Development, Characterization, Optimization, and In Vivo Evaluation of Methacrylic Acid–Ethyl Acrylate Copolymer Nanoparticles Loaded with Glibenclamide in Diabetic Rats for Oral Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Materials and Equipment
2.3. Animals
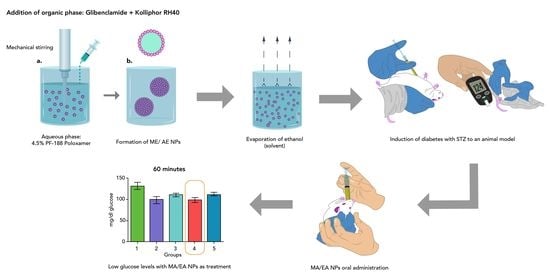
2.4. Nanoparticle Development
2.4.1. Nanoparticles
Aqueous Phase
Organic Phase
NP Obtention
2.5. Design of Experiment (Screening and Optimization)
2.6. Physicochemical Characterization
2.6.1. Particle Size, Polydispersity Index (PDI), and Zeta Potential (ζ-Potential)
2.6.2. Drug Release Profiles
2.6.3. Scanning Electronic Microscopy
2.6.4. Encapsulation Efficiency
2.7. In Vivo Model
3. Results
3.1. Results and Discussion
3.1.1. Screening Design
3.1.2. Optimization of Nanoparticles
3.1.3. Size, PDI, and Zeta Potential
3.1.4. Encapsulation Efficiency
3.1.5. Scanning Electronic Microscopy (SEM)
3.1.6. Drug Release Profiles and Kinetic Drug Release
3.1.7. In Vivo Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Conget, D.I. Diagnosis, classification and pathogenesis of diabetes mellitus. Rev. Esp. Cardiol. 2002, 55, 528–535. [Google Scholar] [CrossRef]
- Sanamé, F.A.R.; Álvarez, M.L.P.; Figueredo, E.A.; Estupiñan, M.R.; Rizo, Y.J. Tratamiento Actual de la Diabetes Mellitus Tipo 2. Correo Científico Médico 2016, 20. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1560-43812016000100009 (accessed on 26 April 2021).
- Cuartero, C.; Glibenclamida, C.; Tabletas, E.N. Validación de un método analítico espectrofotométrico para cuantificar glibenclamida en tabletas de 5 mg Validation of spectrophotometric analytical method to quantify glibenclamide. Rev. Mex. De Cienc. Farm. 2005, 36, 33–41. [Google Scholar]
- Luzi, L.; Pozza, G. Glibenclamide: An old drug with a novel mechanism of action? Acta Diabetol. 1997, 34, 239–244. [Google Scholar] [CrossRef]
- Devarajan, P.V.; Sonavane, G.S. Preparation and in vitro/in vivo evaluation of gliclazide loaded Eudragit nanoparticles as a sustained release carriers. Drug Dev. Ind. Pharm. 2007, 33, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Paolo Fra, G.; Bartoli, E.; Derosa, G. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Winkler, G.; Gerő, L. Pharmacogenetics of insulin secretagogue antidiabetics. Orv. Hetil. 2011, 152, 1651–1660. [Google Scholar] [CrossRef]
- Tessier, D.; Dawson, K.; Tetrault, J.P.; Bravo, G.; Meneilly, G.S. Glibenclamide vs gliclazide in type 2 diabetes of the elderly. Diabet. Med. 1994, 11, 974–980. [Google Scholar] [CrossRef]
- Holstein, A.; Plaschke, A.; Egberts, E.H. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes. Metab. Res. Rev. 2001, 17, 467–473. [Google Scholar] [CrossRef]
- Chamundeeswari, M.; Jeslin, J.; Verma, M.L. Nanocarriers for drug delivery applications. Environ. Chem. Lett. 2019, 17, 849–865. [Google Scholar] [CrossRef]
- Mendes, A.N.; Hubber, I.; Siqueira, M.; Moreno Barbosa, G.; De, D.; Moreira, L.; Holandino, C.; Pinto, J.J.; Nele, M. Preparation and Cytotoxicity of Poly(Methyl Methacrylate) Nanoparticles for Drug Encapsulation. Available online: www.ms-journal.de (accessed on 24 February 2021).
