs | carbon nanotubes |
| Ce6 | chlorin e6 |
| CFU | colony forming unit |
| e− | electron |
| ESKAPE | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. |
| IC | internal conversion |
| ICG | indocyanine green |
| ISC | inter-system crossing |
| GO | graphene oxide |
| H2O2 | hydrogen peroxide |
| HO● | hydroxyl radical |
| MB | methylene blue |
| MDR | multidrug resistance |
| MRSA | methicillin resistant S. aureus |
| MOF | metal organic framework |
| NIR | near infrared |
| NO | nitic oxide |
| NP | nanoparticle |
| O2 | dioxygen |
| O2●− | superoxide anion radical |
| 1O2 | singlet oxygen |
| 3O2 | ground state molecular oxygen |
| PDT | photodynamic therapy |
| PS | photosensitizer |
| PS•− | PS radical anion |
| 1PS | PS in the ground state |
| 1PS* | PS in a first excited singlet state |
| 3PS* | PS in a triplet excited state |
| PEG | poly(ethylene glycol) |
| PCI | photochemical internalization |
| PCL | poly(ε-caprolactone) |
| PLA | poly(lactic acid) |
| pSi | porous silicon |
| PTT | photothermal therapy |
| QD | quantum dot |
| R | reduced molecule |
| R●+ | oxidized molecule |
| RB | rose bengal |
| ROS | reactive oxygen species |
| SS | sonosensitizer |
| SDT | sonodynamic therapy |
| SPDT | sonophotodynamic therapy |
| SPION | superparamagnetic iron oxide NP |
| TB(O) | toluidine blue |
| UCNP | upconversion NP |
| UV | ultraviolet |
| WHO | world health organisation |
References
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to Effective Antimicrobials: A Worldwide Challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Antoñanzas, F.; Goossens, H. The Economics of Antibiotic Resistance: A Claim for Personalised Treatments. Eur. J. Health Econ. 2019, 20, 483–485. [Google Scholar] [CrossRef] [Green Version]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, Present and Future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are We Afraid of the Light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Daniell, M.D.; Hill, J.S. A History of Photodynamic Therapy. Aust. N. Z. J. Surg. 1991, 61, 340–348. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent Advances in Photodynamic Therapy for Cancer and Infectious Diseases. WIREs Nanomed. NanoBiotechnol. 2019, 11, e1560. [Google Scholar] [CrossRef] [PubMed]
- Sabino, C.P.; Wainwright, M.; Ribeiro, M.S.; Sellera, F.P.; dos Anjos, C.; Baptista, M.d.S.; Lincopan, N. Global Priority Multidrug-Resistant Pathogens Do Not Resist Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2020, 208, 111893. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, T.; Lemercier, G.; Chevreux, S.; Tücking, K.-S.; Ravel, J.; Thétiot, F.; Jonas, U.; Schönherr, H.; Montier, T. Ruthenium(II) Polypyridyl Complexes as Photosensitizers for Antibacterial Photodynamic Therapy: A Structure-Activity Study on Clinical Bacterial Strains. ChemMedChem 2018, 13, 2229–2239. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.; Grinholc, M. Combined Antimicrobial Activity of Photodynamic Inactivation and Antimicrobials—State of the Art. Front. Microbiol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Baier, J.; Maier, M.; Engl, R.; Landthaler, M.; Bäumler, W. Time-Resolved Investigations of Singlet Oxygen Luminescence in Water, in Phosphatidylcholine, and in Aqueous Suspensions of Phosphatidylcholine or HT29 Cells. J. Phys. Chem. B 2005, 109, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Baier, J.; Franz, B.; Maier, M.; Landthaler, M.; Szeimies, R.-M.; Bäumler, W. The Role of Singlet Oxygen and Oxygen Concentration in Photodynamic Inactivation of Bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 7223–7228. [Google Scholar] [CrossRef] [Green Version]
- Ogilby, P.R. Singlet Oxygen: There Is Indeed Something New under the Sun. Chem. Soc. Rev. 2010, 39, 3181. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of Type I and Type II Photosensitized Oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef]
- Baptista, M.d.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R.; Abrahamse, H. Oxygen-Independent Antimicrobial Photoinactivation: Type III Photochemical Mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, E.D.; Cormick, M.P.; Pons, P.; Alvarez, M.G.; Durantini, E.N. Mechanistic Aspects of the Photodynamic Inactivation of Candida Albicans Induced by Cationic Porphyrin Derivatives. Eur. J. Med. Chem. 2012, 58, 332–339. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and Targets in Antiviral Phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, A. Photodynamic Inactivation of Bacterial and Yeast Biofilms with a Cationic Porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef]
- Garcez, A.S.; Núñez, S.C.; Azambuja, N.; Fregnani, E.R.; Rodriguez, H.M.H.; Hamblin, M.R.; Suzuki, H.; Ribeiro, M.S. Effects of Photodynamic Therapy on Gram-Positive and Gram-Negative Bacterial Biofilms by Bioluminescence Imaging and Scanning Electron Microscopic Analysis. Photomed. Laser Surg. 2013, 31, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Tome, J.; Almeida, A. An Insight on Bacterial Cellular Targets of Photodynamic Inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [Green Version]
- Lam, M.; Jou, P.C.; Lattif, A.A.; Lee, Y.; Malbasa, C.L.; Mukherjee, P.K.; Oleinick, N.L.; Ghannoum, M.A.; Cooper, K.D.; Baron, E.D. Photodynamic Therapy with Pc 4 Induces Apoptosis of Candida Albicans. Photochem. Photobiol. 2011, 87, 904–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopka, K.; Goslinski, T. Photodynamic Therapy in Dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef]
- Hirakawa, K.; Ota, K.; Hirayama, J.; Oikawa, S.; Kawanishi, S. Nile Blue Can Photosensitize DNA Damage through Electron Transfer. Chem. Res. Toxicol. 2014, 27, 649–655. [Google Scholar] [CrossRef]
- Choi, S.S.; Lee, H.K.; Chae, H.S. In Vitro Photodynamic Antimicrobial Activity of Methylene Blue and Endoscopic White Light against Helicobacter Pylori 26695. J. Photochem. Photobiol. B Biol. 2010, 101, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, H.; Misba, L.; Ahmad, S.; Khan, A.U. Curcumin Induced Photodynamic Therapy Mediated Suppression of Quorum Sensing Pathway of Pseudomonas Aeruginosa: An Approach to Inhibit Biofilm In Vitro. Photodiagn. Photodyn. Ther. 2020, 30, 101645. [Google Scholar] [CrossRef] [PubMed]
- Boluki, E.; Moradi, M.; Azar, P.S.; Fekrazad, R.; Pourhajibagher, M.; Bahador, A. The Effect of Antimicrobial Photodynamic Therapy against Virulence Genes Expression in Colistin-Resistance Acinetobacter Baumannii: APDT in Genes Expression of Colistin-Resistance A. Baumannii. Laser Ther. 2019, 28, 27–33. [Google Scholar] [CrossRef]
- Ghorbanzadeh, R.; Assadian, H.; Chiniforush, N.; Parker, S.; Pourakbari, B.; Ehsani, B.; Alikhani, M.Y.; Bahador, A. Modulation of Virulence in Enterococcus Faecalis Cells Surviving Antimicrobial Photodynamic Inactivation with Reduced Graphene Oxide-Curcumin: An Ex Vivo Biofilm Model. Photodiagn. Photodyn. Ther. 2020, 29, 101643. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Plotino, G.; Chiniforush, N.; Bahador, A. Dual Wavelength Irradiation Antimicrobial Photodynamic Therapy Using Indocyanine Green and Metformin Doped with Nano-Curcumin as an Efficient Adjunctive Endodontic Treatment Modality. Photodiagn. Photodyn. Ther. 2020, 29, 101628. [Google Scholar] [CrossRef] [PubMed]
- Marasini, S.; Leanse, L.G.; Dai, T. Can Microorganisms Develop Resistance against Light Based Anti-Infective Agents? Adv. Drug Deliv. Rev. 2021, 175, 113822. [Google Scholar] [CrossRef] [PubMed]
- Tschowri, N.; Lindenberg, S.; Hengge, R. Molecular Function and Potential Evolution of the Biofilm-Modulating Blue Light-Signalling Pathway of Escherichia Coli. Mol. Microbiol. 2012, 85, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Prates, R.A.; Kato, I.T.; Ribeiro, M.S.; Tegos, G.P.; Hamblin, M.R. Influence of Multidrug Efflux Systems on Methylene Blue-Mediated Photodynamic Inactivation of Candida Albicans. J. Antimicrob. Chemother. 2011, 66, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Tegos, G.P.; Masago, K.; Aziz, F.; Higginbotham, A.; Stermitz, F.R.; Hamblin, M.R. Inhibitors of Bacterial Multidrug Efflux Pumps Potentiate Antimicrobial Photoinactivation. Antimicrob. Agents Chemother. 2008, 52, 3202–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Khan, S.N.; Akhtar, F.; Misba, L.; Meena, R.; Khan, A.U. Inhibition of Multi-Drug Resistant Klebsiella Pneumoniae: Nanoparticles Induced Photoinactivation in Presence of Efflux Pump Inhibitor. Eur. J. Pharm. Biopharm. 2020, 157, 165–174. [Google Scholar] [CrossRef]
- Kashef, N.; Akbarizare, M.; Kamrava, S.K. Effect of Sub-Lethal Photodynamic Inactivation on the Antibiotic Susceptibility and Biofilm Formation of Clinical Staphylococcus Aureus Isolates. Photodiagn. Photodyn. Ther. 2013, 10, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Gollmer, A.; Felgenträger, A.; Bäumler, W.; Maisch, T.; Späth, A. A Novel Set of Symmetric Methylene Blue Derivatives Exhibits Effective Bacteria Photokilling—A Structure–Response Study. Photochem. Photobiol. Sci. 2015, 14, 335–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcez, A.S.; Kaplan, M.; Jensen, G.J.; Scheidt, F.R.; Oliveira, E.M.; Suzuki, S.S. Effects of Antimicrobial Photodynamic Therapy on Antibiotic-Resistant Escherichia Coli. Photodiagn. Photodyn. Ther. 2020, 32, 102029. [Google Scholar] [CrossRef]
- Paziani, M.H.; Tonani, L.; de Menezes, H.D.; Bachmann, L.; Wainwright, M.; Braga, G.Ú.L.; von Zeska Kress, M.R. Antimicrobial Photodynamic Therapy with Phenothiazinium Photosensitizers in Non-Vertebrate Model Galleria Mellonella Infected with Fusarium Keratoplasticum and Fusarium Moniliforme. Photodiagn. Photodyn. Ther. 2019, 25, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Santezi, C.; Reina, B.D.; Dovigo, L.N. Curcumin-Mediated Photodynamic Therapy for the Treatment of Oral Infections—A Review. Photodiagn. Photodyn. Ther. 2018, 21, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieplik, F.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Hiller, K.-A.; Maisch, T.; Karygianni, L. Antimicrobial Photodynamic Therapy as an Adjunct for Treatment of Deep Carious Lesions—A Systematic Review. Photodiagn. Photodyn. Ther. 2017, 18, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, T.; Blunn, G.; Hislop, S.; Ramalhete, R.; Bagley, C.; McKenna, D.; Coathup, M. Antimicrobial Photodynamic Therapy—a Promising Treatment for Prosthetic Joint Infections. Lasers Med. Sci. 2018, 33, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, C.; Cui, Z.; Liang, Y.; Li, Z.; et al. Treatment of MRSA-Infected Osteomyelitis Using Bacterial Capturing, Magnetically Targeted Composites with Microwave-Assisted Bacterial Killing. Nat. Commun. 2020, 11, 4446. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, L.M.; Ray, A.; Santos, D.A.; Cisalpino, P.S.; Friedman, A.J.; Nosanchuk, J.D. Antimicrobial Photodynamic Therapy: An Effective Alternative Approach to Control Fungal Infections. Front. Microbiol. 2015, 6, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrini Carmello, J.; Alves, F.; Basso, F.G.; de Souza Costa, C.A.; Tedesco, A.C.; Lucas Primo, F.; Mima, E.G.d.O.; Pavarina, A.C. Antimicrobial Photodynamic Therapy Reduces Adhesion Capacity and Biofilm Formation of Candida Albicans from Induced Oral Candidiasis in Mice. Photodiagn. Photodyn. Ther. 2019, 27, 402–407. [Google Scholar] [CrossRef]
- Freire, F.; Ferraresi, C.; Jorge, A.O.C.; Hamblin, M.R. Photodynamic Therapy of Oral Candida Infection in a Mouse Model. J. Photochem. Photobiol. B 2016, 159, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leanse, L.G.; Goh, X.S.; Dai, T. Quinine Improves the Fungicidal Effects of Antimicrobial Blue Light: Implications for the Treatment of Cutaneous Candidiasis. Lasers Surg. Med. 2020, 52, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Janeth Rimachi Hidalgo, K.; Cabrini Carmello, J.; Carolina Jordão, C.; Aboud Barbugli, P.; de Sousa Costa, C.A.; Mima, E.G.d.O.; Pavarina, A.C. Antimicrobial Photodynamic Therapy in Combination with Nystatin in the Treatment of Experimental Oral Candidiasis Induced by Candida Albicans Resistant to Fluconazole. Pharmaceuticals 2019, 12, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.J.; Jemec, G.B.E.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic Therapy Treatment of Superficial Fungal Infections: A Systematic Review. Photodiagn. Photodyn. Ther. 2020, 31, 101774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, J.; Sun, Y.; Gao, L. Effects of Photodynamic Inactivation on the Growth and Antifungal Susceptibility of Rhizopus Oryzae. Mycopathologia 2019, 184, 315–319. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, H.; Knüver-Hopf, J.; Gravemann, U.; Redecker-Klein, A.; Müller, T.H. West Nile Virus in Plasma Is Highly Sensitive to Methylene Blue-Light Treatment. Transfusion 2004, 44, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, A.; Hasegawa, T.; Hirasawa, Y.; Tsuchihashi, H.; Ikeda, S. Photodynamic Therapy Using Light-Emitting Diodes for the Treatment of Viral Warts. J. Dermatol. 2009, 36, 525–528. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, A.; Sun, W.; Yue, Y.; Li, H. Efficacy and Safety of Photodynamic Therapy for Cervical Intraepithelial Neoplasia and Human Papilloma Virus Infection. Medicine 2018, 97, e10864. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.F.; Neves, M.G.P.M.S. Antimicrobial Photodynamic Therapy in the Control of COVID-19. Antibiotics 2020, 9, 320. [Google Scholar] [CrossRef]
- Zarubaev, V.V.; Belousova, I.M.; Kiselev, O.I.; Piotrovsky, L.B.; Anfimov, P.M.; Krisko, T.C.; Muraviova, T.D.; Rylkov, V.V.; Starodubzev, A.M.; Sirotkin, A.C. Photodynamic Inactivation of Influenza Virus with Fullerene C60 Suspension in Allantoic Fluid. Photodiagn. Photodyn. Ther. 2007, 4, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Abada, Z.; Cojean, S.; Pomel, S.; Ferrié, L.; Akagah, B.; Lormier, A.T.; Loiseau, P.M.; Figadère, B. Synthesis and Antiprotozoal Activity of Original Porphyrin Precursors and Derivatives. Eur. J. Med. Chem. 2013, 67, 158–165. [Google Scholar] [CrossRef]
- Espitia-Almeida, F.; Díaz-Uribe, C.; Vallejo, W.; Gómez-Camargo, D.; Romero Bohórquez, A.R. In Vitro Anti-Leishmanial Effect of Metallic Meso-Substituted Porphyrin Derivatives against Leishmania Braziliensis and Leishmania Panamensis Promastigotes Properties. Molecules 2020, 25, 1887. [Google Scholar] [CrossRef] [Green Version]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, Â.; Nadais, H.; Almeida, A. Potential Applications of Porphyrins in Photodynamic Inactivation beyond the Medical Scope. J. Photochem. Photobiol. C Photochem. Rev. 2015, 22, 34–57. [Google Scholar] [CrossRef] [Green Version]
- de Souza, L.M.; Venturini, F.P.; Inada, N.M.; Iermak, I.; Garbuio, M.; Mezzacappo, N.F.; de Oliveira, K.T.; Bagnato, V.S. Curcumin in Formulations against Aedes Aegypti: Mode of Action, Photolarvicidal and Ovicidal Activity. Photodiagn. Photodyn. Ther. 2020, 31, 101840. [Google Scholar] [CrossRef]
- Khater, H.; Hendawy, N.; Govindarajan, M.; Murugan, K.; Benelli, G. Photosensitizers in the Fight against Ticks: Safranin as a Novel Photodynamic Fluorescent Acaricide to Control the Camel Tick Hyalomma Dromedarii (Ixodidae). Parasitol. Res. 2016, 115, 3747–3758. [Google Scholar] [CrossRef] [PubMed]