- Dupeyrón, D.; Rieumont, J.; González, M.; Castaño, V.M.; Dupeyr on, D.; Gonz alez, M.; Casta, V.M. Protein Delivery by Enteric Copolymer Nanoparticles. J. Dispers. Sci. Technol. 2009, 30, 1188–1194. [Google Scholar] [CrossRef]
- Urrejola, M.C.; Soto, L.V.; Zumarán, C.C.; Peñaloza, J.P.; Álvarez, B.; Fuentevilla, I.; Haidar, Z.S. Sistemas de Nanopartículas Poliméricas II: Estructura, Métodos de Elaboración, Características, Propiedades, Biofuncionalización y Tecnologías de Auto-Ensamblaje Capa por Capa (Layer-by-Layer Self-Assembly). Int. J. Morphol. 2018, 36, 1463–1471. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Shah, K.W.; Xu, J. Synthesis, morphologies and building applications of nanostructured polymers. Polymers 2017, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Diseños de Cribado—Minitab. Available online: https://support.minitab.com/es-mx/minitab/19/help-and-how-to/statistical-modeling/doe/supporting-topics/factorial-and-screening-designs/screening-designs/ (accessed on 5 March 2021).
- D Dispersión de luz dinámica DLS|Malvern Panalytical. Available online: https://www.malvernpanalytical.com/es/products/technology/light-scattering/dynamic-light-scattering (accessed on 15 May 2021).
- García, M.A.; Soberón, E.; Cortés, M.; Rodríguez, R.; HJ, A.A. Guía de Validación de Métodos Analíticos; Colegio Nacional de Químicos Farmacéuticos Biólogos México AC: Ciudad de México, Mexico, 2002; p. 132. [Google Scholar]
- Chatzigeorgiou, A.; Halapas, A.; Kalafatakis, K.; Kamper, E. The Use of Animal Models in the Study of Diabetes Mellitus. In Vivo 2009, 23, 245–258. Available online: https://iv.iiarjournals.org/content/invivo/23/2/245.full.pdf (accessed on 8 January 2021).
- Like, A.A.; Rossini, A.A. Streptozotocin-induced pancreatic insulitis: New model of diabetes mellitus. Science 1976, 193, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Barriga, S.A. Efecto de la Suplementación con Tributirina vía Oral en Ratas con Diabetes e Insuficiencia Renal. Ph.D. Thesis, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 2020. [Google Scholar]
- Woods, D.C.; Lewis, S.M. Design of Experiments for Screening. In Handbook of Uncertainty Quantification; Ghanem, R., Higdon, D., Owhadi, H., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef] [Green Version]
- Potencial Zeta—Lenntech. Available online: https://www.lenntech.es/potential-zeta.htm (accessed on 19 February 2021).
- Zhou, W.; Apkarian, R.; Wang, Z.L.; Joy, D. Fundamentals of scanning electron microscopy (SEM). In Scanning Microscopy for Nanotechnology; Springer: New York, NY, USA, 2007; pp. 1–40. [Google Scholar]
- Cook, H.J.; de Anda Jáuregui, G.; Carrasco, K.R.; Cruz, L.M. Comparación de Perfiles de Disolución: Impacto de los Criterios de Diferentes Agencias Regulatorias en el Cálculo de ƒ2. 2012. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-01952012000300007 (accessed on 25 July 2021).
- Castañeda, P.S.; Olvera, L.G.; Bernad, M.J.B.; López, H.S.; Escobar-Chávez, J.J. Development of a Spectrophotometric Method for the Determination of Florfenicol in Eudragit Nanocapsules. Pharm. Chem. J. 2021, 54, 1181–1185. [Google Scholar] [CrossRef]
- Minotta, J. Evaluación de la Cinetica de Liberacion de un Fármaco Modelo con Clasificación Biofarmaceutica Clase II, Desde Matrices Comprimidas Compuestas por Materiales Polimericos Anionicos. Available online: https://repository.icesi.edu.co/biblioteca_digital/bitstream/10906/83047/1/jimenez_farmaco_biofarmaceutica_2017.pdf (accessed on 27 May 2021).
- Sonaje, K.; Lin, K.-J.; Wang, J.-J.; Mi, F.-L.; Chen, C.-T.; Juang, J.-H.; Sung, H.-W. Self-Assembled pH-Sensitive Nanoparticles: A Platform for Oral Delivery of Protein Drugs. Adv. Funct. Mater. 2010, 20, 3695–3700. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, Q. pH-sensitive polymeric nanoparticles to improve oral bioavailability of peptide/protein drugs and poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2012, 82, 219–229. [Google Scholar] [CrossRef]
- Graham, M.L.; Janecek, J.L.; Kittredge, J.A.; Hering, B.J.; Schuurman, H.J. The streptozotocin-induced diabetic nude mouse model: Differences between animals from different sources. Comp. Med. 2011, 61, 356–360. [Google Scholar] [PubMed]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.; Jamshidi, S.; Farhangi, A.; Allah Verdi, A.; Mofidian, S.; Lame Rad, B.; Akbarzadeh, A. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikebukuro, K.; Adachi, Y.; Yamada, Y.; Fujimoto, S.; Seino, Y.; Oyaizu, H.; Hioki, K.; Ikehara, S. Treatment of streptozotocin-induced diabetes mellitus by transplantation of islet cells plus bone marrow cells via portal vein in rats. Transplantation 2002, 73, 512–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]


















| Factors | Low | High | Units |
|---|---|---|---|
| Drug/polymer | 50 | 150 | Mg |
| PF-188 | 1.5 | 4.5 | % |
| RH-40 | 1.5 | 4.5 | G |
| Stirring speed | 2000 | 6000 | Rpm |
| Stirring time | 2.0 | 6.0 | Min |
| Volume of water | 45 | 75 | mL |
| Response | Units |
|---|---|
| Size | Nm |
| Zeta potential | mV |
| PDI | -- |
| Encapsulation efficiency (EE) | % |
| Factors | Low | High | Units | Continuous |
|---|---|---|---|---|
| Drug/Polymer | 40 | 120 | mg | Yes |
| RH-40 | 1 | 3 | g | Yes |
| Evaporation temperature | −1 | 1 | °C | Yes |
| Response | Units |
|---|---|
| Size | Nm |
| Zeta potential | mV |
| PDI | -- |
| EE | % |
| Animals Were Divided into 5 Different Groups with 5 Individuals Each as Follows: |
|---|
| Group 1: untreated diabetic rats |
| Group 2: diabetic rats with conventional pharmaceutical form (tablets) |
| Group 3: diabetic rats administered with NP without glibenclamide |
| Group 4: diabetic rats administered with NP with glibenclamide |
| Group 5: control |
| Drug/Polymer | PF-188 | Kolliphor RH-40® | Stirring | Stirring Time | Water Volume | Size | Zeta Pot | PDI | EE |
|---|---|---|---|---|---|---|---|---|---|
| mg | % | G | rpm | min | mL | Nm | mV | % | |
| 50 | 4.5 | 4.5 | 6000 | 2 | 75 | 24.59 | −11.42 | 1.2 | 75.253666 |
| 150 | 1.5 | 4.5 | 6000 | 2 | 75 | 4687 | −10.14 | 0.2939 | 63.397299 |
| 150 | 4.5 | 1.5 | 6000 | 6 | 45 | 3891 | −16.22 | 0.1027 | 99.312885 |
| 50 | 1.5 | 1.5 | 6000 | 6 | 75 | 1634 | −23.44 | 0.4802 | 87.46687 |
| 150 | 1.5 | 4.5 | 2000 | 2 | 45 | 5117 | −11.54 | 0.189 | 94.337278 |
| 150 | 4.5 | 1.5 | 6000 | 2 | 45 | 3664 | −20.68 | 0.2228 | 98.847868 |
| 100 | 3 | 3 | 4000 | 4 | 60 | 5136 | −10.69 | 0.3943 | 97.082929 |