- Biel, M.A.; Pedigo, L.; Gibbs, A.; Loebel, N. Photodynamic Therapy of Antibiotic Resistant Biofilms in a Maxillary Sinus Model. Int. Forum Allergy Rhinol. 2013, 3, 468–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biel, M.A.; Sievert, C.; Usacheva, M.; Teichert, M.; Wedell, E.; Loebel, N.; Rose, A.; Zimmermann, R. Reduction of Endotracheal Tube Biofilms Using Antimicrobial Photodynamic Therapy. Lasers Surg. Med. 2011, 43, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Karner, L.; Drechsler, S.; Metzger, M.; Hacobian, A.; Schädl, B.; Slezak, P.; Grillari, J.; Dungel, P. Antimicrobial Photodynamic Therapy Fighting Polymicrobial Infections—A Journey from In Vitro to In Vivo. Photochem. Photobiol. Sci. 2020, 19, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.G.; Venturini, M.; Capezzera, R.; Sala, R.; Zane, C. Photodynamic Therapy of Interdigital Mycoses of the Feet with Topical Application of 5-Aminolevulinic Acid. Photodermatol. Photoimmunol. Photomed. 2004, 20, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wan, M.T. Current Evidence and Applications of Photodynamic Therapy in Dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannucci, E.; Genovese, S.; Monami, M.; Navalesi, G.; Dotta, F.; Anichini, R.; Romagnoli, F.; Gensini, G. Photodynamic Topical Antimicrobial Therapy for Infected Foot Ulcers in Patients with Diabetes: A Randomized, Double-Blind, Placebo-Controlled Study—The D.A.N.T.E (Diabetic Ulcer Antimicrobial New Topical Treatment Evaluation) Study. Acta Diabetol. 2014, 51, 435–440. [Google Scholar] [CrossRef]
- Morley, S.; Griffiths, J.; Philips, G.; Moseley, H.; O’Grady, C.; Mellish, K.; Lankester, C.L.; Faris, B.; Young, R.J.; Brown, S.B.; et al. Phase IIa Randomized, Placebo-Controlled Study of Antimicrobial Photodynamic Therapy in Bacterially Colonized, Chronic Leg Ulcers and Diabetic Foot Ulcers: A New Approach to Antimicrobial Therapy. Br. J. Dermatol. 2013, 168, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Adami, F.; Correa, J.A.; Pinhal, M.A.S.; Baptista, M.S. A Clinical Trial Testing the Efficacy of PDT in Preventing Amputation in Diabetic Patients. Photodiagn. Photodyn. Ther. 2014, 11, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Viveiros, J.; Yang, C.; Ahmadi, A.; Ganz, R.A.; Tolkoff, M.J. Helicobacter Pylori Accumulates Photoactive Porphyrins and Is Killed by Visible Light. Antimicrob. Agents Chemother. 2005, 49, 2822–2827. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, R.; Fayyaz, F.; Rassa, M. The Study of Cellulosic Fabrics Impregnated with Porphyrin Compounds for Use as Photo-Bactericidal Polymers. Mater. Sci. Eng. C 2016, 59, 661–668. [Google Scholar] [CrossRef]
- Fonseca, G.A.M.D.; Dourado, D.C.; Barreto, M.P.; Cavalcanti, M.F.X.B.; Pavelski, M.D.; Ribeiro, L.B.Q.; Frigo, L. Antimicrobial Photodynamic Therapy (APDT) for Decontamination of High-Speed Handpieces: A Comparative Study. Photodiagn. Photodyn. Ther. 2020, 30, 101686. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in Antibacterial Photodynamic Therapy: An Overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. Phthalocyanines Derivatives as Control Approach for Antimicrobial Photodynamic Therapy. J. Am. J. Clin. Microbiol. Antimicrob. 2019, 2, 8. [Google Scholar]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.-M.; Lee, H.-S.; Jeong, D.; Oh, H.-K.; Ra, K.-H.; Lee, M.-Y. Antimicrobial Photodynamic Therapy Using Chlorin E6 with Halogen Light for Acne Bacteria-Induced Inflammation. Life Sci. 2015, 124, 56–63. [Google Scholar] [CrossRef]
- Li, X.; Lee, D.; Huang, J.-D.; Yoon, J. Phthalocyanine-Assembled Nanodots as Photosensitizers for Highly Efficient Type I Photoreactions in Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 9885–9890. [Google Scholar] [CrossRef]
- Terra Garcia, M.; Correia Pereira, A.H.; Figueiredo-Godoi, L.M.A.; Jorge, A.O.C.; Strixino, J.F.; Junqueira, J.C. Photodynamic Therapy Mediated by Chlorin-Type Photosensitizers against Streptococcus Mutans Biofilms. Photodiagn. Photodyn. Ther. 2018, 24, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lovell, J.F. Recent Applications of Phthalocyanines and Naphthalocyanines for Imaging and Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1420. [Google Scholar] [CrossRef] [Green Version]
- Hirose, M.; Yoshida, Y.; Horii, K.; Hasegawa, Y.; Shibuya, Y. Efficacy of Antimicrobial Photodynamic Therapy with Rose Bengal and Blue Light against Cariogenic Bacteria. Arch Oral Biol. 2021, 122, 105024. [Google Scholar] [CrossRef] [PubMed]
- Galdino, D.Y.T.; da Rocha Leódido, G.; Pavani, C.; Gonçalves, L.M.; Bussadori, S.K.; Benini Paschoal, M.A. Photodynamic Optimization by Combination of Xanthene Dyes on Different Forms of Streptococcus Mutans: An In Vitro Study. Photodiagnosis Photodyn. Ther. 2021, 33, 102191. [Google Scholar] [CrossRef]
- Silva, A.F.; Dos Santos, A.R.; Trevisan, D.A.C.; Bonin, E.; Freitas, C.F.; Batista, A.F.P.; Hioka, N.; Simões, M.; Graton Mikcha, J.M. Xanthene Dyes and Green LED for the Inactivation of Foodborne Pathogens in Planktonic and Biofilm States. Photochem Photobiol 2019, 95, 1230–1238. [Google Scholar] [CrossRef]
- Mizuno, K.; Zhiyentayev, T.; Huang, L.; Khalil, S.; Nasim, F.; Tegos, G.P.; Gali, H.; Jahnke, A.; Wharton, T.; Hamblin, M.R. Antimicrobial Photodynamic Therapy with Functionalized Fullerenes: Quantitative Structure-Activity Relationships. J. Nanomed. Nanotechnol. 2011, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Chiang, L.Y.; Hamblin, M.R. Photodynamic Therapy with Fullerenes In Vivo: Reality or a Dream? Nanomedicine 2011, 6, 1813–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, R.; Hamblin, M. Antimicrobial Photosensitizers: Drug Discovery Under the Spotlight. Curr. Med. Chem. 2015, 22, 2159–2185. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Alsehli, M.; Scotti, L.; Tullius Scotti, M.; Tsai, S.-T.; Yu, R.-S.; Hsieh, M.F.; Chen, J.-C. Progress in Polymeric Nano-Medicines for Theranostic Cancer Treatment. Polymers 2020, 12, 598. [Google Scholar] [CrossRef] [Green Version]
- Josefsen, L.B.; Boyle, R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met.-Based Drugs 2008, 2008, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal Complexes as a Promising Source for New Antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, C.N.; Prosser, K.E.; Stokes, R.W.; Cordes, A.; Metzler-Nolte, N.; Cohen, S.M. Expanding Medicinal Chemistry into 3D Space: Metallofragments as 3D Scaffolds for Fragment-Based Drug Discovery. Chem. Sci. 2020, 11, 1216–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloway, W.R.J.D.; Isidro-Llobet, A.; Spring, D.R. Diversity-Oriented Synthesis as a Tool for the Discovery of Novel Biologically Active Small Molecules. Nat. Commun. 2010, 1, 80. [Google Scholar] [CrossRef] [Green Version]
- Hung, A.W.; Ramek, A.; Wang, Y.; Kaya, T.; Wilson, J.A.; Clemons, P.A.; Young, D.W. Route to Three-Dimensional Fragments Using Diversity-Oriented Synthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 6799–6804. [Google Scholar] [CrossRef] [Green Version]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 56. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium Complexes as Antimicrobial Agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [Green Version]
- Deng, T.; Zhao, H.; Shi, M.; Qiu, Y.; Jiang, S.; Yang, X.; Zhao, Y.; Zhang, Y. Photoactivated Trifunctional Platinum Nanobiotics for Precise Synergism of Multiple Antibacterial Modes. Small 2019, 15, 1902647. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Zelenka, J.; Rumlová, M.; Cvačka, J.; Ruml, T.; Lang, K. Cationic Octahedral Molybdenum Cluster Complexes Functionalized with Mitochondria-Targeting Ligands: Photodynamic Anticancer and Antibacterial Activities. Biomater. Sci. 2019, 7, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Sudhamani, C.N.; Bhojya Naik, H.S.; Sangeetha Gowda, K.R.; Girija, D.; Giridhar, M. DNA Binding, Prominent Photonuclease Activity and Antibacterial PDT of Cobalt(II) Complexes of Phenanthroline Based Photosensitizers. Nucleosides Nucleotides Nucleic Acids 2018, 37, 546–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Monro, S.; Cui, P.; Yin, H.; Liu, B.; Cameron, C.G.; Xu, W.; Hetu, M.; Fuller, A.; Kilina, S.; et al. Heteroleptic Ir(III)N 6 Complexes with Long-Lived Triplet Excited States and In Vitro Photobiological Activities. ACS Appl. Mater. Interfaces 2019, 11, 3629–3644. [Google Scholar] [CrossRef]
- Munteanu, A.-C.; Uivarosi, V. Ruthenium Complexes in the Fight against Pathogenic Microorganisms. An Extensive Review. Pharmaceutics 2021, 13, 874. [Google Scholar] [CrossRef]
- Jain, A.; Garrett, N.T.; Malone, Z.P. Ruthenium-based Photoactive Metalloantibiotics. Photochem. Photobiol. 2021, php.13435. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Rubbiani, R.; Tubafard, S.; Blacque, O.; Anstaett, P.; Felgenträger, A.; Maisch, T.; Spiccia, L.; Gasser, G. Synthesis, Characterization, and Biological Evaluation of New Ru(II) Polypyridyl Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2014, 57, 7280–7292. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Jian, Y.; Zhou, M.; Yao, Y.; Tian, N.; Li, C.; Chen, J.; Wang, X.; Zhou, Q. Selective and Efficient Photoinactivation of Intracellular Staphylococcus Aureus and MRSA with Little Accumulation of Drug Resistance: Application of a Ru(II) Complex with Photolabile Ligands. J. Med. Chem. 2021, 64, 7359–7370. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elshahat, M.E.; Elfiky, A.A. COVID-19 Spike-Host Cell Receptor GRP78 Binding Site Prediction. J. Infect. 2020, 80, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Khorsandi, K.; Fekrazad, S.; Vahdatinia, F.; Farmany, A.; Fekrazad, R. Nano Antiviral Photodynamic Therapy: A Probable Biophysicochemical Management Modality in SARS-CoV-2. Expert Opin. Drug Deliv. 2021, 18, 265–272. [Google Scholar] [CrossRef]
- Perni, S.; Prokopovich, P.; Pratten, J.; Parkin, I.P.; Wilson, M. Nanoparticles: Their Potential Use in Antibacterial Photodynamic Therapy. Photochem. Photobiol. Sci. 2011, 10, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Baek, K.-H. Multimetallic Nanoparticles as Alternative Antimicrobial Agents: Challenges and Perspectives. Molecules 2021, 26, 912. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, S.; Sui, M.; Chen, S.; Huang, L.; Xu, H.; Jiang, T. Multifunctional Nanocomplex for Surface-Enhanced Raman Scattering Imaging and near-Infrared Photodynamic Antimicrobial Therapy of Vancomycin-Resistant Bacteria. Colloids Surf. B Biointerfaces 2018, 161, 394–402. [Google Scholar] [CrossRef]
- Gupta, A.; Landis, R.F.; Rotello, V.M. Nanoparticle-Based Antimicrobials: Surface Functionality Is Critical. F1000Res 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Alfirdous, R.A.; Garcia, I.M.; Balhaddad, A.A.; Collares, F.M.; Martinho, F.C.; Melo, M.A.S. Advancing Photodynamic Therapy for Endodontic Disinfection with Nanoparticles: Present Evidence and Upcoming Approaches. Appl. Sci. 2021, 11, 4759. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Soares, A.B.; Mima, E.G.d.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Morsy, M.A.; Shinu, P.; Kotta, S.; Chandrasekaran, M.; Tahir, A. Advances of Non-iron Metal Nanoparticles in Biomedicine. J. Pharm. Pharm. Sci. 2021, 24, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Belekov, E.; Kholikov, K.; Cooper, L.; Banga, S.; Er, A.O. Improved Antimicrobial Properties of Methylene Blue Attached to Silver Nanoparticles. Photodiagn. Photodyn. Ther. 2020, 32, 102012. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottais, A.; Berchel, M.; Sibiril, Y.; Laurent, V.; Gill, D.; Hyde, S.; Jaffrès, P.-A.; Montier, T.; Le Gall, T. Antibacterial Effect and DNA Delivery Using a Combination of an Arsonium-Containing Lipophosphoramide with an N-Heterocyclic Carbene-Silver Complex—Potential Benefits for Cystic Fibrosis Lung Gene Therapy. Int. J. Pharm. 2018, 536, 29–41. [Google Scholar] [CrossRef]
- Ribeiro, M.S.; de Melo, L.S.A.; Farooq, S.; Baptista, A.; Kato, I.T.; Núñez, S.C.; de Araujo, R.E. Photodynamic Inactivation Assisted by Localized Surface Plasmon Resonance of Silver Nanoparticles: In Vitro Evaluation on Escherichia Coli and Streptococcus Mutans. Photodiagn. Photodyn. Ther. 2018, 22, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Shitomi, K.; Miyata, S.; Miyaji, H.; Aota, H.; Kawasaki, H. Bovine Serum Albumin-Capped Gold Nanoclusters Conjugating with Methylene Blue for Efficient 1O2 Generation via Energy Transfer. J. Colloid Interface Sci. 2018, 510, 221–227. [Google Scholar] [CrossRef]
- Rizzi, V.; Vurro, D.; Placido, T.; Fini, P.; Petrella, A.; Semeraro, P.; Cosma, P. Gold-Chlorophyll a-Hybrid Nanoparticles and Chlorophyll a/Cetyltrimethylammonium Chloride Self-Assembled-Suprastructures as Novel Carriers for Chlorophyll a Delivery in Water Medium: Photoactivity and Photostability. Colloids Surf. B Biointerfaces 2018, 161, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Miyaji, H.; Kawasaki, H.; Yamamoto, M.; Nishida, E.; Takita, H.; Akasaka, T.; Ushijima, N.; Iwanaga, T.; Sugaya, T. Antimicrobial Photodynamic Activity and Cytocompatibility of Au25(Capt)18 Clusters Photoexcited by Blue LED Light Irradiation. Int. J. Nanomed. 2017, 12, 2703–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubheka, G.; Uddin, I.; Amuhaya, E.; Mack, J.; Nyokong, T. Synthesis and Photophysicochemical Properties of BODIPY Dye Functionalized Gold Nanorods for Use in Antimicrobial Photodynamic Therapy. J. Porphyr. Phthalocyanines 2016, 20, 1016–1024. [Google Scholar] [CrossRef]
- Okamoto, I.; Miyaji, H.; Miyata, S.; Shitomi, K.; Sugaya, T.; Ushijima, N.; Akasaka, T.; Enya, S.; Saita, S.; Kawasaki, H. Antibacterial and Antibiofilm Photodynamic Activities of Lysozyme-Au Nanoclusters/Rose Bengal Conjugates. ACS Omega 2021, 6, 9279–9290. [Google Scholar] [CrossRef]
- Rigotto Caruso, G.; Tonani, L.; Marcato, P.D.; von Zeska Kress, M.R. Phenothiazinium Photosensitizers Associated with Silver Nanoparticles in Enhancement of Antimicrobial Photodynamic Therapy. Antibiotics 2021, 10, 569. [Google Scholar] [CrossRef]
- Morales-de-Echegaray, A.V.; Lin, L.; Sivasubramaniam, B.; Yermembetova, A.; Wang, Q.; Abutaleb, N.S.; Seleem, M.N.; Wei, A. Antimicrobial Photodynamic Activity of Gallium-Substituted Haemoglobin on Silver Nanoparticles. Nanoscale 2020, 12, 21734–21742. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, P.; Thamanna, R.Y.; Shaji, C.; Sharan, A.; Bahkali, A.H.; Al-Harthi, H.F.; Syed, A.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Biogenic Silver Nanoparticles Decorated with Methylene Blue Potentiated the Photodynamic Inactivation of Pseudomonas Aeruginosa and Staphylococcus Aureus. Pharmaceutics 2020, 12, 709. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, L.; Chen, J.; Liu, W.; Zhang, D.; Xu, P.; Dai, T.; Shang, L.; Yang, Y.; Tang, S.; et al. Composite of Silver Nanoparticles and Photosensitizer Leads to Mutual Enhancement of Antimicrobial Efficacy and Promotes Wound Healing. Chem. Eng. J. 2019, 374, 1373–1381. [Google Scholar] [CrossRef]
- Sen, P.; Nyokong, T. Enhanced Photodynamic Inactivation of Staphylococcus Aureus with Schiff Base Substituted Zinc Phthalocyanines through Conjugation to Silver Nanoparticles. J. Mol. Struct. 2021, 1232, 130012. [Google Scholar] [CrossRef]
- Shabangu, S.M.; Babu, B.; Soy, R.C.; Managa, M.; Sekhosana, K.E.; Nyokong, T. Photodynamic Antimicrobial Chemotherapy of Asymmetric Porphyrin-Silver Conjugates towards Photoinactivation of Staphylococcus Aureus. J. Coord. Chem. 2020, 73, 593–608. [Google Scholar] [CrossRef]
- Wanarska, E.; Maliszewska, I. Gold Nanoparticles in an Enhancement of Antimicrobial Activity. Physicochem. Probl. Miner. Process. 2020, 56, 269–279. [Google Scholar] [CrossRef]
- Managa, M.; Antunes, E.; Nyokong, T. Conjugates of Platinum Nanoparticles with Gallium Tetra—(4-Carboxyphenyl) Porphyrin and Their Use in Photodynamic Antimicrobial Chemotherapy When in Solution or Embedded in Electrospun Fiber. Polyhedron 2014, 76, 94–101. [Google Scholar] [CrossRef]
- Ermini, M.L.; Voliani, V. Antimicrobial Nano-Agents: The Copper Age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef]
- Zhen, X.; Chudal, L.; Pandey, N.K.; Phan, J.; Ran, X.; Amador, E.; Huang, X.; Johnson, O.; Ran, Y.; Chen, W.; et al. A Powerful Combination of Copper-Cysteamine Nanoparticles with Potassium Iodide for Bacterial Destruction. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110659. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.; Wu, J.; Vanasse, A.; Pandey, N.K.; Chudal, L.; Huang, Z.; Song, W.; Yu, H.; Ma, L.; Chen, W.; et al. Effects of Nanoparticle Size and Radiation Energy on Copper-Cysteamine Nanoparticles for X-Ray Induced Photodynamic Therapy. Nanomaterials 2020, 10, 1087. [Google Scholar] [CrossRef] [PubMed]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal Oxide Nanoparticles Against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef] [PubMed]
- Namanga, J.; Foba, J.; Ndinteh, D.T.; Yufanyi, D.M.; Krause, R.W.M. Synthesis and Magnetic Properties of a Superparamagnetic Nanocomposite "Pectin-Magnetite Nanocomposite". J. Nanomater. 2013, 2013, 87. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub, M.; Güvener, N.; Engelmann, U.; et al. Size-Isolation of Superparamagnetic Iron Oxide Nanoparticles Improves MRI, MPI and Hyperthermia Performance. J. Nanobiotechnol. 2020, 18, 22. [Google Scholar] [CrossRef]
- Alves, E.; Rodrigues, J.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Lin, Z.; Cunha, Â.; Nadais, M.H.; Tomé, J.P.C.; Almeida, A. A New Insight on Nanomagnet–Porphyrin Hybrids for Photodynamic Inactivation of Microorganisms. Dye. Pigment. 2014, 110, 80–88. [Google Scholar] [CrossRef]
- Bilici, K.; Atac, N.; Muti, A.; Baylam, I.; Dogan, O.; Sennaroglu, A.; Can, F.; Yagci Acar, H. Broad Spectrum Antibacterial Photodynamic and Photothermal Therapy Achieved with Indocyanine Green Loaded SPIONs under near Infrared Irradiation. Biomater. Sci. 2020, 8, 4616–4625. [Google Scholar] [CrossRef]
- de Santana, W.M.O.S.; Caetano, B.L.; de Annunzio, S.R.; Pulcinelli, S.H.; Ménager, C.; Fontana, C.R.; Santilli, C.V. Conjugation of Superparamagnetic Iron Oxide Nanoparticles and Curcumin Photosensitizer to Assist in Photodynamic Therapy. Colloids Surf. B Biointerfaces 2020, 196, 111297. [Google Scholar] [CrossRef] [PubMed]
- Dube, E.; Soy, R.; Shumba, M.; Nyokong, T. Photophysicochemical Behaviour of Phenoxy Propanoic Acid Functionalised Zinc Phthalocyanines When Grafted onto Iron Oxide and Silica Nanoparticles: Effects in Photodynamic Antimicrobial Chemotherapy. J. Lumin. 2021, 234, 117939. [Google Scholar] [CrossRef]
- Mapukata, S.; Nwahara, N.; Nyokong, T. The Photodynamic Antimicrobial Chemotherapy of Stapphylococcus Aureus Using an Asymmetrical Zinc Phthalocyanine Conjugated to Silver and Iron Oxide Based Nanoparticles. J. Photochem. Photobiol. A Chem. 2020, 402, 112813. [Google Scholar] [CrossRef]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Heredia, D.A.; Durantini, A.M.; Durantini, E.N. Magnetic Nanoplatforms for In Situ Modification of Macromolecules: Synthesis, Characterization, and Photoinactivating Power of Cationic Nanoiman–Porphyrin Conjugates. ACS Appl. Bio Mater. 2020, 3, 5930–5940. [Google Scholar] [CrossRef]
- Scanone, A.C.; Santamarina, S.C.; Heredia, D.A.; Durantini, E.N.; Durantini, A.M. Functionalized Magnetic Nanoparticles with BODIPYs for Bioimaging and Antimicrobial Therapy Applications. ACS Appl. Bio Mater. 2020, 3, 1061–1070. [Google Scholar] [CrossRef]
- Sun, X.; Wang, L.; Lynch, C.D.; Sun, X.; Li, X.; Qi, M.; Ma, C.; Li, C.; Dong, B.; Zhou, Y.; et al. Nanoparticles Having Amphiphilic Silane Containing Chlorin E6 with Strong Anti-Biofilm Activity against Periodontitis-Related Pathogens. J. Dent. 2019, 81, 70–84. [Google Scholar] [CrossRef]
- Toledo, V.H.; Yoshimura, T.M.; Pereira, S.T.; Castro, C.E.; Ferreira, F.F.; Ribeiro, M.S.; Haddad, P.S. Methylene Blue-Covered Superparamagnetic Iron Oxide Nanoparticles Combined with Red Light as a Novel Platform to Fight Non-Local Bacterial Infections: A Proof of Concept Study against Escherichia Coli. J. Photochem. Photobiol. B 2020, 209, 111956. [Google Scholar] [CrossRef]
- Qi, M.; Chi, M.; Sun, X.; Xie, X.; Weir, M.D.; Oates, T.W.; Zhou, Y.; Wang, L.; Bai, Y.; Xu, H.H. Novel Nanomaterial-Based Antibacterial Photodynamic Therapies to Combat Oral Bacterial Biofilms and Infectious Diseases. Int. J. Nanomed. 2019, 14, 6937–6956. [Google Scholar] [CrossRef] [Green Version]
- Collen Makola, L.; Nyokong, T.; Amuhaya, E.K. Impact of Axial Ligation on Photophysical and Photodynamic Antimicrobial Properties of Indium (III) Methylsulfanylphenyl Porphyrin Complexes Linked to Silver-Capped Copper Ferrite Magnetic Nanoparticles. Polyhedron 2021, 193, 114882. [Google Scholar] [CrossRef]
- Makola, L.C.; Managa, M.; Nyokong, T. Enhancement of Photodynamic Antimicrobialtherapy through the Use of Cationic Indium Porphyrin Conjugated to Ag/CuFe2O4 Nanoparticles. Photodiagn. Photodyn. Ther. 2020, 30, 101736. [Google Scholar] [CrossRef]
- Philip, S.; Kuriakose, S. Photodynamic Antifungal Activity of a Superparamagnetic and Fluorescent Drug Carrier System against Antibiotic-Resistant Fungal Strains. Cellulose 2021, 28, 9091–9102. [Google Scholar] [CrossRef]
- Sun, X.; Sun, J.; Sun, Y.; Li, C.; Fang, J.; Zhang, T.; Wan, Y.; Xu, L.; Zhou, Y.; Wang, L.; et al. Oxygen Self-Sufficient Nanoplatform for Enhanced and Selective Antibacterial Photodynamic Therapy against Anaerobe-Induced Periodontal Disease. Adv. Funct. Mater. 2021, 31, 2101040. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship Between Structure and Antimicrobial Activity of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joe, A.; Park, S.-H.; Shim, K.-D.; Kim, D.-J.; Jhee, K.-H.; Lee, H.-W.; Heo, C.-H.; Kim, H.-M.; Jang, E.-S. Antibacterial Mechanism of ZnO Nanoparticles under Dark Conditions. J. Ind. Eng. Chem. 2017, 45, 430–439. [Google Scholar] [CrossRef]
- Liou, J.-W.; Chang, H.-H. Bactericidal Effects and Mechanisms of Visible Light-Responsive Titanium Dioxide Photocatalysts on Pathogenic Bacteria. Arch. Immunol. Ther. Exp. 2012, 60, 267–275. [Google Scholar] [CrossRef]
- Oves, M.; Arshad, M.; Khan, M.S.; Ahmed, A.S.; Azam, A.; Ismail, I.M.I. Anti-Microbial Activity of Cobalt Doped Zinc Oxide Nanoparticles: Targeting Water Borne Bacteria. J. Saudi Chem. Soc. 2015, 19, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Raut, A.V.; Yadav, H.M.; Gnanamani, A.; Pushpavanam, S.; Pawar, S.H. Synthesis and Characterization of Chitosan-TiO2:Cu Nanocomposite and Their Enhanced Antimicrobial Activity with Visible Light. Colloids Surf. B Biointerfaces 2016, 148, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Dutta, A.; Khurgin, J.; Wei, A.; Shalaev, V.M.; Boltasseva, A. TiN@TiO2 Core–Shell Nanoparticles as Plasmon-Enhanced Photosensitizers: The Role of Hot Electron Injection. Laser Photonics Rev. 2020, 14, 1900376. [Google Scholar] [CrossRef]
- Page, K.; Wilson, M.; Parkin, I.P. Antimicrobial Surfaces and Their Potential in Reducing the Role of the Inanimate Environment in the Incidence of Hospital-Acquired Infections. J. Mater. Chem. 2009, 19, 3819–3831. [Google Scholar] [CrossRef]
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and Antimicrobial Activities of Chitosan-TiO2 Nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef]
- Pantaroto, H.N.; Ricomini-Filho, A.P.; Bertolini, M.M.; Dias da Silva, J.H.; Azevedo Neto, N.F.; Sukotjo, C.; Rangel, E.C.; Barão, V.A.R. Antibacterial Photocatalytic Activity of Different Crystalline TiO2 Phases in Oral Multispecies Biofilm. Dent. Mater. 2018, 34, e182–e195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suketa, N.; Sawase, T.; Kitaura, H.; Naito, M.; Baba, K.; Nakayama, K.; Wennerberg, A.; Atsuta, M. An Antibacterial Surface on Dental Implants, Based on the Photocatalytic Bactericidal Effect. Clin. Implant. Dent. Relat. Res. 2005, 7, 105–111. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef] [PubMed]
- De Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial Effect of Titanium Dioxide Nanoparticles; IntechOpen: London, UK, 2020; ISBN 978-1-83962-433-9. [Google Scholar]
- Farkhonde Masoule, S.; Pourhajibagher, M.; Safari, J.; Khoobi, M. Base-Free Green Synthesis of Copper(II) Oxide Nanoparticles Using Highly Cross-Linked Poly(Curcumin) Nanospheres: Synergistically Improved Antimicrobial Activity. Res. Chem. Intermed. 2019, 45, 4449–4462. [Google Scholar] [CrossRef]
- Varaprasad, K.; López, M.; Núñez, D.; Jayaramudu, T.; Sadiku, E.R.; Karthikeyan, C.; Oyarzúnc, P. Antibiotic Copper Oxide-Curcumin Nanomaterials for Antibacterial Applications. J. Mol. Liq. 2020, 300, 112353. [Google Scholar] [CrossRef]
- Xiu, W.; Gan, S.; Wen, Q.; Qiu, Q.; Dai, S.; Dong, H.; Li, Q.; Yuwen, L.; Weng, L.; Teng, Z.; et al. Biofilm Microenvironment-Responsive Nanotheranostics for Dual-Mode Imaging and Hypoxia-Relief-Enhanced Photodynamic Therapy of Bacterial Infections. Research 2020, 2020, 9426453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambhaji, R.B.; Vijay, J.S. Folate Tethered Gd2O3 Nanoparticles Exhibit Photoactive Antimicrobial Effects and pH Responsive Delivery of 5-Fluorouracil into MCF-7 Cells. Drug Deliv. Lett. 2019, 9, 58–68. [Google Scholar]
- Hossain, M.K.; Khan, M.I.; El-Denglawey, A. A Review on Biomedical Applications, Prospects, and Challenges of Rare Earth Oxides. Appl. Mater. Today 2021, 24, 101104. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Qin, Z.; Yin, S.; Sun, H. Transition Metal Oxide and Chalcogenide-Based Nanomaterials for Antibacterial Activities: An Overview. Nanoscale 2021, 13, 6373–6388. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCollum, C.R.; Levy, M.; Bertram, J.R.; Nagpal, P.; Chatterjee, A. Photoexcited Quantum Dots as Efficacious and Nontoxic Antibiotics in an Animal Model. ACS Biomater. Sci. Eng. 2021, 7, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Nain, A.; Wei, S.-C.; Lin, Y.-F.; Tseng, Y.-T.; Mandal, R.P.; Huang, Y.-F.; Huang, C.-C.; Tseng, F.-G.; Chang, H.-T. Copper Sulfide Nanoassemblies for Catalytic and Photoresponsive Eradication of Bacteria from Infected Wounds. ACS Appl. Mater. Interfaces 2021, 13, 7865–7878. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Scholle, F.; Ghiladi, R.A.; Dai, T.; Wu, M.X.; Popp, J. Mn-Doped Zn/S Quantum Dots as Photosensitizers for Antimicrobial Photodynamic Inactivation. In Photonic Diagnosis and Treatment of Infections and Inflammatory Diseases II; SPIE BiOS: San Francisco, CA, USA, 2019; p. 10863. [Google Scholar] [CrossRef]
- McCollum, C.R.; Bertram, J.R.; Nagpal, P.; Chatterjee, A. Photoactivated Indium Phosphide Quantum Dots Treat Multidrug-Resistant Bacterial Abscesses In Vivo. ACS Appl. Mater. Interfaces 2021, 13, 30404–30419. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Sun, Y.; Fan, S.; Boudreau, M.D.; Chen, C.; Ge, C.; Yin, J.-J. Photogenerated Charge Carriers in Molybdenum Disulfide Quantum Dots with Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2019, 11, 4858–4866. [Google Scholar] [CrossRef]
- Narband, N.; Mubarak, M.; Ready, D.; Parkin, I.P.; Nair, S.P.; Green, M.A.; Beeby, A.; Wilson, M. Quantum Dots as Enhancers of the Efficacy of Bacterial Lethal Photosensitization. Nanotechnology 2008, 19, 445102. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, J.; Chibli, H.; Carlini, L. Photosensitization of InP/ZnS Quantum Dots for Anti-Cancer and Anti-Microbial Applications. In Proceedings of the Colloidal Nanocrystals for Biomedical Applications VII; SPIE: San Francisco, CA, USA, 2012; Volume 8232, pp. 87–95. [Google Scholar]
- Liu, J.; Cheng, W.; Wang, Y.; Fan, X.; Shen, J.; Liu, H.; Wang, A.; Hui, A.; Nichols, F.; Chen, S. Cobalt-Doped Zinc Oxide Nanoparticle–MoS2 Nanosheet Composites as Broad-Spectrum Bactericidal Agents. ACS Appl. Nano Mater. 2021, 4, 4361–4370. [Google Scholar] [CrossRef]
- Courtney, C.M.; Goodman, S.M.; McDaniel, J.A.; Madinger, N.E.; Chatterjee, A.; Nagpal, P. Photoexcited Quantum Dots for Killing Multidrug-Resistant Bacteria. Nat. Mater. 2016, 15, 529–534. [Google Scholar] [CrossRef]
- Goodman, S.M.; Levy, M.; Li, F.-F.; Ding, Y.; Courtney, C.M.; Chowdhury, P.P.; Erbse, A.; Chatterjee, A.; Nagpal, P. Designing Superoxide-Generating Quantum Dots for Selective Light-Activated Nanotherapy. Front. Chem. 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, P.; Mathew, S.; Clarizia, L.; Kumar, R.S.; Akande, A.; Hinder, S.; Breen, A.; Pillai, S.C. Theoretical and Experimental Investigation of Visible Light Responsive AgBiS2-TiO2 Heterojunctions for Enhanced Photocatalytic Applications. Appl. Catal. B Environ. 2019, 253, 401–418. [Google Scholar] [CrossRef]
- Nazir, M.; Aziz, M.I.; Ali, I.; Basit, M.A. Revealing Antimicrobial and Contrasting Photocatalytic Behavior of Metal Chalcogenide Deposited P25-TiO2 Nanoparticles. Photonics Nanostruct.-Fundam. Appl. 2019, 36, 100721. [Google Scholar] [CrossRef]
- Suganya, M.; Balu, A.R.; Balamurugan, S.; Srivind, J.; Narasimman, V.; Manjula, N.; Rajashree, C.; Nagarethinam, V.S. Photoconductive, Photocatalytic and Antifungal Properties of PbS:Mo Nanoparticles Synthesized via Precipitation Method. Surf. Interfaces 2018, 13, 148–156. [Google Scholar] [CrossRef]
- Schlachter, A.; Asselin, P.; Harvey, P.D. Porphyrin-Containing MOFs and COFs as Heterogeneous Photosensitizers for Singlet Oxygen-Based Antimicrobial Nanodevices. ACS Appl. Mater. Interfaces 2021, 13, 26651–26672. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Song, L.; Sun, L.; Zhang, X.; Xin, Z.; Yin, J.; Luan, S. Metal-Organic Framework/Poly (ε-Caprolactone) Hybrid Electrospun Nanofibrous Membranes with Effective Photodynamic Antibacterial Activities. J. Photochem. Photobiol. A Chem. 2020, 400, 112626. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Mensah, A.; Wang, Q.; Huang, F.; Wei, Q. FRET as a Novel Strategy to Enhance the Singlet Oxygen Generation of Porphyrinic MOF Decorated Self-Disinfecting Fabrics. Chem. Eng. J. 2020, 395, 125012. [Google Scholar] [CrossRef]
- Yu, P.; Han, Y.; Han, D.; Liu, X.; Liang, Y.; Li, Z.; Zhu, S.; Wu, S. In-Situ Sulfuration of Cu-Based Metal-Organic Framework for Rapid near-Infrared Light Sterilization. J. Hazard. Mater. 2020, 390, 122126. [Google Scholar] [CrossRef]
- Xiong, K.; Li, J.; Tan, L.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y.; Zhu, S.; Liu, X. Ag2S Decorated Nanocubes with Enhanced Near-Infrared Photothermal and Photodynamic Properties for Rapid Sterilization. Colloid Interface Sci. Commun. 2019, 33, 100201. [Google Scholar] [CrossRef]
- Bagchi, D.; Bhattacharya, A.; Dutta, T.; Nag, S.; Wulferding, D.; Lemmens, P.; Pal, S.K. Nano MOF Entrapping Hydrophobic Photosensitizer for Dual-Stimuli-Responsive Unprecedented Therapeutic Action against Drug-Resistant Bacteria. ACS Appl. Bio Mater. 2019, 2, 1772–1780. [Google Scholar] [CrossRef]
- Golmohamadpour, A.; Bahramian, B.; Khoobi, M.; Pourhajibagher, M.; Barikani, H.R.; Bahador, A. Antimicrobial Photodynamic Therapy Assessment of Three Indocyanine Green-Loaded Metal-Organic Frameworks against Enterococcus Faecalis. Photodiagn. Photodyn. Ther. 2018, 23, 331–338. [Google Scholar] [CrossRef]
- Wang, M.; Nian, L.; Cheng, Y.; Yuan, B.; Cheng, S.; Cao, C. Encapsulation of Colloidal Semiconductor Quantum Dots into Metal-Organic Frameworks for Enhanced Antibacterial Activity through Interfacial Electron Transfer. Chem. Eng. J. 2021, 426, 130832. [Google Scholar] [CrossRef]
- Hynek, J.; Zelenka, J.; Rathouský, J.; Kubát, P.; Ruml, T.; Demel, J.; Lang, K. Designing Porphyrinic Covalent Organic Frameworks for the Photodynamic Inactivation of Bacteria. ACS Appl. Mater. Interfaces 2018, 10, 8527–8535. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent Progress in the Development of Upconversion Nanomaterials in Bioimaging and Disease Treatment. J. Nanobiotechnol. 2020, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ying, D.; Qi, M.; Li, X.; Fu, L.; Sun, X.; Wang, L.; Zhou, Y. Anti-Biofilm Property of Bioactive Upconversion Nanocomposites Containing Chlorin E6 against Periodontal Pathogens. Molecules 2019, 24, 2692. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Wang, F. Emerging Frontiers of Upconversion Nanoparticles. Trends Chem. 2020, 2, 427–439. [Google Scholar] [CrossRef]
- Yao, J.; Huang, C.; Liu, C.; Yang, M. Upconversion Luminescence Nanomaterials: A Versatile Platform for Imaging, Sensing, and Therapy. Talanta 2020, 208, 120157. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, S.; Liu, W.; Dai, T.; Ke, J.; Li, X.; Li, R.; Zhang, Y.; Chen, Z.; Chen, X. Synergistic Lysozyme-Photodynamic Therapy Against Resistant Bacteria Based on an Intelligent Upconversion Nanoplatform. Angew. Chem. Int. Ed. 2021, 60, 19201–19206. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Zhao, Y.; Hu, M.; Ma, D.; Zhang, P.; Xue, Y.; Li, X. Photomagnetic Nanoparticles in Dual-Modality Imaging and Photo-Sonodynamic Activity against Bacteria. Chem. Eng. J. 2019, 356, 811–818. [Google Scholar] [CrossRef]