| 150 | 4.5 | 4.5 | 2000 | 6 | 75 | 14.79 | −11.54 | 0.4279 | 88.191434 |
| 150 | 1.5 | 1.5 | 2000 | 6 | 75 | 1893 | −27 | 0.0299 | 101.33701 |
| 50 | 1.5 | 1.5 | 2000 | 2 | 45 | 2245 | −18.03 | 0.3975 | 90.442499 |
| 50 | 4.5 | 1.5 | 2000 | 2 | 75 | 37.11 | −17 | 0.5533 | 70.439951 |
| 100 | 3 | 3 | 4000 | 4 | 60 | 4577 | −14.36 | 0.7203 | 74.129591 |
| 50 | 1.5 | 4.5 | 6000 | 6 | 45 | 22.13 | −5.168 | 0.969 | 62.067704 |
| 100 | 3 | 3 | 4000 | 4 | 60 | 12.74 | −12.66 | 0.3544 | 62.876815 |
| 50 | 4.5 | 4.5 | 2000 | 6 | 45 | 21.3 | −5.812 | 0.3075 | 86.575837 |
| Block | Drug/Polymer | Kolliphor RH-40® | Evaporation Temperature | Size | Zeta Potential | PDI | EE |
|---|---|---|---|---|---|---|---|
| Mg | G | °C | nm | mV | % | ||
| 1 | 40 | 1 | 1 | 1565 | −18.19 | 0.6814 | 79.491841 |
| 1 | 80 | 2 | 1.68179 | 756 | −18.93 | 0.6576 | 83.9436238 |
| 1 | 120 | 1 | −1 | 1823 | −24.94 | 4.418 | 93.0083276 |
| 1 | 80 | 3.68179 | 0 | 13.97 | −13.35 | 0.2083 | 76.8001864 |
| 1 | 147.272 | 2 | 0 | 1250 | −17.75 | 0.3323 | 80.6717907 |
| 1 | 120 | 1 | 1 | 3979 | −25.41 | 0.04271 | 94.2851601 |
| 1 | 80 | 2 | 0 | 34.43 | −13.67 | 0.3513 | 47.9191043 |
| 1 | 40 | 1 | −1 | 32.4 | −23.82 | 1.374 | 79.6281078 |
| 1 | 120 | 3 | −1 | 746 | 0.02799 | 0.9255 | 88.8181854 |
| 1 | 120 | 3 | 1 | 22.02 | −13.71 | 0.4585 | 87.1761386 |
| 1 | 80 | 2 | −1.68179 | 15.83 | −15.17 | 0.3648 | 10.3197805 |
| 1 | 80 | 2 | 0 | 27.75 | −18.33 | 0.08396 | 63.2713165 |
| 1 | 40 | 3 | −1 | 15.93 | −12.29 | 0.3544 | 43.862202 |
| 1 | 12.7283 | 2 | 0 | 19.16 | −13.77 | 0.3824 | 44.8243383 |
| 1 | 40 | 3 | 1 | 14.97 | −12.66 | 0.3222 | 23.1320136 |
| 1 | 80 | 0.318207 | 0 | 1653 | −39.36 | 0.4928 | 99.8984444 |
| Expected Size | Size (nm) | PDI | Zeta Potential |
|---|---|---|---|
| 50 nm | 18.98 +/− 9.14 | 0.37085 +/− 0.014 | −13.7125 +/− 1.82 |
| Absorbance 305 nm | Dilution | Quantification (µg/mL) | Dilution Factor | Quantification (mg) | Theoretical Load (mg) | Load Capacity (mg) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|---|---|
| 1.4907 1.4865 1.4907 | (1:2) | 251.118644 | 502.237288 | 42.6901695 | 77 | 34.3098305 | 44.5582214 |
| (1:2) | 250.40678 | 500.813559 | 42.5691525 | 77 | 34.4308475 | 44.7153863 | |
| (1:2) | 251.118644 | 502.237288 | 42.6901695 | 77 | 34.3098305 | 44.5582214 | |
| 1.4893 | (1:2) | 250.881356 | 501.762712 | 42.6498305 | 77 | 34.3501695 | 44.61 +/- 0.22 |
| Tukey’s Multiple Comparison Test | Significant p < 0.05 | Summary | 95% CI of diff |
|---|---|---|---|
| Group 1 vs. Group 2 | No | ns | −13.78 to 12.00 |
| Group 1 vs. Group 3 | No | ns | −8.380 to 17.40 |
| Group 1 vs. Group 4 | No | ns | −15.03 to 10.75 |
| Group 1 vs. Group 5 | No | ns | −0.9797 to 24.80 |
| Group 2 vs. Group 3 | No | ns | −6.755 to 17.55 |
| Group 2 vs. Group 4 | No | ns | −13.40 to 10.90 |
| Group 2 vs. Group 5 | Yes | * | 0.6451 to 24.95 |
| Group 3 vs. Group 4 | No | ns | −18.80 to 5.505 |