- Li, S.; Cui, S.; Yin, D.; Zhu, Q.; Ma, Y.; Qian, Z.; Gu, Y. Dual Antibacterial Activities of a Chitosan-Modified Upconversion Photodynamic Therapy System against Drug-Resistant Bacteria in Deep Tissue. Nanoscale 2017, 9, 3912–3924. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, P.; Wang, D.; Chen, J.; Liu, W.; Hu, P.; Huang, M.; Chen, X.; Chen, Z. Near-Infrared-Triggered Antibacterial and Antifungal Photodynamic Therapy Based on Lanthanide-Doped Upconversion Nanoparticles. Nanoscale 2018, 10, 15485–15495. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Kaur, G.; Chaudhary, G.R.; Gawali, S.L.; Hassan, P.A. High Antimicrobial Photodynamic Activity of Photosensitizer Encapsulated Dual-Functional Metallocatanionic Vesicles against Drug-Resistant Bacteria S. Aureus. Biomater. Sci. 2020, 8, 2905–2920. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Berwal, K.; Sharma, B.; Chaudhary, G.R.; Gawali, S.L.; Hassan, P.A. Enhanced Antimicrobial Photodynamic Activity of Photosensitizer Encapsulated Copper Based Metallocatanionic Vesicles against E. coli Using Visible Light. J. Mol. Liq. 2021, 324, 114688. [Google Scholar] [CrossRef]
- Wang, D.; Niu, L.; Qiao, Z.-Y.; Cheng, D.-B.; Wang, J.; Zhong, Y.; Bai, F.; Wang, H.; Fan, H. Synthesis of Self-Assembled Porphyrin Nanoparticle Photosensitizers. ACS Nano 2018, 12, 3796–3803. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Yoo, J.; Kang, M.-H.; Kwon, W.; Joo, J. Photoluminescent and Biodegradable Porous Silicon Nanoparticles for Biomedical Imaging. J. Mater. Chem. B 2019, 7, 6271–6292. [Google Scholar] [CrossRef]
- Jabir, M.S.; Nayef, U.M.; Jawad, K.H.; Taqi, Z.J.; Buthenhia, A.H.; Ahmed, N.R. Porous Silicon Nanoparticles Prepared via an Improved Method: A Developing Strategy for a Successful Antimicrobial Agent against Escherichia Coli and Staphylococcus Aureus. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012077. [Google Scholar] [CrossRef]
- Xiao, L.; Gu, L.; Howell, S.B.; Sailor, M.J. Porous Silicon Nanoparticle Photosensitizers for Singlet Oxygen and Their Phototoxicity against Cancer Cells. ACS Nano 2011, 5, 3651–3659. [Google Scholar] [CrossRef]
- Secret, E.; Maynadier, M.; Gallud, A.; Gary-Bobo, M.; Chaix, A.; Belamie, E.; Maillard, P.; Sailor, M.J.; Garcia, M.; Durand, J.-O.; et al. Anionic Porphyrin-Grafted Porous Silicon Nanoparticles for Photodynamic Therapy. Chem. Commun. 2013, 49, 4202–4204. [Google Scholar] [CrossRef] [Green Version]
- Chaix, A.; Cheikh, K.E.; Bouffard, E.; Maynadier, M.; Aggad, D.; Stojanovic, V.; Knezevic, N.; Garcia, M.; Maillard, P.; Morère, A.; et al. Mesoporous Silicon Nanoparticles for Targeted Two-Photon Theranostics of Prostate Cancer. J. Mater. Chem. B 2016, 4, 3639–3642. [Google Scholar] [CrossRef]
- Näkki, S.; Martinez, J.O.; Evangelopoulos, M.; Xu, W.; Lehto, V.-P.; Tasciotti, E. Chlorin E6 Functionalized Theranostic Multistage Nanovectors Transported by Stem Cells for Effective Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 23441–23449. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Stojanovic, V.; Chaix, A.; Bouffard, E.; Cheikh, K.E.; Morère, A.; Maynadier, M.; Lemercier, G.; Garcia, M.; Gary-Bobo, M.; et al. Ruthenium(II) Complex-Photosensitized Multifunctionalized Porous Silicon Nanoparticles for Two-Photon near-Infrared Light Responsive Imaging and Photodynamic Cancer Therapy. J. Mater. Chem. B 2016, 4, 1337–1342. [Google Scholar] [CrossRef]
- Capeletti, L.B.; de Oliveira, L.F.; Gonçalves, K.d.A.; de Oliveira, J.F.A.; Saito, Â.; Kobarg, J.; dos Santos, J.H.Z.; Cardoso, M.B. Tailored Silica–Antibiotic Nanoparticles: Overcoming Bacterial Resistance with Low Cytotoxicity. Langmuir 2014, 30, 7456–7464. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Rogelj, S.; Zhang, P. Rose Bengal-Decorated Silica Nanoparticles as Photosensitizers for Inactivation of Gram-Positive Bacteria. Nanotechnology 2010, 21, 065102. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, P.; Qiao, Y.; Kang, Y.; Yan, C.; Yu, Z.; Wang, J.; He, X.; Wu, H. PH Responsive Superporogen Combined with PDT Based on Poly Ce6 Ionic Liquid Grafted on SiO2 for Combating MRSA Biofilm Infection. Theranostics 2020, 10, 4795–4808. [Google Scholar] [CrossRef]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Photodynamic Properties and Photoinactivation of Microorganisms Mediated by 5,10,15,20-Tetrakis(4-Carboxyphenyl)Porphyrin Covalently Linked to Silica-Coated Magnetite Nanoparticles. J. Photochem. Photobiol. A Chem. 2017, 346, 452–461. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Pires, S.M.G.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Daniel-da-Silva, A.L.; Almeida, A.; et al. Pyrrolidine-Fused Chlorin Photosensitizer Immobilized on Solid Supports for the Photoinactivation of Gram Negative Bacteria. Dye. Pigment. 2014, 110, 123–133. [Google Scholar] [CrossRef]
- Baigorria, E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Silica Nanoparticles Embedded with Water Insoluble Phthalocyanines for the Photoinactivation of Microorganisms. Photodiagn. Photodyn. Ther. 2018, 23, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Kaduskar, D.V. Comparative Study on Photodynamic Activation of Ortho-Toluidine Blue and Methylene Blue Loaded Mesoporous Silica Nanoparticles Against Resistant Microorganisms. Recent Pat. Drug Deliv. Formul. 2018, 12, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Paramanantham, P.; Siddhardha, B.; Sb, S.L.; Sharan, A.; Alyousef, A.A.; Dosary, M.S.A.; Arshad, M.; Syed, A. Antimicrobial Photodynamic Therapy on Staphylococcus Aureus and Escherichia Coli Using Malachite Green Encapsulated Mesoporous Silica Nanoparticles: An In Vitro Study. PeerJ 2019, 7, e7454. [Google Scholar] [CrossRef] [Green Version]
- Parasuraman, P.; Antony, A.P.; S.B, S.L.; Sharan, A.; Siddhardha, B.; Kasinathan, K.; Bahkali, N.A.; Dawoud, T.M.S.; Syed, A. Antimicrobial Photodynamic Activity of Toluidine Blue Encapsulated in Mesoporous Silica Nanoparticles against Pseudomonas Aeruginosa and Staphylococcus Aureus. Biofouling 2019, 35, 89–103. [Google Scholar] [CrossRef] [Green Version]
- Wysocka-Król, K.; Olsztyńska-Janus, S.; Plesch, G.; Plecenik, A.; Podbielska, H.; Bauer, J. Nano-Silver Modified Silica Particles in Antibacterial Photodynamic Therapy. Appl. Surf. Sci. 2018, 461, 260–268. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Xiao, Y.; Chen, F.; Xiao, F. A Multifunctional Nanoplatform Based on Mesoporous Silica Nanoparticles for Imaging-Guided Chemo/Photodynamic Synergetic Therapy. RSC Adv. 2017, 7, 31133–31141. [Google Scholar] [CrossRef] [Green Version]
- Grüner, M.C.; Arai, M.S.; Carreira, M.; Inada, N.; de Camargo, A.S.S. Functionalizing the Mesoporous Silica Shell of Upconversion Nanoparticles to Enhance Bacterial Targeting and Killing via Photosensitizer-Induced Antimicrobial Photodynamic Therapy. ACS Appl. Bio Mater. 2018, 1, 1028–1036. [Google Scholar] [CrossRef]
- Mapukata, S.; Britton, J.; Osifeko, O.L.; Nyokong, T. The Improved Antibacterial Efficiency of a Zinc Phthalocyanine When Embedded on Silver Nanoparticle Modified Silica Nanofibers. Photodiagn. Photodyn. Ther. 2021, 33, 102100. [Google Scholar] [CrossRef]
- Al-Omari, S. Toward a Molecular Understanding of the Photosensitizer-Copper Interaction for Tumor Destruction. Biophys. Rev. 2013, 5, 305–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckl, D.B.; Dengler, L.; Nemmert, M.; Eichner, A.; Bäumler, W.; Huber, H. A Closer Look at Dark Toxicity of the Photosensitizer TMPyP in Bacteria. Photochem. Photobiol. 2018, 94, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Liu, C.; Yan, K. CQDs-MoS2 QDs Loaded on Dendritic Fibrous Nanosilica/Hydrophobic Waterborne Polyurethane Acrylate for Antibacterial Coatings. Chem. Eng. J. 2022, 429, 132170. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, R.; Wang, J.; Wu, H.; Wang, Y.; Chen, A.; Shao, S.; Li, Y.-Q. A Bacteria-Activated Photodynamic Nanosystem Based on Polyelectrolyte-Coated Silica Nanoparticles. J. Mater. Chem. B 2017, 5, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, Photophysical Characterization, and Photoinduced Antibacterial Activity of Methylene Blue-Loaded Amino- and Mannose-Targeted Mesoporous Silica Nanoparticles. Molecules 2015, 20, 6284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramanantham, P.; Anju, V.T.; Dyavaiah, M.; Siddhardha, B. Applications of Carbon-Based Nanomaterials for Antimicrobial Photodynamic Therapy. In Microbial Nanobionics: Volume 2, Basic Research and Applications; Prasad, R., Ed.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 237–259. ISBN 978-3-030-16534-5. [Google Scholar]
- Alavi, M.; Jabari, E.; Jabbari, E. Functionalized Carbon-Based Nanomaterials and Quantum Dots with Antibacterial Activity: A Review. Expert Rev. Anti-Infect. Ther. 2021, 19, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of Nanocellulose/Nanocarbon Composites: Focus on Biotechnology and Medicine. Nanomaterials 2020, 10, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agazzi, M.L.; Durantini, J.E.; Gsponer, N.S.; Durantini, A.M.; Bertolotti, S.G.; Durantini, E.N. Light-Harvesting Antenna and Proton-Activated Photodynamic Effect of a Novel BODIPY−Fullerene C60 Dyad as Potential Antimicrobial Agent. ChemPhysChem 2019, 20, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, M.L.; Almodovar, V.A.S.; Gsponer, N.S.; Bertolotti, S.; Tomé, A.C.; Durantini, E.N. Diketopyrrolopyrrole–Fullerene C60 Architectures as Highly Efficient Heavy Atom-Free Photosensitizers: Synthesis, Photophysical Properties and Photodynamic Activity. Org. Biomol. Chem. 2020, 18, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, M.L.; Durantini, J.E.; Quiroga, E.D.; Alvarez, M.G.; Durantini, E.N. A Novel Tricationic Fullerene C60 as Broad-Spectrum Antimicrobial Photosensitizer: Mechanisms of Action and Potentiation with Potassium Iodide. Photochem. Photobiol. Sci. 2021, 20, 327–341. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Sharma, S.K.; Yin, R.; Agrawal, T.; Chiang, L.Y.; Hamblin, M.R. Functionalized Fullerenes in Photodynamic Therapy. J. Biomed. Nanotechnol. 2014, 10, 1918–1936. [Google Scholar] [CrossRef] [PubMed]
- Palacios, Y.B.; Durantini, J.E.; Heredia, D.A.; Martínez, S.R.; González de la Torre, L.; Durantini, A.M. Tuning the Polarity of Fullerene C60 Derivatives for Enhanced Photodynamic Inactivation. Photochem. Photobiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Reynoso, E.; Durantini, A.M.; Solis, C.A.; Macor, L.P.; Otero, L.A.; Gervaldo, M.A.; Durantini, E.N.; Heredia, D.A. Photoactive Antimicrobial Coating Based on a PEDOT-Fullerene C60 Polymeric Dyad. RSC Adv. 2021, 11, 23519–23532. [Google Scholar] [CrossRef]
- Spesia, M.B.; Milanesio, M.E.; Durantini, E.N. Chapter 18—Fullerene Derivatives in Photodynamic Inactivation of Microorganisms. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 413–433. ISBN 978-0-323-46152-8. [Google Scholar]
- Anju, V.T.; Paramanantham, P.; S.B, S.L.; Sharan, A.; Syed, A.; Bahkali, N.A.; Alsaedi, M.H.; K., K.; Busi, S. Antimicrobial Photodynamic Activity of Toluidine Blue-Carbon Nanotube Conjugate against Pseudomonas Aeruginosa and Staphylococcus Aureus—Understanding the Mechanism of Action. Photodiagn. Photodyn. Ther. 2019, 27, 305–316. [Google Scholar] [CrossRef]
- Tondro, G.H.; Behzadpour, N.; Keykhaee, Z.; Akbari, N.; Sattarahmady, N. Carbon@polypyrrole Nanotubes as a Photosensitizer in Laser Phototherapy of Pseudomonas Aeruginosa. Colloids Surf. B Biointerfaces 2019, 180, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Anju, V.T.; Paramanantham, P.; Siddhardha, B.; Lal, S.S.; Sharan, A.; Alyousef, A.A.; Arshad, M.; Syed, A. Malachite Green-Conjugated Multi-Walled Carbon Nanotubes Potentiate Antimicrobial Photodynamic Inactivation of Planktonic Cells and Biofilms of Pseudomonas Aeruginosa and Staphylococcus Aureus. Int. J. Nanomed. 2019, 14, 3861–3874. [Google Scholar] [CrossRef] [Green Version]
- Parasuraman, P.; Anju, V.T.; Lal, S.S.; Sharan, A.; Busi, S.; Kaviyarasu, K.; Arshad, M.; Dawoud, T.M.S.; Syed, A. Synthesis and Antimicrobial Photodynamic Effect of Methylene Blue Conjugated Carbon Nanotubes on E. Coli and S. Aureus. Photochem. Photobiol. Sci. 2019, 18, 563–576. [Google Scholar] [CrossRef]
- Vt, A.; Paramanantham, P.; Sb, S.L.; Sharan, A.; Alsaedi, M.H.; Dawoud, T.M.S.; Asad, S.; Busi, S. Antimicrobial Photodynamic Activity of Rose Bengal Conjugated Multi Walled Carbon Nanotubes against Planktonic Cells and Biofilm of Escherichia Coli. Photodiagn. Photodyn. Ther. 2018, 24, 300–310. [Google Scholar] [CrossRef]
- Sah, U.; Sharma, K.; Chaudhri, N.; Sankar, M.; Gopinath, P. Antimicrobial Photodynamic Therapy: Single-Walled Carbon Nanotube (SWCNT)-Porphyrin Conjugate for Visible Light Mediated Inactivation of Staphylococcus Aureus. Colloids Surf. B Biointerfaces 2018, 162, 108–117. [Google Scholar] [CrossRef]
- Szunerits, S.; Barras, A.; Boukherroub, R. Antibacterial Applications of Nanodiamonds. Int. J. Environ. Res Public Health 2016, 13, 413. [Google Scholar] [CrossRef]
- Hurtado, C.R.; Hurtado, G.R.; de Cena, G.L.; Queiroz, R.C.; Silva, A.V.; Diniz, M.F.; dos Santos, V.R.; Trava-Airoldi, V.; Baptista, M.d.S.; Tsolekile, N.; et al. Diamond Nanoparticles-Porphyrin MTHPP Conjugate as Photosensitizing Platform: Cytotoxicity and Antibacterial Activity. Nanomaterials 2021, 11, 1393. [Google Scholar] [CrossRef]
- Openda, Y.I.; Nyokong, T. Enhanced Photo-Ablation Effect of Positively Charged Phthalocyanines-Detonation Nanodiamonds Nanoplatforms for the Suppression of Staphylococcus Aureus and Escherichia Coli Planktonic Cells and Biofilms. J. Photochem. Photobiol. A Chem. 2021, 411, 113200. [Google Scholar] [CrossRef]
- Openda, Y.I.; Nyokong, T. Detonation Nanodiamonds-Phthalocyanine Photosensitizers with Enhanced Photophysicochemical Properties and Effective Photoantibacterial Activity. Photodiagn. Photodyn. Ther. 2020, 32, 102072. [Google Scholar] [CrossRef]
- Openda, Y.I.; Ngoy, B.P.; Nyokong, T. Photodynamic Antimicrobial Action of Asymmetrical Porphyrins Functionalized Silver-Detonation Nanodiamonds Nanoplatforms for the Suppression of Staphylococcus Aureus Planktonic Cells and Biofilms. Front. Chem. 2021, 9, 97. [Google Scholar] [CrossRef]
- Gayen, B.; Palchoudhury, S.; Chowdhury, J. Carbon Dots: A Mystic Star in the World of Nanoscience. J. Nanomater. 2019, 2019, e3451307. [Google Scholar] [CrossRef] [Green Version]
- Knoblauch, R.; Geddes, C.D. Carbon Nanodots in Photodynamic Antimicrobial Therapy: A Review. Materials 2020, 13, 4004. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Lakshmi, B.A.; Kim, S.; Kim, J. Preparation of Shape-Specific (Trilateral and Quadrilateral) Carbon Quantum Dots towards Multiple Color Emission. Nanoscale 2020, 12, 11947–11959. [Google Scholar] [CrossRef]
- Dong, X.; Ge, L.; Abu Rabe, D.I.; Mohammed, O.O.; Wang, P.; Tang, Y.; Kathariou, S.; Yang, L.; Sun, Y.-P. Photoexcited State Properties and Antibacterial Activities of Carbon Dots Relevant to Mechanistic Features and Implications. Carbon 2020, 170, 137–145. [Google Scholar] [CrossRef]
- Knoblauch, R.; Harvey, A.; Geddes, C.D. Metal-Enhanced Photosensitization of Singlet Oxygen (ME1O2) from Brominated Carbon Nanodots on Silver Nanoparticle Substrates. Plasmonics 2021, 16, 1765–1772. [Google Scholar] [CrossRef]
- Kováčová, M.; Špitalská, E.; Markovic, Z.; Špitálský, Z. Carbon Quantum Dots As Antibacterial Photosensitizers and Their Polymer Nanocomposite Applications. Part. Part. Syst. Charact. 2020, 37, 1900348. [Google Scholar] [CrossRef]
- Marković, Z.M.; Kováčová, M.; Humpolíček, P.; Budimir, M.D.; Vajďák, J.; Kubát, P.; Mičušík, M.; Švajdlenková, H.; Danko, M.; Capáková, Z.; et al. Antibacterial Photodynamic Activity of Carbon Quantum Dots/Polydimethylsiloxane Nanocomposites against Staphylococcus Aureus, Escherichia Coli and Klebsiella Pneumoniae. Photodiagn. Photodyn. Ther. 2019, 26, 342–349. [Google Scholar] [CrossRef]
- Tejwan, N.; Saini, A.K.; Sharma, A.; Singh, T.A.; Kumar, N.; Das, J. Metal-Doped and Hybrid Carbon Dots: A Comprehensive Review on Their Synthesis and Biomedical Applications. J. Control. Release 2021, 330, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, X.; Tang, D.; Wu, S.; Hou, X.; Liu, J.; Wu, P. Phosphorescent Carbon Dots for Highly Efficient Oxygen Photosensitization and as Photo-Oxidative Nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, J.; Chen, Y.; Fu, F.; Chi, Y.; Chen, G. Graphene Quantum Dots, Graphene Oxide, Carbon Quantum Dots and Graphite Nanocrystals in Coals. Nanoscale 2014, 6, 7410–7415. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene Quantum Dots from Chemistry to Applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Cui, F.; Ye, Y.; Ping, J.; Sun, X. Carbon Dots: Current Advances in Pathogenic Bacteria Monitoring and Prospect Applications. Biosens. Bioelectron. 2020, 156, 112085. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Wei, S.-C.; Chou, C.P.; Zhang, L.-Z.; Huang, C.-C. Graphene Oxide and Carbon Dots as Broad-Spectrum Antimicrobial Agents—A Minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Karahan, H.E.; Wiraja, C.; Xu, C.; Wei, J.; Wang, Y.; Wang, L.; Liu, F.; Chen, Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, 1701406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, W.-S.; Shao, Y.-T.; Huang, K.-S.; Chou, T.-M.; Yang, C.-H. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef]
- Ristic, B.Z.; Milenkovic, M.M.; Dakic, I.R.; Todorovic-Markovic, B.M.; Milosavljevic, M.S.; Budimir, M.D.; Paunovic, V.G.; Dramicanin, M.D.; Markovic, Z.M.; Trajkovic, V.S. Photodynamic Antibacterial Effect of Graphene Quantum Dots. Biomaterials 2014, 35, 4428–4435. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Nwahara, N.; Nyokong, T. Photodynamic Antimicrobial Activity of Benzimidazole Substituted Phthalocyanine When Conjugated to Nitrogen Doped Graphene Quantum Dots against Staphylococcus Aureus. Main Group Chem. 2021, 20, 175–191. [Google Scholar] [CrossRef]
- Yu, Y.; Mei, L.; Shi, Y.; Zhang, X.; Cheng, K.; Cao, F.; Zhang, L.; Xu, J.; Li, X.; Xu, Z. Ag-Conjugated Graphene Quantum Dots with Blue Light-Enhanced Singlet Oxygen Generation for Ternary-Mode Highly-Efficient Antimicrobial Therapy. J. Mater. Chem. B 2020, 8, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Ferro, S.; Ricchelli, F.; Mancini, G.; Tognon, G.; Jori, G. Inactivation of Methicillin-Resistant Staphylococcus Aureus (MRSA) by Liposome-Delivered Photosensitising Agents. J. Photochem. Photobiol. B Biol. 2006, 83, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Lee, J.; Im, B.N.; Park, H.; Na, K. Combined Photodynamic and Antibiotic Therapy for Skin Disorder via Lipase-Sensitive Liposomes with Enhanced Antimicrobial Performance. Biomaterials 2017, 141, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Miretti, M.; Juri, L.; Cosiansi, M.C.; Tempesti, T.C.; Baumgartner, M.T. Antimicrobial Effects of ZnPc Delivered into Liposomes on Multidrug Resistant (MDR)-Mycobacterium Tuberculosis. ChemistrySelect 2019, 4, 9726–9730. [Google Scholar] [CrossRef]
- Plenagl, N.; Seitz, B.S.; Duse, L.; Pinnapireddy, S.R.; Jedelska, J.; Brüßler, J.; Bakowsky, U. Hypericin Inclusion Complexes Encapsulated in Liposomes for Antimicrobial Photodynamic Therapy. Int. J. Pharm. 2019, 570, 118666. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, L.; Dlugaszewska, J.; Ziental, D.; Szczolko, W.; Koczorowski, T.; Goslinski, T.; Mielcarek, J. Optical Properties of a Series of Pyrrolyl-Substituted Porphyrazines and Their Photoinactivation Potential against Enterococcus Faecalis after Incorporation into Liposomes. J. Photochem. Photobiol. A Chem. 2019, 368, 104–109. [Google Scholar] [CrossRef]
- Wieczorek, E.; Mlynarczyk, D.T.; Kucinska, M.; Dlugaszewska, J.; Piskorz, J.; Popenda, L.; Szczolko, W.; Jurga, S.; Murias, M.; Mielcarek, J.; et al. Photophysical Properties and Photocytotoxicity of Free and Liposome-Entrapped Diazepinoporphyrazines on LNCaP Cells under Normoxic and Hypoxic Conditions. Eur. J. Med. Chem. 2018, 150, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Babaie, S.; Bakhshayesh, A.R.D.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A Novel Nanocarrier for Transdermal Drug Delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, J.; Jeong, S.; Im, B.N.; Kim, M.-K.; Yang, S.-G.; Na, K. Lipase-Sensitive Transfersomes Based on Photosensitizer/Polymerizable Lipid Conjugate for Selective Antimicrobial Photodynamic Therapy of Acne. Adv. Healthc. Mater. 2016, 5, 3139–3147. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Zhang, F.; Zeng, K.; Zhu, X. Antifungal Photodynamic Activity of Hexyl-Aminolevulinate Ethosomes Against Candida Albicans Biofilm. Front. Microbiol. 2020, 11, 2052. [Google Scholar] [CrossRef]
- Caruso, E.; Orlandi, V.T.; Malacarne, M.C.; Martegani, E.; Scanferla, C.; Pappalardo, D.; Vigliotta, G.; Izzo, L. Bodipy-Loaded Micelles Based on Polylactide as Surface Coating for Photodynamic Control of Staphylococcus Aureus. Coatings 2021, 11, 223. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Costa, L.D.; Prandini, J.A.; Biazzotto, J.C.; Tomé, A.C.; Hamblin, M.R.; da Graça, P.M.S. Neves, M.; Faustino, M.A.F.; da Silva, R.S. The Photosensitizing Efficacy of Micelles Containing a Porphyrinic Photosensitizer and KI against Resistant Melanoma Cells. Chem.—A Eur. J. 2021, 27, 1990–1994. [Google Scholar] [CrossRef]
- Dantas Lopes dos Santos, D.; Besegato, J.F.; de Melo, P.B.G.; Oshiro Junior, J.A.; Chorilli, M.; Deng, D.; Bagnato, V.S.; Rastelli, A.N.d.S. Curcumin-Loaded Pluronic® F-127 Micelles as a Drug Delivery System for Curcumin-Mediated Photodynamic Therapy for Oral Application. Photochem. Photobiol. 2021, 97, 1072–1088. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Do Bonfim, C.M.; Monteleoni, L.F.; Calmon, M.d.F.; Cândido, N.M.; Provazzi, P.J.S.; de Souza Lino, V.; Rabachini, T.; Sichero, L.; Villa, L.L.; Quintana, S.M.; et al. Antiviral Activity of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Vulvar Cell Lines Transducing Different Variants of HPV-16. Artif. Cells Nanomed. Biotechnol. 2020, 48, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Osorno, L.L.; Brandley, A.N.; Maldonado, D.E.; Yiantsos, A.; Mosley, R.J.; Byrne, M.E. Review of Contemporary Self-Assembled Systems for the Controlled Delivery of Therapeutics in Medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, X.; Yang, Y.; Bai, H.; Cui, Q.; Sun, H.; Li, L.; Wang, S. Conjugated Polymer with Aggregation-Directed Intramolecular Förster Resonance Energy Transfer Enabling Efficient Discrimination and Killing of Microbial Pathogens. Chem. Mater. 2018, 30, 3244–3253. [Google Scholar] [CrossRef]
- Meng, Z.; Hou, W.; Zhou, H.; Zhou, L.; Chen, H.; Wu, C. Therapeutic Considerations and Conjugated Polymer-Based Photosensitizers for Photodynamic Therapy. Macromol. Rapid Commun. 2018, 39, 1700614. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, W.; Manghnani, P.; Xu, S.; Wang, Y.; Goh, C.C.; Ng, L.G.; Liu, B. Polymerization-Enhanced Two-Photon Photosensitization for Precise Photodynamic Therapy. ACS Nano 2019, 13, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Mao, D.; Xu, S.; Kenry; Hu, F.; Li, X.; Kong, D.; Liu, B. Polymerization-Enhanced Photosensitization. Chem 2018, 4, 1937–1951. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; He, P.; Wang, J.; Chen, H.; Lv, F.; Liu, L.; Wang, S.; Yoon, J. Antimicrobial Activity of a Conjugated Polymer with Cationic Backbone. Dye. Pigment. 2019, 160, 519–523. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, D. New Photosensitizer Design Concept: Polymerization-Enhanced Photosensitization. Chem 2018, 4, 2013–2015. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Hu, R.; Wang, L.; Qiu, Y.; Zhang, G.; Deng, Q.; Zhang, H.; Yin, P.; Situ, B.; Zhan, C.; et al. An AIE-Active Conjugated Polymer with High ROS-Generation Ability and Biocompatibility for Efficient Photodynamic Therapy of Bacterial Infections. Angew. Chem. Int. Ed. 2020, 59, 9952–9956. [Google Scholar] [CrossRef]
- Huang, L.; Zhiyentayev, T.; Xuan, Y.; Azhibek, D.; Kharkwal, G.B.; Hamblin, M.R. Photodynamic Inactivation of Bacteria Using Polyethylenimine–Chlorin(E6) Conjugates: Effect of Polymer Molecular Weight, Substitution Ratio of Chlorin(E6) and PH. Lasers Surg. Med. 2011, 43, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Gavara, R.; de Llanos, R.; Pérez-Laguna, V.; Arnau del Valle, C.; Miravet, J.F.; Rezusta, A.; Galindo, F. Broad-Spectrum Photo-Antimicrobial Polymers Based on Cationic Polystyrene and Rose Bengal. Front. Med. 2021, 8, 641646. [Google Scholar] [CrossRef]
- Yang, Y.; Cai, Z.; Huang, Z.; Tang, X.; Zhang, X. Antimicrobial Cationic Polymers: From Structural Design to Functional Control. Polym. J. 2018, 50, 33–44. [Google Scholar] [CrossRef]
- Ahmetali, E.; Sen, P.; Süer, N.C.; Aksu, B.; Nyokong, T.; Eren, T.; Şener, M.K. Enhanced Light-Driven Antimicrobial Activity of Cationic Poly(Oxanorbornene)s by Phthalocyanine Incorporation into Polymer as Pendants. Macromol. Chem. Phys. 2020, 221, 2000386. [Google Scholar] [CrossRef]
- Li, T.; Yan, L. Functional Polymer Nanocarriers for Photodynamic Therapy. Pharmaceuticals 2018, 11, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.-Y.; Shen, S.; Xu, C.-F.; Li, H.-J.; Liu, Y.; Cao, Z.-T.; Yang, X.-Z.; Xia, J.-X.; Wang, J. Tumor Acidity-Sensitive Polymeric Vector for Active Targeted SiRNA Delivery. J. Am. Chem. Soc. 2015, 137, 15217–15224. [Google Scholar] [CrossRef]
- Zhou, J.; Li, T.; Zhang, C.; Xiao, J.; Cui, D.; Cheng, Y. Charge-Switchable Nanocapsules with Multistage PH-Responsive Behaviours for Enhanced Tumour-Targeted Chemo/Photodynamic Therapy Guided by NIR/MR Imaging. Nanoscale 2018, 10, 9707–9719. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharmacol. 2017, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Svenson, S. Dendrimers as Versatile Platform in Drug Delivery Applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Khanna, S.N. A Systematic Framework and Nanoperiodic Concept for Unifying Nanoscience: Hard/Soft Nanoelements, Superatoms, Meta-Atoms, New Emerging Properties, Periodic Property Patterns, and Predictive Mendeleev-like Nanoperiodic Tables. Chem. Rev. 2016, 116, 2705–2774. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.-O.; Majoral, J.-P. Biological Properties of Water-Soluble Phosphorhydrazone Dendrimers. Braz. J. Pharm. Sci. 2013, 49, 33–44. [Google Scholar] [CrossRef]
- Staegemann, M.H.; Gräfe, S.; Gitter, B.; Achazi, K.; Quaas, E.; Haag, R.; Wiehe, A. Hyperbranched Polyglycerol Loaded with (Zinc-)Porphyrins: Photosensitizer Release Under Reductive and Acidic Conditions for Improved Photodynamic Therapy. Biomacromolecules 2018, 19, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Staegemann, M.H.; Gitter, B.; Dernedde, J.; Kuehne, C.; Haag, R.; Wiehe, A. Mannose-Functionalized Hyperbranched Polyglycerol Loaded with Zinc Porphyrin: Investigation of the Multivalency Effect in Antibacterial Photodynamic Therapy. Chem.—A Eur. J. 2017, 23, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Kubát, P.; Henke, P.; Raya, R.K.; Štěpánek, M.; Mosinger, J. Polystyrene and Poly(Ethylene Glycol)-b-Poly(ε-Caprolactone) Nanoparticles with Porphyrins: Structure, Size, and Photooxidation Properties. Langmuir 2020, 36, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Gualdesi, M.S.; Vara, J.; Aiassa, V.; Alvarez Igarzabal, C.I.; Ortiz, C.S. New Poly(Acrylamide) Nanoparticles in the Development of Third Generation Photosensitizers. Dye. Pigment. 2021, 184, 108856. [Google Scholar] [CrossRef]
- Malá, Z.; Žárská, L.; Malina, L.; Langová, K.; Večeřová, R.; Kolář, M.; Henke, P.; Mosinger, J.; Kolářová, H. Photodynamic Effect of TPP Encapsulated in Polystyrene Nanoparticles toward Multi-Resistant Pathogenic Bacterial Strains: AFM Evaluation. Sci. Rep. 2021, 11, 6786. [Google Scholar] [CrossRef]
- Ahmadi, H.; Haddadi-Asl, V.; Ghafari, H.-A.; Ghorbanzadeh, R.; Mazlum, Y.; Bahador, A. Shear Bond Strength, Adhesive Remnant Index, and Anti-Biofilm Effects of a Photoexcited Modified Orthodontic Adhesive Containing Curcumin Doped Poly Lactic-Co-Glycolic Acid Nanoparticles: An Ex-Vivo Biofilm Model of S. Mutans on the Enamel Slab Bonded Brackets. Photodiagn. Photodyn. Ther. 2020, 30, 101674. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Yuan, H.; Lu, W.; Li, Z.; Wang, L.; Gao, L.-H. Conjugated Polymer and Triphenylamine Derivative Codoped Nanoparticles for Photothermal and Photodynamic Antimicrobial Therapy. ACS Appl. Bio. Mater. 2020, 3, 3494–3499. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Zhao, H.; Qi, R.; Chen, Z.; Yuan, H.; Liang, H.; Wang, L. Dual-Mode Antibacterial Conjugated Polymer Nanoparticles for Photothermal and Photodynamic Therapy. Macromol. Biosci. 2020, 20, e1900301. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Kirar, S.; Thakur, N.S.; Laha, J.K.; Banerjee, U.C. Porphyrin Functionalized Gelatin Nanoparticle-Based Biodegradable Phototheranostics: Potential Tools for Antimicrobial Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 4202–4212. [Google Scholar] [CrossRef]
- Barbosa, P.M.; Campanholi, K.d.S.S.; Vilsinski, B.H.; Ferreira, S.B.d.S.; Gonçalves, R.S.; de Castro-Hoshino, L.V.; de Oliveira, A.C.V.; Sato, F.; Baesso, M.L.; Bruschi, M.L.; et al. Aluminum Phthalocyanine Hydroxide-Loaded Thermoresponsive Biomedical Hydrogel: A Design for Targeted Photosensitizing Drug Delivery. J. Mol. Liq. 2021, 341, 117421. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, E.; Weng, Q.; Zhou, L.; Li, Q. Optimization of Hydrogel Containing Toluidine Blue O for Photodynamic Therapy in Treating Acne. Lasers Med. Sci. 2019, 34, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, K.; He, Y.; Du, B.; Hong, J.; Yin, H.; Lu, D.; Luo, F.; Li, Z.; Li, J.; et al. Tough and Biodegradable Polyurethane-Curcumin Composited Hydrogel with Antioxidant, Antibacterial and Antitumor Properties. Mater. Sci. Eng. C 2021, 121, 111820. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.M.; Garcia, M.T.; de Barros, P.P.; Pedroso, L.L.C.; Ward, R.A.d.C.; Strixino, J.F.; Melo, V.M.M.; Junqueira, J.C. Chitosan Enhances the Antimicrobial Photodynamic Inactivation Mediated by Photoditazine® against Streptococcus Mutans. Photodiagn. Photodyn. Ther. 2020, 32, 102001. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. ISBN 9789811502637. [Google Scholar]
- Pourhajibagher, M.; Rokn, A.R.; Rostami-Rad, M.; Barikani, H.R.; Bahador, A. Monitoring of Virulence Factors and Metabolic Activity in Aggregatibacter Actinomycetemcomitans Cells Surviving Antimicrobial Photodynamic Therapy via Nano-Chitosan Encapsulated Indocyanine Green. Front. Phys. 2018, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Rad, M.R.; Pourhajibagher, M.; Rokn, A.R.; Barikani, H.R.; Bahador, A. Effect of Antimicrobial Photodynamic Therapy Using Indocyanine Green Doped with Chitosan Nanoparticles on Biofilm Formation-Related Gene Expression of Aggregatibacter Actinomycetemcomitans. Front. Dent. 2019, 16, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Kishen, A. Polycationic Chitosan-Conjugated Photosensitizer for Antibacterial Photodynamic Therapy. Photochem. Photobiol. 2012, 88, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Zhou, M.; Wang, C.; Feng, P.; Miao, W.; Huang, H. Photodynamic Chitosan Nano-Assembly as a Potent Alternative Candidate for Combating Antibiotic-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 26711–26721. [Google Scholar] [CrossRef]
- Rizzi, V.; Fini, P.; Fanelli, F.; Placido, T.; Semeraro, P.; Sibillano, T.; Fraix, A.; Sortino, S.; Agostiano, A.; Giannini, C.; et al. Molecular Interactions, Characterization and Photoactivity of Chlorophyll a/Chitosan/2-HP-β-Cyclodextrin Composite Films as Functional and Active Surfaces for ROS Production. Food Hydrocoll. 2016, 58, 98–112. [Google Scholar] [CrossRef]