| Group 3 vs. Group 5 | No | ns | −4.755 to 19.55 |
| Group 4 vs. Group 5 | Yes | * | 1.895 to 26.20 |
| Tukey’s Multiple Comparison Test | Significant p < 0.05 | Summary | 95% CI of diff |
|---|---|---|---|
| Group 1 vs. Group 2 | Yes | *** | 15.42 to 61.18 |
| Group 1 vs. Group 3 | No | ns | −4.005 to 41.76 |
| Group 1 vs. Group 4 | Yes | *** | 11.52 to 57.29 |
| Group 1 vs. Group 5 | No | ns | −3.008 to 42.23 |
| Group 2 vs. Group 3 | No | ns | −41.30 to 2.460 |
| Group 2 vs. Group 4 | No | ns | −25.78 to 17.99 |
| Group 2 vs. Group 5 | No | ns | −40.29 to 2.919 |
| Group 3 vs. Group 4 | No | ns | −6.354 to 37.41 |
| Group 3 vs. Group 5 | No | ns | −20.87 to 22.34 |
| Group 4 vs. Group 5 | No | ns | −36.40 to 6.813 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guadarrama-Escobar, O.R.; Sánchez-Vázquez, I.; Serrano-Castañeda, P.; Chamorro-Cevallos, G.A.; Rodríguez-Cruz, I.M.; Sánchez-Padrón, A.Y.; Anguiano-Almazán, E.; Peña-Juárez, M.C.; Méndez-Albores, A.; Domínguez-Delgado, C.L.; et al. Development, Characterization, Optimization, and In Vivo Evaluation of Methacrylic Acid–Ethyl Acrylate Copolymer Nanoparticles Loaded with Glibenclamide in Diabetic Rats for Oral Administration. Pharmaceutics 2021, 13, 2023. https://doi.org/10.3390/pharmaceutics13122023
Guadarrama-Escobar OR, Sánchez-Vázquez I, Serrano-Castañeda P, Chamorro-Cevallos GA, Rodríguez-Cruz IM, Sánchez-Padrón AY, Anguiano-Almazán E, Peña-Juárez MC, Méndez-Albores A, Domínguez-Delgado CL, et al. Development, Characterization, Optimization, and In Vivo Evaluation of Methacrylic Acid–Ethyl Acrylate Copolymer Nanoparticles Loaded with Glibenclamide in Diabetic Rats for Oral Administration. Pharmaceutics. 2021; 13(12):2023. https://doi.org/10.3390/pharmaceutics13122023
Chicago/Turabian StyleGuadarrama-Escobar, Omar Rodrigo, Ivonne Sánchez-Vázquez, Pablo Serrano-Castañeda, German Alberto Chamorro-Cevallos, Isabel Marlen Rodríguez-Cruz, Adalí Yisell Sánchez-Padrón, Ericka Anguiano-Almazán, Ma. Concepción Peña-Juárez, Abraham Méndez-Albores, Clara Luisa Domínguez-Delgado, and et al. 2021. "Development, Characterization, Optimization, and In Vivo Evaluation of Methacrylic Acid–Ethyl Acrylate Copolymer Nanoparticles Loaded with Glibenclamide in Diabetic Rats for Oral Administration" Pharmaceutics 13, no. 12: 2023. https://doi.org/10.3390/pharmaceutics13122023
APA StyleGuadarrama-Escobar, O. R., Sánchez-Vázquez, I., Serrano-Castañeda, P., Chamorro-Cevallos, G. A., Rodríguez-Cruz, I. M., Sánchez-Padrón, A. Y., Anguiano-Almazán, E., Peña-Juárez, M. C., Méndez-Albores, A., Domínguez-Delgado, C. L., Mercado-Márquez, C., Rodríguez-Pérez, B., & Escobar-Chávez, J. J. (2021). Development, Characterization, Optimization, and In Vivo Evaluation of Methacrylic Acid–Ethyl Acrylate Copolymer Nanoparticles Loaded with Glibenclamide in Diabetic Rats for Oral Administration. Pharmaceutics, 13(12), 2023. https://doi.org/10.3390/pharmaceutics13122023