- Sharma, M.; Dube, A.; Majumder, S.K. Antibacterial Photodynamic Activity of Photosensitizer-Embedded Alginate-Pectin-Carboxymethyl Cellulose Composite Biopolymer Films. Lasers Med. Sci. 2021, 36, 763–772. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Abdelraof, M.; Fikry, M.; Shaker, Y.M.; Sweed, A.M.K.; Senge, M.O. Development of Antimicrobial Laser-Induced Photodynamic Therapy Based on Ethylcellulose/Chitosan Nanocomposite with 5,10,15,20-Tetrakis(m-Hydroxyphenyl)Porphyrin. Molecules 2021, 26, 3551. [Google Scholar] [CrossRef]
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart Hydrogel-Based DVDMS/BFGF Nanohybrids for Antibacterial Phototherapy with Multiple Damaging Sites and Accelerated Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169. [Google Scholar] [CrossRef]
- Müller, A.; Preuß, A.; Bornhütter, T.; Thomas, I.; Prager, A.; Schulze, A.; Röder, B. Electron Beam Functionalized Photodynamic Polyethersulfone Membranes—Photophysical Characterization and Antimicrobial Activity. Photochem. Photobiol. Sci. 2018, 17, 1346–1354. [Google Scholar] [CrossRef]
- Martínez, S.R.; Palacios, Y.B.; Heredia, D.A.; Aiassa, V.; Bartolilla, A.; Durantini, A.M. Self-Sterilizing 3D-Printed Polylactic Acid Surfaces Coated with a BODIPY Photosensitizer. ACS Appl. Mater. Interfaces 2021, 13, 11597–11608. [Google Scholar] [CrossRef]
- Santos, M.R.E.; Mendonça, P.V.; Branco, R.; Sousa, R.; Dias, C.; Serra, A.C.; Fernandes, J.R.; Magalhães, F.D.; Morais, P.V.; Coelho, J.F.J. Light-Activated Antimicrobial Surfaces Using Industrial Varnish Formulations to Mitigate the Incidence of Nosocomial Infections. ACS Appl. Mater. Interfaces 2021, 13, 7567–7579. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Contreras, A.; Andaloussi, S.A.; Coradin, T.; Hélary, C.; Razza, N.; Sangermano, M.; Mazeran, P.-E.; Malval, J.-P.; Versace, D.-L. Eosin-Mediated Synthesis of Polymer Coatings Combining Photodynamic Inactivation and Antimicrobial Properties. J. Mater. Chem. B 2017, 5, 7572–7582. [Google Scholar] [CrossRef] [PubMed]
- Kováčová, M.; Kleinová, A.; Vajďák, J.; Humpolíček, P.; Kubát, P.; Bodík, M.; Marković, Z.; Špitálský, Z. Photodynamic-Active Smart Biocompatible Material for an Antibacterial Surface Coating. J. Photochem. Photobiol. B Biol. 2020, 211, 112012. [Google Scholar] [CrossRef] [PubMed]
- Wylie, M.P.; Irwin, N.J.; Howard, D.; Heydon, K.; McCoy, C.P. Hot-Melt Extrusion of Photodynamic Antimicrobial Polymers for Prevention of Microbial Contamination. J. Photochem. Photobiol. B Biol. 2021, 214, 112098. [Google Scholar] [CrossRef] [PubMed]
- Moor, K.J.; Osuji, C.O.; Kim, J.-H. Dual-Functionality Fullerene and Silver Nanoparticle Antimicrobial Composites via Block Copolymer Templates. ACS Appl. Mater. Interfaces 2016, 8, 33583–33591. [Google Scholar] [CrossRef]
- Mafukidze, D.M.; Sindelo, A.; Nyokong, T. Spectroscopic Characterization and Photodynamic Antimicrobial Chemotherapy of Phthalocyanine-Silver Triangular Nanoprism Conjugates When Supported on Asymmetric Polymer Membranes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 219, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fan, Y.; Zhang, P.; Zhang, X.; Zhou, Q.; Zhao, J.; Ren, L. Self-Enriched Mesoporous Silica Nanoparticle Composite Membrane with Remarkable Photodynamic Antimicrobial Performances. J. Colloid Interface Sci. 2020, 559, 197–205. [Google Scholar] [CrossRef]
- Heredia, D.A.; Martínez, S.R.; Durantini, A.M.; Pérez, M.E.; Mangione, M.I.; Durantini, J.E.; Gervaldo, M.A.; Otero, L.A.; Durantini, E.N. Antimicrobial Photodynamic Polymeric Films Bearing Biscarbazol Triphenylamine End-Capped Dendrimeric Zn(II) Porphyrin. ACS Appl. Mater. Interfaces 2019, 11, 27574–27587. [Google Scholar] [CrossRef] [PubMed]
- Peddinti, B.S.T.; Scholle, F.; Ghiladi, R.A.; Spontak, R.J. Photodynamic Polymers as Comprehensive Anti-Infective Materials: Staying Ahead of a Growing Global Threat. ACS Appl. Mater. Interfaces 2018, 10, 25955–25959. [Google Scholar] [CrossRef]
- Baigorria, E.; Milanesio, M.E.; Durantini, E.N. Synthesis, Spectroscopic Properties and Photodynamic Activity of Zn(II) Phthalocyanine-Polymer Conjugates as Antimicrobial Agents. Eur. Polym. J. 2020, 134, 109816. [Google Scholar] [CrossRef]
- Ambrósio, J.A.R.; Pinto, B.C.D.S.; da Silva, B.G.M.; Passos, J.C.d.S.; Beltrame Junior, M.; Costa, M.S.; Simioni, A.R. BSA Nanoparticles Loaded-Methylene Blue for Photodynamic Antimicrobial Chemotherapy (PACT): Effect on Both Growth and Biofilm Formation by Candida Albicans. J. Biomater. Sci. Polym. Ed. 2020, 31, 2182–2198. [Google Scholar] [CrossRef]
- Silva, E.P.O.; Ribeiro, N.M.; Cardoso, M.A.G.; Pacheco-Soares, C.; Jr, M.B. Photodynamic Therapy Using Silicon Phthalocyanine Conjugated with Bovine Serum Albumin as a Drug Delivery System. Laser Phys. 2021, 31, 075601. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Specific Drug Delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Dolanský, J.; Henke, P.; Malá, Z.; Žárská, L.; Kubát, P.; Mosinger, J. Antibacterial Nitric Oxide- and Singlet Oxygen-Releasing Polystyrene Nanoparticles Responsive to Light and Temperature Triggers. Nanoscale 2018, 10, 2639–2648. [Google Scholar] [CrossRef]
- Ren, B.; Li, K.; Liu, Z.; Liu, G.; Wang, H. White Light-Triggered Zwitterionic Polymer Nanoparticles Based on an AIE-Active Photosensitizer for Photodynamic Antimicrobial Therapy. J. Mater. Chem. B 2020, 8, 10754–10763. [Google Scholar] [CrossRef]
- Wang, H.; He, J.; Zhang, M.; Tao, Y.; Li, F.; Tam, K.C.; Ni, P. Biocompatible and Acid-Cleavable Poly(ε-Caprolactone)-Acetal-Poly(Ethylene Glycol)-Acetal-Poly(ε-Caprolactone) Triblock Copolymers: Synthesis, Characterization and PH-Triggered Doxorubicin Delivery. J. Mater. Chem. B 2013, 1, 6596–6607. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-Responsive Polymeric Micelles for Drug Delivery and Cancer Therapy. IJN 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, Y.; Eisenberg, A. Self-Assembly of Block Copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Shen, H.; Eisenberg, A. Morphological Phase Diagram for a Ternary System of Block Copolymer PS310-b-PAA52/Dioxane/H2O. J. Phys. Chem. B 1999, 103, 9473–9487. [Google Scholar] [CrossRef]
- Zhang, L.; Eisenberg, A. Multiple Morphologies of “Crew-Cut” Aggregates of Polystyrene-b-Poly(Acrylic Acid) Block Copolymers. Science 1995, 268, 1728–1731. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, e3702518. [Google Scholar] [CrossRef]
- Won, Y.-Y.; Brannan, A.K.; Davis, H.T.; Bates, F.S. Cryogenic Transmission Electron Microscopy (Cryo-TEM) of Micelles and Vesicles Formed in Water by Poly(Ethylene Oxide)-Based Block Copolymers. J. Phys. Chem. B 2002, 106, 3354–3364. [Google Scholar] [CrossRef]
- Haas, S.; Chen, Y.; Fuchs, C.; Handschuh, S.; Steuber, M.; Schönherr, H. Amphiphilic Block Copolymer Vesicles for Active Wound Dressings: Synthesis of Model Systems and Studies of Encapsulation and Release. Macromol. Symp. 2013, 328, 73–79. [Google Scholar] [CrossRef]
- Haas, S.; Hain, N.; Raoufi, M.; Handschuh-Wang, S.; Wang, T.; Jiang, X.; Schönherr, H. Enzyme Degradable Polymersomes from Hyaluronic Acid-Block-Poly(ε-Caprolactone) Copolymers for the Detection of Enzymes of Pathogenic Bacteria. Biomacromolecules 2015, 16, 832–841. [Google Scholar] [CrossRef]
- Handschuh-Wang, S.; Wesner, D.; Wang, T.; Lu, P.; Tücking, K.-S.; Haas, S.; Druzhinin, S.I.; Jiang, X.; Schönherr, H. Determination of the Wall Thickness of Block Copolymer Vesicles by Fluorescence Lifetime Imaging Microscopy. Macromol. Chem. Phys. 2017, 218, 1600454. [Google Scholar] [CrossRef]
- Tücking, K.-S.; Handschuh-Wang, S.; Schönherr, H.; Tücking, K.-S.; Handschuh-Wang, S.; Schönherr, H. Bacterial Enzyme Responsive Polymersomes: A Closer Look at the Degradation Mechanism of PEG-Block-PLA Vesicles. Aust. J. Chem. 2014, 67, 578–584. [Google Scholar] [CrossRef]
- Tang, Q.; Hu, P.; Peng, H.; Zhang, N.; Zheng, Q.; He, Y. Near-Infrared Laser-Triggered, Self-Immolative Smart Polymersomes for in Vivo Cancer Therapy. Int. J. Nanomed. 2020, 15, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Knop, K.; Mingotaud, A.-F.; El-Akra, N.; Violleau, F.; Souchard, J.-P. Monomeric Pheophorbide(a)-Containing Poly(Ethyleneglycol-b-ε-Caprolactone) Micelles for Photodynamic Therapy. Photochem. Photobiol. Sci. 2009, 8, 396–404. [Google Scholar] [CrossRef]
- Zhang, G.-D.; Harada, A.; Nishiyama, N.; Jiang, D.-L.; Koyama, H.; Aida, T.; Kataoka, K. Polyion Complex Micelles Entrapping Cationic Dendrimer Porphyrin: Effective Photosensitizer for Photodynamic Therapy of Cancer. J. Control. Release 2003, 93, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Xiao-ying, Z.; Pei-ying, Z. Polymersomes in Nanomedicine—A Review. Curr. Nanosci. 2017, 13, 124–129. [Google Scholar]
- Lanzilotto, A.; Kyropoulou, M.; Constable, E.C.; Housecroft, C.E.; Meier, W.P.; Palivan, C.G. Porphyrin-Polymer Nanocompartments: Singlet Oxygen Generation and Antimicrobial Activity. J. Biol. Inorg. Chem. 2018, 23, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Fan, F.; Ma, J.; Yin, W.; Zhu, D.; Zhang, L.; Wang, Z. Oxygen- and Bubble-Generating Polymersomes for Tumor-Targeted and Enhanced Photothermal–Photodynamic Combination Therapy. Biomater. Sci. 2021, 9, 5841–5853. [Google Scholar] [CrossRef]
- Avci, P.; Erdem, S.S.; Hamblin, M.R. Photodynamic Therapy: One Step Ahead with Self-Assembled Nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1937–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibot, L.; Demazeau, M.; Pimienta, V.; Mingotaud, A.-F.; Vicendo, P.; Collin, F.; Martins-Froment, N.; Dejean, S.; Nottelet, B.; Roux, C.; et al. Role of Polymer Micelles in the Delivery of Photodynamic Therapy Agent to Liposomes and Cells. Cancers 2020, 12, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karges, J.; Tharaud, M.; Gasser, G. Polymeric Encapsulation of a Ru(II)-Based Photosensitizer for Folate-Targeted Photodynamic Therapy of Drug Resistant Cancers. J. Med. Chem. 2021, 64, 4612–4622. [Google Scholar] [CrossRef] [PubMed]
- Kashef, N.; Huang, Y.-Y.; Hamblin, M.R. Advances in Antimicrobial Photodynamic Inactivation at the Nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef] [Green Version]
- Vilsinski, B.H.; Gerola, A.P.; Enumo, J.A.; Campanholi, K.d.S.S.; Pereira, P.C.d.S.; Braga, G.; Hioka, N.; Kimura, E.; Tessaro, A.L.; Caetano, W. Formulation of Aluminum Chloride Phthalocyanine in Pluronic(TM) P-123 and F-127 Block Copolymer Micelles: Photophysical Properties and Photodynamic Inactivation of Microorganisms. Photochem. Photobiol. 2015, 91, 518–525. [Google Scholar] [CrossRef]
- Tsai, T.; Yang, Y.-T.; Wang, T.-H.; Chien, H.-F.; Chen, C.-T. Improved Photodynamic Inactivation of Gram-Positive Bacteria Using Hematoporphyrin Encapsulated in Liposomes and Micelles. Lasers Surg. Med. 2009, 41, 316–322. [Google Scholar] [CrossRef]
- Teng, F.; Deng, P.; Song, Z.; Zhou, F.; Feng, R. Enhanced Effect in Combination of Curcumin- and Ketoconazole-Loaded Methoxy Poly (Ethylene Glycol)-Poly (ε-Caprolactone) Micelles. Biomed. Pharmacother. 2017, 88, 43–51. [Google Scholar] [CrossRef]
- Yi, X.; Hu, J.-J.; Dai, J.; Lou, X.; Zhao, Z.; Xia, F.; Tang, B.Z. Self-Guiding Polymeric Prodrug Micelles with Two Aggregation-Induced Emission Photosensitizers for Enhanced Chemo-Photodynamic Therapy. ACS Nano 2021, 15, 3026–3037. [Google Scholar] [CrossRef]
- Demir, B.; Barlas, F.B.; Gumus, Z.P.; Unak, P.; Timur, S. Theranostic Niosomes as a Promising Tool for Combined Therapy and Diagnosis: “All-in-One” Approach. ACS Appl. Nano Mater. 2018, 1, 2827–2835. [Google Scholar] [CrossRef]
- Fadel, M.A.; Tawfik, A.A. New Topical Photodynamic Therapy for Treatment of Hidradenitis Suppurativa Using Methylene Blue Niosomal Gel: A Single-Blind, Randomized, Comparative Study. Clin. Exp. Dermatol. 2015, 40, 116–122. [Google Scholar] [CrossRef]
- Belali, S.; Karimi, A.R.; Hadizadeh, M. Novel Nanostructured Smart, Photodynamic Hydrogels Based on Poly(N-Isopropylacrylamide) Bearing Porphyrin Units in Their Crosslink Chains: A Potential Sensitizer System in Cancer Therapy. Polymer 2017, 109, 93–105. [Google Scholar] [CrossRef]
- Preis, E.; Anders, T.; Širc, J.; Hobzova, R.; Cocarta, A.-I.; Bakowsky, U.; Jedelská, J. Biocompatible Indocyanine Green Loaded PLA Nanofibers for In Situ Antimicrobial Photodynamic Therapy. Mater. Sci. Eng. C 2020, 115, 111068. [Google Scholar] [CrossRef]
- Stoll, K.R.; Scholle, F.; Zhu, J.; Zhang, X.; Ghiladi, R.A. BODIPY-Embedded Electrospun Materials in Antimicrobial Photodynamic Inactivation. Photochem. Photobiol. Sci. 2019, 18, 1923–1932. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Li, L.; Wang, X.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Wool/Acrylic Blended Fabrics as Next-Generation Photodynamic Antimicrobial Materials. ACS Appl. Mater. Interfaces 2019, 11, 29557–29568. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Raxworthy, M.J.; Wood, S.; Schiffman, J.D.; Tronci, G. Photodynamically Active Electrospun Fibers for Antibiotic-Free Infection Control. ACS Appl. Bio Mater. 2019, 2, 4258–4270. [Google Scholar] [CrossRef]
- Czapka, T.; Winkler, A.; Maliszewska, I.; Kacprzyk, R. Fabrication of Photoactive Electrospun Cellulose Acetate Nanofibers for Antibacterial Applications. Energies 2021, 14, 2598. [Google Scholar] [CrossRef]
- Managa, M.; Amuhaya, E.K.; Nyokong, T. Photodynamic Antimicrobial Chemotherapy Activity of (5,10,15,20-Tetrakis(4-(4-Carboxyphenycarbonoimidoyl)Phenyl)Porphyrinato) Chloro Gallium(III). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Sindelo, A.; Nyokong, T. Magnetic Nanoparticle—Indium Phthalocyanine Conjugate Embedded in Electrospun Fiber for Photodynamic Antimicrobial Chemotherapy and Photodegradation of Methyl Red. Heliyon 2019, 5, e02352. [Google Scholar] [CrossRef] [Green Version]
- Barra, F.; Roscetto, E.; Soriano, A.A.; Vollaro, A.; Postiglione, I.; Pierantoni, G.M.; Palumbo, G.; Catania, M.R. Photodynamic and Antibiotic Therapy in Combination to Fight Biofilms and Resistant Surface Bacterial Infections. Int. J. Mol. Sci. 2015, 16, 20417–20430. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.J.; de Avila, E.D.; Klein, M.I.; Panariello, B.H.D.; Spolidório, D.M.P.; Pavarina, A.C. Antimicrobial Photodynamic Therapy Alone or in Combination with Antibiotic Local Administration against Biofilms of Fusobacterium Nucleatum and Porphyromonas Gingivalis. J. Photochem. Photobiol. B Biol. 2018, 188, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Pan, W.; Shi, E.; Bai, L.; Liu, H.; Li, C.; Wang, Y.; Deng, J.; Wang, Y. A Multifunctional Nanosystem Based on Bacterial Cell-Penetrating Photosensitizer for Fighting Periodontitis via Combining Photodynamic and Antibiotic Therapies. ACS Biomater. Sci. Eng. 2021, 7, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; García-Luque, I.; Ballesta, S.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; Rezusta, A.; Gilaberte, Y. Photodynamic Therapy Using Methylene Blue, Combined or Not with Gentamicin, against Staphylococcus Aureus and Pseudomonas Aeruginosa. Photodiagn. Photodyn. Ther. 2020, 31, 101810. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Palanisami, A.; Ashraf, S.; Bhayana, B.; Hasan, T. Photodynamic Inactivation of Bacterial Carbapenemases Restores Bacterial Carbapenem Susceptibility and Enhances Carbapenem Antibiotic Effectiveness. Photodiagn. Photodyn. Ther. 2020, 30, 101693. [Google Scholar] [CrossRef]
- Núñez, C.; Palavecino, A.; González, I.A.; Dreyse, P.; Palavecino, C.E. Effective Photodynamic Therapy with Ir(III) for Virulent Clinical Isolates of Extended-Spectrum Beta-Lactamase Klebsiella Pneumoniae. Pharmaceutics 2021, 13, 603. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.M.; Lorenzón, E.N.; Cilli, E.M.; de Oliveira, K.T.; Fontana, C.R.; Mang, T.S. Photodynamic and Peptide-Based Strategy to Inhibit Gram-Positive Bacterial Biofilm Formation. Biofouling 2019, 35, 742–757. [Google Scholar] [CrossRef]
- Cantelli, A.; Piro, F.; Pecchini, P.; Di Giosia, M.; Danielli, A.; Calvaresi, M. Concanavalin A-Rose Bengal Bioconjugate for Targeted Gram-Negative Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2020, 206, 111852. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; Dong, P.-T.; Goh, X.S.; Lu, M.; Cheng, J.-X.; Hooper, D.C.; Dai, T. Quinine Enhances Photo-Inactivation of Gram-Negative Bacteria. J. Infect. Dis. 2020, 221, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, X.; Lu, S.; Hamblin, M.R.; Mylonakis, E.; Zhang, J.; Xi, L. Photodynamic Therapy Combined with Terbinafine Against Chromoblastomycosis and the Effect of PDT on Fonsecaea Monophora In Vitro. Mycopathologia 2015, 179, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.; Lu, S.; Zheng, B.; Tang, Z.; Li, J.; Zhang, J. Combinatory Effect of ALA-PDT and Itraconazole Treatment for Trichosporon Asahii. Lasers Surg. Med. 2020, 53, 722–730. [Google Scholar] [CrossRef]
- Ayaz, F.; Gonul, İ.; Demirbag, B.; Ocakoglu, K. Differential Immunomodulatory Activities of Schiff Base Complexes Depending on Their Metal Conjugation. Inflammation 2019, 42, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Sadraeian, M.; Bahou, C.; da Cruz, E.F.; Janini, L.M.R.; Sobhie Diaz, R.; Boyle, R.W.; Chudasama, V.; Eduardo Gontijo Guimarães, F. Photoimmunotherapy Using Cationic and Anionic Photosensitizer-Antibody Conjugates against HIV Env-Expressing Cells. Int. J. Mol. Sci. 2020, 21, 9151. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Pavarina, A.C.; Mima, E.G.d.O.; McHale, A.P.; Callan, J.F. Antimicrobial Sonodynamic and Photodynamic Therapies against Candida Albicans. Biofouling 2018, 34, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Hu, M.; Liu, C.; Choi, S.K. Yolk-Structured Multifunctional Up-Conversion Nanoparticles for Synergistic Photodynamic–Sonodynamic Antibacterial Resistance Therapy. Biomater. Sci. 2017, 5, 678–685. [Google Scholar] [CrossRef] [PubMed]
- de Melo, W.d.C.M.A.; Lee, A.N.; Perussi, J.R.; Hamblin, M.R. Electroporation Enhances Antimicrobial Photodynamic Therapy Mediated by the Hydrophobic Photosensitizer, Hypericin. Photodiagn. Photodyn. Ther. 2013, 10, 647–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Yang, Y.; Guo, Y.; Lu, S.; Du, Y.; Li, J.-J.; Zhang, X.; Leung, N.L.C.; Zhao, Z.; Niu, G.; et al. Phage-Guided Targeting, Discriminative Imaging, and Synergistic Killing of Bacteria by AIE Bioconjugates. J. Am. Chem. Soc. 2020, 142, 3959–3969. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Shi, H.; Zhang, X.; Chen, X.; Cao, D.; Mao, C.; Gao, X.; Wang, L. Difunctional Bacteriophage Conjugated with Photosensitizers for Candida Albicans-Targeting Photodynamic Inactivation. Int. J. Nanomed. 2018, 13, 2199–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suci, P.A.; Varpness, Z.; Gillitzer, E.; Douglas, T.; Young, M. Targeting and Photodynamic Killing of a Microbial Pathogen Using Protein Cage Architectures Functionalized with a Photosensitizer. Langmuir 2007, 23, 12280–12286. [Google Scholar] [CrossRef]
- Tosato, M.G.; Schilardi, P.; Lorenzo de Mele, M.F.; Thomas, A.H.; Lorente, C.; Miñán, A. Synergistic Effect of Carboxypterin and Methylene Blue Applied to Antimicrobial Photodynamic Therapy against Mature Biofilm of Klebsiella Pneumoniae. Heliyon 2020, 6, e03522. [Google Scholar] [CrossRef] [PubMed]
- Openda, Y.I.; Sen, P.; Managa, M.; Nyokong, T. Acetophenone Substituted Phthalocyanines and Their Graphene Quantum Dots Conjugates as Photosensitizers for Photodynamic Antimicrobial Chemotherapy against Staphylococcus Aureus. Photodiagn. Photodyn. Ther. 2020, 29, 101607. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Viana, S.M.; Ng, D.K.P.; Kolli, B.K.; Chang, K.P.; de Oliveira, C.I. Photodynamic Inactivation of Leishmania Braziliensis Doubly Sensitized with Uroporphyrin and Diamino-Phthalocyanine Activates Effector Functions of Macrophages In Vitro. Sci. Rep. 2020, 10, 17065. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, G.; Pan, J.; Chen, X.; Huang, N.; Wang, X.; Liu, J. Effective PDT/PTT Dual-Modal Phototherapeutic Killing of Pathogenic Bacteria by Using Ruthenium Nanoparticles. J. Mater. Chem. B 2016, 4, 6258–6270. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.P.; Marangoni, V.S.; de Faria, C.G.; Leite, I.S.; Silva, C.; de Carvalho Castro e Silva, C.; Maroneze, C.M.; Pereira-da-Silva, M.A.; Bagnato, V.S.; Inada, N.M. Graphene Oxide Mediated Broad-Spectrum Antibacterial Based on Bimodal Action of Photodynamic and Photothermal Effects. Front. Microbiol. 2020, 10, 2995. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Tao, B.; He, Y.; Mu, C.; Liu, G.; Zhang, J.; Liao, Q.; Liu, P.; Cai, K. Remote Eradication of Biofilm on Titanium Implant via Near-Infrared Light Triggered Photothermal/Photodynamic Therapy Strategy. Biomaterials 2019, 223, 119479. [Google Scholar] [CrossRef]
- Dolanský, J.; Henke, P.; Kubát, P.; Fraix, A.; Sortino, S.; Mosinger, J. Polystyrene Nanofiber Materials for Visible-Light-Driven Dual Antibacterial Action via Simultaneous Photogeneration of NO and O2((1)Δg). ACS Appl. Mater. Interfaces 2015, 7, 22980–22989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, H.; Tao, X.; Xie, Y.; Yang, L.; Mao, Z.; Xia, W. Light-Triggered Nitric Oxide Release by a Photosensitizer to Combat Bacterial Biofilm Infections. Chem. Eur. J. 2021, 27, 5453–5460. [Google Scholar] [CrossRef]
- Miñán, A.; Lorente, C.; Ipiña, A.; Thomas, A.H.; Fernández Lorenzo de Mele, M.; Schilardi, P.L. Photodynamic Inactivation Induced by Carboxypterin: A Novel Non-Toxic Bactericidal Strategy against Planktonic Cells and Biofilms of Staphylococcus Aureus. Biofouling 2015, 31, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Potentiation of Antimicrobial Photodynamic Inactivation by Inorganic Salts. Expert Rev. Anti-Infect. Ther. 2017, 15, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; St. Denis, T.G.; Xuan, Y.; Huang, Y.-Y.; Tanaka, M.; Zadlo, A.; Sarna, T.; Hamblin, M.R. Paradoxical Potentiation of Methylene Blue-Mediated Antimicrobial Photodynamic Inactivation by Sodium Azide: Role of Ambient Oxygen and Azide Radicals. Free Radic. Biol. Med. 2012, 53, 2062–2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasimova, K.R.; Sadasivam, M.; Landi, G.; Sarna, T.; Hamblin, M.R. Potentiation of Photoinactivation of Gram-Positive and Gram-Negative Bacteria Mediated by Six Phenothiazinium Dyes by Addition of Azide Ion. Photochem. Photobiol. Sci. 2014, 13, 1541–1548. [Google Scholar] [CrossRef]
- Vecchio, D.; Gupta, A.; Huang, L.; Landi, G.; Avci, P.; Rodas, A.; Hamblin, M.R. Bacterial Photodynamic Inactivation Mediated by Methylene Blue and Red Light Is Enhanced by Synergistic Effect of Potassium Iodide. Antimicrob. Agents Chemother. 2015, 59, 5203–5212. [Google Scholar] [CrossRef] [Green Version]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight Into the Potentiation Effect of Potassium Iodide on APDT Efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [Green Version]
- Reynoso, E.; Quiroga, E.D.; Agazzi, M.L.; Ballatore, M.B.; Bertolotti, S.G.; Durantini, E.N. Photodynamic Inactivation of Microorganisms Sensitized by Cationic BODIPY Derivatives Potentiated by Potassium Iodide. Photochem. Photobiol. Sci. 2017, 16, 1524–1536. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.E.; Durantini, J.E.; Reynoso, E.; Alvarez, M.G.; Milanesio, M.E.; Durantini, E.N. Porphyrin–Schiff Base Conjugates Bearing Basic Amino Groups as Antimicrobial Phototherapeutic Agents. Molecules 2021, 26, 5877. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Inorganic Salts and Antimicrobial Photodynamic Therapy: Mechanistic Conundrums? Molecules 2018, 23, 3190. [Google Scholar] [CrossRef] [Green Version]
- Gsponer, N.S.; Spesia, M.B.; Durantini, E.N. Effects of Divalent Cations, EDTA and Chitosan on the Uptake and Photoinactivation of Escherichia Coli Mediated by Cationic and Anionic Porphyrins. Photodiagn. Photodyn. Ther. 2015, 12, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R. A Combination of Photodynamic Therapy and Antimicrobial Compounds to Treat Skin and Mucosal Infections: A Systematic Review. Photochem. Photobiol. Sci. 2019, 18, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Cahan, R.; Swissa, N.; Gellerman, G.; Nitzan, Y. Photosensitizer–Antibiotic Conjugates: A Novel Class of Antibacterial Molecules. Photochem. Photobiol. 2010, 86, 418–425. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, T.; Huang, L.; Kurup, D.B.; Tegos, G.P.; Jahnke, A.; Wharton, T.; Hamblin, M.R. Photodynamic Therapy with a Cationic Functionalized Fullerene Rescues Mice from Fatal Wound Infections. Nanomedicine 2010, 5, 1525–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, T.L.; Markus, E.A.; Hassett, D.J.; Robinson, J.B. The Effect of a Cationic Porphyrin on Pseudomonas Aeruginosa Biofilms. Curr. Microbiol. 2010, 61, 411–416. [Google Scholar] [CrossRef]
- Almeida, J.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Costa, L.; Faustino, M.A.F.; Almeida, A. Photodynamic Inactivation of Multidrug-Resistant Bacteria in Hospital Wastewaters: Influence of Residual Antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Di Poto, A.; Sbarra, M.S.; Provenza, G.; Visai, L.; Speziale, P. The Effect of Photodynamic Treatment Combined with Antibiotic Action or Host Defence Mechanisms on Staphylococcus Aureus Biofilms. Biomaterials 2009, 30, 3158–3166. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Mroz, P.; Dai, T.; Huang, L.; Morimoto, Y.; Kinoshita, M.; Yoshihara, Y.; Shinomiya, N.; Seki, S.; Nemoto, K.; et al. Linezolid and Vancomycin Decrease the Therapeutic Effect of Methylene Blue-Photodynamic Therapy in a Mouse Model of MRSA Bacterial Arthritis. Photochem. Photobiol. 2013, 89, 679–682. [Google Scholar] [CrossRef]
- Jiang, Q.; Fangjie, E.; Tian, J.; Yang, J.; Zhang, J.; Cheng, Y. Light-Excited Antibiotics for Potentiating Bacterial Killing via Reactive Oxygen Species Generation. ACS Appl. Mater. Interfaces 2020, 12, 16150–16158. [Google Scholar] [CrossRef] [PubMed]
- Morschhäuser, J. The Development of Fluconazole Resistance in Candida Albicans—An Example of Microevolution of a Fungal Pathogen. J. Microbiol. 2016, 54, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, E.D.; Mora, S.J.; Alvarez, M.G.; Durantini, E.N. Photodynamic Inactivation of Candida Albicans by a Tetracationic Tentacle Porphyrin and Its Analogue without Intrinsic Charges in Presence of Fluconazole. Photodiagn. Photodyn. Ther. 2016, 13, 334–340. [Google Scholar] [CrossRef]
- Hu, Y.; Qi, X.; Sun, H.; Lu, Y.; Hu, Y.; Chen, X.; Liu, K.; Yang, Y.; Mao, Z.; Wu, Z.; et al. Photodynamic Therapy Combined with Antifungal Drugs against Chromoblastomycosis and the Effect of ALA-PDT on Fonsecaea In Vitro. PLoS Negl. Trop. Dis. 2019, 13, e0007849. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, S.; Wu, M.; Kenry; Huang, Z.; Lee, C.; Liu, B. Membrane-Anchoring Photosensitizer with Aggregation-Induced Emission Characteristics for Combating Multidrug-Resistant Bacteria. Angew. Chem. Int. Ed. 2020, 59, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, T.; Berchel, M.; Le Hir, S.; Fraix, A.; Salaün, J.Y.; Férec, C.; Lehn, P.; Jaffrès, P.-A.; Montier, T. Arsonium-Containing Lipophosphoramides, Poly-Functional Nano-Carriers for Simultaneous Antibacterial Action and Eukaryotic Cell Transfection. Adv. Healthc. Mater. 2013, 2, 1513–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Liu, C.; Han, X.; Zhong, H.; Cheng, C. Viral Nanoparticle System: An Effective Platform for Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 1728. [Google Scholar] [CrossRef]
- Hosseini, N.; Pourhajibagher, M.; Chiniforush, N.; Hosseinkhan, N.; Rezaie, P.; Bahador, A. Modulation of Toxin-Antitoxin System Rnl AB Type II in Phage-Resistant Gammaproteobacteria Surviving Photodynamic Treatment. J. Lasers Med. Sci. 2018, 10, 21–28. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.-C.; Roach, D.R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Chen, W.; Liu, L.; Yu, F. Light and Sound to Trigger the Pandora’s Box against Breast Cancer: A Combination Strategy of Sonodynamic, Photodynamic and Photothermal Therapies. Biomaterials 2020, 232, 119685. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.; Wang, X.; Liu, Q.; Zhang, K.; Wang, P. The Application of DVDMS as a Sensitizing Agent for Sono-/Photo-Therapy. Front. Pharm. 2020, 11, 19. [Google Scholar] [CrossRef] [Green Version]
- Fraix, A.; Sortino, S. Combination of PDT Photosensitizers with NO Photodononors. Photochem. Photobiol. Sci. 2018, 17, 1709–1727. [Google Scholar] [CrossRef]
- Parisi, C.; Failla, M.; Fraix, A.; Rescifina, A.; Rolando, B.; Lazzarato, L.; Cardile, V.; Graziano, A.C.E.; Fruttero, R.; Gasco, A.; et al. A Molecular Hybrid Producing Simultaneously Singlet Oxygen and Nitric Oxide by Single Photon Excitation with Green Light. Bioorganic Chem. 2019, 85, 18–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maya, R.; Ladeira, L.L.C.; Maya, J.E.P.; Mail, L.M.G.; Bussadori, S.K.; Paschoal, M.A.B. The Combination of Antimicrobial Photodynamic Therapy and Photobiomodulation Therapy for the Treatment of Palatal Ulcers: A Case Report. J. Lasers Med. Sci. 2020, 11, 228–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqerban, A. Efficacy of Antimicrobial Photodynamic and Photobiomodulation Therapy against Treponema Denticola, Fusobacterium Nucleatum and Human Beta Defensin-2 Levels in Patients with Gingivitis Undergoing Fixed Orthodontic Treatment: A Clinic-Laboratory Study. Photodiagn. Photodyn. Ther. 2020, 29, 101659. [Google Scholar] [CrossRef]
- Fekrazad, R. Photobiomodulation and Antiviral Photodynamic Therapy as a Possible Novel Approach in COVID-19 Management. Photobiomodulation Photomed. Laser Surg. 2020, 38, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Lago, A.D.N.; Fortes, A.B.C.; Furtado, G.S.; Menezes, C.F.S.; Gonçalves, L.M. Association of Antimicrobial Photodynamic Therapy and Photobiomodulation for Herpes Simplex Labialis Resolution: Case Series. Photodiagn. Photodyn. Ther. 2020, 32, 102070. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, I.S.; Leal, F.S.; Tateno, R.Y.; Palma, L.F.; Campos, L. Photobiomodulation Therapy and Antimicrobial Photodynamic Therapy for Orofacial Lesions in Patients with COVID-19: A Case Series. Photodiagn. Photodyn. Ther. 2021, 34, 102281. [Google Scholar] [CrossRef]
- Ma, X.; Pan, H.; Wu, G.; Yang, Z.; Yi, J. Ultrasound May Be Exploited for the Treatment of Microbial Diseases. Med. Hypotheses 2009, 73, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Giuntini, F. Sonodynamic Antimicrobial Chemotherapy: First Steps towards a Sound Approach for Microbe Inactivation. J. Photochem. Photobiol. B Biol. 2015, 150, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Costley, D.; Ewan, C.M.; Fowley, C.; McHale, A.P.; Atchison, J.; Nomikou, N.; Callan, J.F. Treating Cancer with Sonodynamic Therapy: A Review. Int. J. Hyperth. 2015, 31, 107–117. [Google Scholar] [CrossRef]
- Alves, F.; Gomes Guimarães, G.; Mayumi Inada, N.; Pratavieira, S.; Salvador Bagnato, V.; Kurachi, C. Strategies to Improve the Antimicrobial Efficacy of Photodynamic, Sonodynamic, and Sonophotodynamic Therapies. Lasers Surg. Med. 2021, lsm.23383. [Google Scholar] [CrossRef]
- Erriu, M.; Blus, C.; Szmukler-Moncler, S.; Buogo, S.; Levi, R.; Barbato, G.; Madonnaripa, D.; Denotti, G.; Piras, V.; Orrù, G. Microbial Biofilm Modulation by Ultrasound: Current Concepts and Controversies. Ultrason. Sonochemistry 2014, 21, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.; Dennison, S.R.; Phoenix, D.A. Using Sound for Microbial Eradication—Light at the End of the Tunnel? FEMS Microbiol. Lett. 2014, 356, 20–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourhajibagher, M.; reza Rokn, A.; reza Barikani, H.; Bahador, A. Photo-Sonodynamic Antimicrobial Chemotherapy via Chitosan Nanoparticles-Indocyanine Green against Polymicrobial Periopathogenic Biofilms: Ex Vivo Study on Dental Implants. Photodiagn. Photodyn. Ther. 2020, 31, 101834. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. Attenuation of Aggregatibacter Actinomycetemcomitans Virulence Using Curcumin-Decorated Nanophytosomes-Mediated Photo-Sonoantimicrobial Chemotherapy. Sci. Rep. 2021, 11, 6012. [Google Scholar] [CrossRef] [PubMed]
- Traitcheva, N.; Berg, H. Electroporation and Alternating Current Cause Membrane Permeation of Photodynamic Cytotoxins Yielding Necrosis and Apoptosis of Cancer Cells. Bioelectrochemistry 2010, 79, 257–260. [Google Scholar] [CrossRef]
- Weżgowiec, J.; Kulbacka, J.; Saczko, J.; Rossowska, J.; Chodaczek, G.; Kotulska, M. Biological Effects in Photodynamic Treatment Combined with Electropermeabilization in Wild and Drug Resistant Breast Cancer Cells. Bioelectrochemistry 2018, 123, 9–18. [Google Scholar] [CrossRef]
- Reinhard, A.; Sandborn, W.J.; Melhem, H.; Bolotine, L.; Chamaillard, M.; Peyrin-Biroulet, L. Photodynamic Therapy as a New Treatment Modality for Inflammatory and Infectious Conditions. Expert Rev. Clin. Immunol. 2015, 11, 637–657. [Google Scholar] [CrossRef] [PubMed]
- McFarland, S.A.; Mandel, A.; Dumoulin-White, R.; Gasser, G. Metal-Based Photosensitizers for Photodynamic Therapy: The Future of Multimodal Oncology? Curr. Opin. Chem. Biol. 2019, 56, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Gupta, A.; Sasmal, P.K. Aggregation-Induced Emission Active Metal Complexes: A Promising Strategy to Tackle Bacterial Infections. Chem. Commun. 2021, 57, 174–186. [Google Scholar] [CrossRef]
- Gao, S.; Yan, X.; Xie, G.; Zhu, M.; Ju, X.; Stang, P.J.; Tian, Y.; Niu, Z. Membrane Intercalation-Enhanced Photodynamic Inactivation of Bacteria by a Metallacycle and TAT-Decorated Virus Coat Protein. Proc. Natl. Acad. Sci. USA 2019, 116, 23437–23443. [Google Scholar] [CrossRef]
- Jerjes, W.; Theodossiou, T.A.; Hirschberg, H.; Høgset, A.; Weyergang, A.; Selbo, P.K.; Hamdoon, Z.; Hopper, C.; Berg, K. Photochemical Internalization for Intracellular Drug Delivery. From Basic Mechanisms to Clinical Research. J. Clin. Med. 2020, 9, 528. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; de Boer, L.; Heiliegers, L.; Man-Bovenkerk, S.; Selbo, P.K.; Drijfhout, J.W.; Høgset, A.; Zaat, S.A.J. Photochemical Internalization Enhances Cytosolic Release of Antibiotic and Increases Its Efficacy against Staphylococcal Infection. J. Control. Release 2018, 283, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Hilgers, F.; Bitzenhofer, N.L.; Ackermann, Y.; Burmeister, A.; Grünberger, A.; Jaeger, K.-E.; Drepper, T. Genetically Encoded Photosensitizers as Light-Triggered Antimicrobial Agents. Int. J. Mol. Sci. 2019, 20, 4608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorbachev, D.A.; Staroverov, D.B.; Lukyanov, K.A.; Sarkisyan, K.S. Genetically Encoded Red Photosensitizers with Enhanced Phototoxicity. Int. J. Mol. Sci. 2020, 21, 8800. [Google Scholar] [CrossRef] [PubMed]
- Endres, S.; Wingen, M.; Torra, J.; Ruiz-González, R.; Polen, T.; Bosio, G.; Bitzenhofer, N.L.; Hilgers, F.; Gensch, T.; Nonell, S.; et al. An Optogenetic Toolbox of LOV-Based Photosensitizers for Light-Driven Killing of Bacteria. Sci. Rep. 2018, 8, 15021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y. Design and Applications of Metastable-State Photoacids. Acc. Chem. Res. 2017, 50, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.; Peng, P.; Hossain, M.; Jiang, T.; Fu, W.; Liao, Y.; Su, M. Visible Light Mediated Killing of Multidrug-Resistant Bacteria Using Photoacids. J. Mater. Chem. B 2013, 1, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-C.; Chen, C.-J.; Ding, S.-J.; Chen, C.-C. Antimicrobial Efficacy of Methylene Blue-Mediated Photodynamic Therapy on Titanium Alloy Surfaces In Vitro. Photodiagn. Photodyn. Ther. 2019, 25, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Goetzfried, M.A.; Hidaka, K.; You, M.; Tan, W.; Sugiyama, H.; Endo, M. Direct Visualization of Walking Motions of Photocontrolled Nanomachine on the DNA Nanostructure. Nano Lett. 2015, 15, 6672–6676. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Ma, X.; Xue, X.; Jiang, Q.; Song, L.; Dai, L.; Zhang, C.; Jin, S.; Yang, K.; Ding, B.; et al. A Photosensitizer-Loaded DNA Origami Nanosystem for Photodynamic Therapy. ACS Nano 2016, 10, 3486–3495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal Photodynamic Therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pereira Rosa, L. Antimicrobial Photodynamic Therapy: A New Therapeutic Option to Combat Infections. J. Med. Microb. Diagn. 2014, 3, 1. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, F.; Karges, J.; Gasser, G. Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy. Acc. Chem. Res. 2017, 50, 2727–2736. [Google Scholar] [CrossRef]
- Boca, S.C.; Four, M.; Bonne, A.; van der Sanden, B.; Astilean, S.; Baldeck, P.L.; Lemercier, G. An Ethylene-Glycol Decorated Ruthenium(II) Complex for Two-Photon Photodynamic Therapy. Chem. Commun. 2009, 30, 4590–4592. [Google Scholar] [CrossRef] [PubMed]
- Blum, N.T.; Zhang, Y.; Qu, J.; Lin, J.; Huang, P. Recent Advances in Self-Exciting Photodynamic Therapy. Front. Bioeng. Biotechnol. 2020, 8, 594491. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yuan, H.; Bai, H.; Zhang, P.; Lv, F.; Liu, L.; Dai, Z.; Bao, J.; Wang, S. Electrochemiluminescence for Electric-Driven Antibacterial Therapeutics. J. Am. Chem. Soc. 2018, 140, 2284–2291. [Google Scholar] [CrossRef]
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS Nano 2016, 10, 3918–3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamanli, A.F.; Çetinel, G. Comparison of Pulse and Super Pulse Radiation Modes’ Singlet Oxygen Production Effect in Antimicrobial Photodynamic Therapy (AmPDT). Photodiagn. Photodyn Ther. 2020, 30, 101706. [Google Scholar] [CrossRef]
- Hou, X.; Tao, Y.; Li, X.; Pang, Y.; Yang, C.; Jiang, G.; Liu, Y. CD44-Targeting Oxygen Self-Sufficient Nanoparticles for Enhanced Photodynamic Therapy Against Malignant Melanoma. Int. J. Nanomed. 2020, 15, 10401–10416. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Chen, S.; Xie, Y.; Fan, Q.; Zhou, J.; Bao, J.; Wei, T.; Dai, Z. Dual-Path Modulation of Hydrogen Peroxide to Ameliorate Hypoxia for Enhancing Photodynamic/Starvation Synergistic Therapy. J. Mater. Chem. B 2020, 8, 9933–9942. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, H.; Phua, S.Z.F.; Yang, G.; Qi Lim, W.; Gu, L.; Qian, C.; Wang, H.; Guo, Z.; Chen, H.; et al. Self-Assembled Single-Atom Nanozyme for Enhanced Photodynamic Therapy Treatment of Tumor. Nat. Commun. 2020, 11, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonell, S.; Ferreras, L.R.; Cañete, A.; Lemp, E.; Günther, G.; Pizarro, N.; Zanocco, A.L. Photophysics and Photochemistry of Naphthoxazinone Derivatives. J. Org. Chem. 2008, 73, 5371–5378. [Google Scholar] [CrossRef]
- Lamarque, G.C.C.; Méndez, D.A.C.; Gutierrez, E.; Dionisio, E.J.; Machado, M.A.A.M.; Oliveira, T.M.; Rios, D.; Cruvinel, T. Could Chlorhexidine Be an Adequate Positive Control for Antimicrobial Photodynamic Therapy in-in Vitro Studies? Photodiagn. Photodyn. Ther. 2019, 25, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Bahador, A. Computational Biology Analysis of COVID-19 Receptor-Binding Domains: A Target Site for Indocyanine Green Through Antimicrobial Photodynamic Therapy. J. Lasers Med. Sci. 2020, 11, 433–441. [Google Scholar] [CrossRef]


| Medical Conditions | Target Micro-Organism(s) | Photosensitizer | Trial Phase | Number and Year |
|---|---|---|---|---|
| Acne | Propionibacterium acnes | Butenyl ALA | N.A. | NCT02313467, 2014 |
| Lemuteporfin | Phase 1/2 | NCT01490736, 2011 | ||
| 5-ALA | Phase 2 | NCT01689935, 2012 | ||
| Methyl aminolevulinate | Phase 2 | NCT00673933, 2013 | ||
| Dental caries | Streptococcus mutans, Streptococcus sobrinus, Lactobacillus casei, Fusobacterium nucleatum, and Atopobium rimae | TBO | Phase 1 | NCT02479958, 2015 |
| MB | Phase 1 | NCT02479958, 2015 | ||
| Aggregatibacter actinomycetemcomitans, Tannerella forsythia and Porphyromonas gingivalis | N.C. | N.A. | NCT03309748, 2017 | |
| Denture-related stomatitis | Candida albicans | MB | Phase 4 | NCT02642900, 2015 |
| Orthodontic | N.D. | Curcumin | Phase 1 | NCT02337192, 2015 |
| Peri-implantitis | N.D. | N.D. | Phase 3 | NCT02848482, 2016 |
| Periodontic | Aggregatibacter Actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia and Treponema denticola | MB | N.A. | NCT03750162, 2018 |
| ICG | Phase 2 | NCT02043340, 2014 | ||
| Methyl aminolevulinate | Phase 2 | NCT00933543, 2013 | ||
| MB | N.A. | NCT03262077, 2017 | ||
| MB | Phase 2 | NCT03074136, 2017 | ||
| Phenothiazine hydrochloride | Phase 4 | NCT03498404, 2018 | ||
| TB | Phase 4 | NCT03412331, 2018 | ||
| Distal subungual onychomycosis | Fungi infecting nails | 5-ALA | Phase 2 | NCT02355899, 2015 |
| Endodontic | E. faecalis and C. albicans | MB | Phase 2 | NCT02824601, 2016 |
| HPV infection | Human Papillomavirus (HPV) | 5-ALA | Phase 2 | NCT02631863, 2015 |
| Leg ulcers | Streptococci, anaerobes, coliform, S. aureus, P. aeruginosa, yeast, and diphtheroids | PPA904 | Phase 2 | NCT00825760, 2009 |
| Combination with Antibiotics | Target(s) | In Vitro and/or In Vivo Effect(s) | Reference |
| 5-ALA + Gentamicin | S. aureus and S. epidermidis | In vitro: antibiofilm synergistic effect | [392] |
| Photodithazine + Metronidazole | F. nucleatum and P. gingivalis | In vitro: improvement of antibiofilm effect | [393] |
| Ce6 NP + Tinidazole | Periodontal pathogenic bacteria | In vitro: synergistic antiperiodontitis effects; in vivo: reduced adsorption of alveolar bone in a rat model of periodontitis | [394] |
| MB + Clindamycin/Amoxicillin | E. coli | In vitro: enhancement of antibiotic susceptibility following aPDT treatment; in vivo: prolonged survival of infected G. mellonella larvae | [43] |
| MB + Gentamicin | S. aureus and P. aeruginosa | In vitro: synergistic effect on planctonik cultures of both bacteria; positive effect on P. aeruginosa biofilm | [395] |
| MB + Carbapenem | S. marcescens, K. pneumoniae and E. aerogenes | In vitro: impairment of the enzymatic activity and genetic determinants of carbapenemases; restoration of the susceptibility to Carbapenem | [396] |
| [Ir(ppy)2 (ppdh)]PF6) + Cefotaxime | K. pneumoniae | In vitro: synergistic aPDI effect with Cefotaxime | [397] |
| Combination with other antibacterial compounds | Target(s) | In vitro and/or in vivo effect(s) | Reference |
| MB or Ce6 + aurein 1.2 monomer or aurein 1.2 C-terminal dimer | E. faecalis | In vitro: prevention of biofilm formation with all treatments; improvement of aurein monomer effect when combined with Ce6-PDT | [398] |
| RB + Concanavalin A | E. coli | In vitro: improvement of RB uptake, increased membrane damages and enhanced PDT effect | [399] |
| MB@GNPDEX-ConA + Carbonyl cyanide m-chlorophenylhydrazone | K. pneumoniae | In vitro: enhancement of the MB-NPs mediated phototoxicity with the efflux pump inhibitor CCCP | [40] |
| Quinine hydrochloride + antimicrobial blue light | MDR P. aeruginosa and A. baumannii | In vitro: photo-inactivation of planktonic cells and biofilms; in vivo: potentiation of aBL effect in a mouse skin abrasion infection model | [400] |
| Combination with other antifungal treatment compounds | Target(s) | In vitro and/or in vivo effect(s) | Reference |
| 5-ALA + ITZ, itraconazole; TBF, terbinafine; VOR, voriconazole | Candida species, dermatophytes, A. fumigatus and F. monophora | In vitro: reduction/improvement of lesions, disappearance of plaque | [401] |
| Photodithazine + Nystatin | Fluconazole-resistant C. albicans | In vitro: reduction of fungal viability, decrease in oral lesions and inflammatory reaction; in vivo: decrease in tongue lesions | [54] |
| 5-ALA + Itraconazole | Trichosporon asahii | In vitro: better elimination of planktonic and biofilms fungi than single therapy | [402] |
| Combination with immunotherapy | Target(s) | In vitro and/or in vivo effect(s) | Reference |
| Schiff base complexes | E. coli et S. aureus | In vitro: blockage of the production of inflammatory TNFα cytokine | [403] |
| Porphyrin + phtalocyanine | HIV-infected cells | In vitro: specific phototoxicity against infected cells | [404] |
| Combination with sonodynamic therapy (SDT) | Target(s) | In vitro and/or in vivo effect(s) | Reference |
| Ce6 derivative Photodithazine + RB | C. albicans | In vitro: inactivation of biofilm (viability and total biomass) | [405] |
| UCNPs + hematoporphyrin + SiO2-RB 1 | Antibiotic-resistant bacteria | In vitro: greater antibacterial effect with SDT and PDT at once | [406] |
| Combination with electrochemotherapy | Target(s) | In vitro and/or in vivo effect(s) | Reference |
| Hypericin | E. coli and S. aureus | In vitro: better bacterial inactivation with combined therapies | [407] |
| Combination with viral NPs | Target(s) | In vitro and/or in vivo effect(s) | Reference |
| TVP-A (luminogen) + PAP phage | P. aeruginosa | In vitro: synergistic bacterial recognizing and killing; in vivo: acceleration of healing rates | [408] |
| Pheophorbide A (chlorophyll) + JM-phage | C. albicans | In vitro: better specificity of PS targeting | [409] |
| Ru(bpy2)phen-IA + Cowpea chlorotic mottle virus | S. aureus | In vitro: targeted bacterial photodynamic inactivation | [410] |
| Combination of several PSs | Target(s) | In vitro and/or in vivo effect(s) | Reference |
| Carboxypterin + MB | K. pneumoniae | In vitro: better biofilm eradication | [411] |
| Phthalocyanines + Graphene QDs | S. aureus | In vitro: better bacterial photoinactivation | [412] |
| ICG + Metformin + Curcumin | E. faecalis | In vitro: better biofilm eradication | [35] |
| Porphyrin + Phthalocyanine | Leishmania braziliensis | In vitro: better assimilation of photo-inactivated parasites by macrophages | [413] |
| Combination with photothermal therapy (PTT) | Target(s) | In vitro and/or in vivo effect(s) | Reference |
| Ruthenium NPs | Pathogenic bacteria | In vitro: bacterial inhibition; in vivo: reduction of bacterial load and repair of infected wounds | [414] |
| Graphene oxide | E. coli and S. aureus | In vitro: efficient vector for both PDT and PTT | [415] |
| ICG + SPIONs | E. coli, K. pneumoniae, P. aeruginosa, and S. epidermis | In vitro: antimicrobial and antibiofilm activity at a low dose | [148] |
| Ag-conjugated graphene QDs | E. coli and S. aureus | In vitro: efficient photoinactivation by PDT and PTT; in vivo: promoted healing in bacteria-infected rat wounds | [280] |
| PDPPTT (photothermal agent) + MEH-PPV (PS) 2 | E. coli | In vitro: better inhibition rate than PTT/PDT systems used alone | [324] |
| Mesoporous polydopamine NPs + ICG | S. aureus | In vivo: eradication of S. aureus biofilm on titanium implant | [416] |
| Combination with NO phototherapy | Target(s) | In vitro and/or in vivo effect(s) | Reference |
| N-(3-aminopropyl)-3-(trifluoromethyl)-4-nitrobenzenamine + TMPyP/ZnPc | E. coli | In vitro: dual-mode photoantibacterial action | [417] |
| Sulfonated polystyrene NPs (NO photodonor + porphyrin/phthalocyanine) | E. coli | In vitro: strong antibacterial action | [355] |
| [Ru(bpy)3]Cl2 | P. aeruginosa | In vitro: PDT/NO synergistic antibiofilm effect | [418] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. https://doi.org/10.3390/pharmaceutics13121995
Youf R, Müller M, Balasini A, Thétiot F, Müller M, Hascoët A, Jonas U, Schönherr H, Lemercier G, Montier T, et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics. 2021; 13(12):1995. https://doi.org/10.3390/pharmaceutics13121995
Chicago/Turabian StyleYouf, Raphaëlle, Max Müller, Ali Balasini, Franck Thétiot, Mareike Müller, Alizé Hascoët, Ulrich Jonas, Holger Schönherr, Gilles Lemercier, Tristan Montier, and et al. 2021. "Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies" Pharmaceutics 13, no. 12: 1995. https://doi.org/10.3390/pharmaceutics13121995
APA StyleYouf, R., Müller, M., Balasini, A., Thétiot, F., Müller, M., Hascoët, A., Jonas, U., Schönherr, H., Lemercier, G., Montier, T., & Le Gall, T. (2021). Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics, 13(12), 1995. https://doi.org/10.3390/pharmaceutics13121995