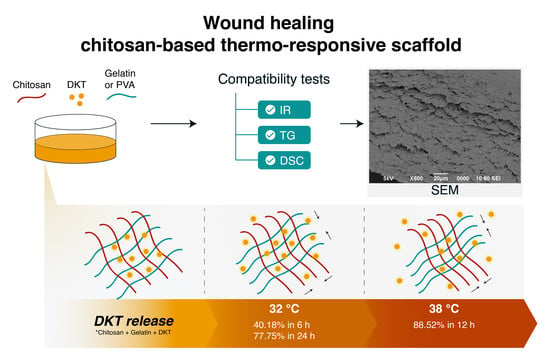
Topical Chitosan-Based Thermo-Responsive Scaffold Provides Dexketoprofen Trometamol Controlled Release for 24 h Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preformulation and Formulation
2.3. Compatibility Tests
2.3.1. Thermal Characterization
2.3.2. Fourier Transformed Infrared Spectroscopy
2.4. Microscopic Characterization
2.5. In Vitro Release Studies
2.6. Kinetic Release Models
3. Results and Discussion
3.1. Preformulation and Formulation
3.2. Compatibility Tests
3.3. Scanning Electron Microscopy Characterization
3.4. In Vitro Release Studies
3.5. Kinetic Release Models
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sahana, T.G.; Rekha, P.D. Biopolymers: Applications in Wound Healing and Skin Tissue Engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Singh, B.N.; Panda, N.N.; Mund, R.; Pramanik, K. Carboxymethyl Cellulose Enables Silk Fibroin Nanofibrous Scaffold with Enhanced Biomimetic Potential for Bone Tissue Engineering Application. Carbohydr. Polym. 2016, 151, 335–347. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Calori, I.R.; Braga, G.; de Jesus, P.D.C.C.; Bi, H.; Tedesco, A.C. Polymer Scaffolds as Drug Delivery Systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive Polymers and Their Biomedical Application in Tissue Engineering—A Review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Argüelles-Monal, W.; Recillas-Mota, M.; Fernández-Quiroz, D. Chitosan-Based Thermosensitive Materials; IntechOpen: London, UK, 2017; ISBN 978-953-51-2860-1. [Google Scholar]
- Grinberg, V.Y.; Burova, T.V.; Grinberg, N.V.; Tikhonov, V.E.; Dubovik, A.S.; Moskalets, A.P.; Khokhlov, A.R. Thermodynamic Insight into the Thermoresponsive Behavior of Chitosan in Aqueous Solutions: A Differential Scanning Calorimetry Study. Carbohydr. Polym. 2020, 229, 115558. [Google Scholar] [CrossRef]
- Bao, H.; Li, L.; Leong, W.C.; Gan, L.H. Thermo-Responsive Association of Chitosan-Graft-Poly(N-Isopropylacrylamide) in Aqueous Solutions. J. Phys. Chem. B 2010, 114, 10666–10673. [Google Scholar] [CrossRef]
- Magli, S.; Rossi, G.B.; Risi, G.; Bertini, S.; Cosentino, C.; Crippa, L.; Ballarini, E.; Cavaletti, G.; Piazza, L.; Masseroni, E.; et al. Design and Synthesis of Chitosan—Gelatin Hybrid Hydrogels for 3D Printable in vitro Models. Front. Chem. 2020, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Soloman, P.A.; Rejini, V.O. Preparation of Chitosan-Polyvinyl Alcohol Blends and Studies on Thermal and Mechanical Properties. Procedia Technol. 2016, 24, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Haley, R.M.; von Recum, H.A. Localized and Targeted Delivery of NSAIDs for Treatment of Inflammation: A Review. Exp. Biol. Med. 2019, 244, 433–444. [Google Scholar] [CrossRef]
- Bermejo, M.; Kuminek, G.; Al-Gousous, J.; Ruiz-Picazo, A.; Tsume, Y.; Garcia-Arieta, A.; González-Alvarez, I.; Hens, B.; Mudie, D.; Amidon, G.E.; et al. Exploring Bioequivalence of Dexketoprofen Trometamol Drug Products with the Gastrointestinal Simulator (GIS) and Precipitation Pathways Analyses. Pharmaceutics 2019, 11, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, M.V.; Briand, L.E. Relevance and Bio-Catalytic Strategies for the Kinetic Resolution of Ketoprofen towards Dexketoprofen. Crit. Rev. Biotechnol. 2018, 38, 778–800. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.A.; Kıyan, H.T. Treatment of Oxidative Stress-Induced Pain and Inflammation with Dexketoprofen Trometamol Loaded Different Molecular Weight Chitosan Nanoparticles: Formulation, Characterization and Anti-Inflammatory Activity by Using in Vivo HET-CAM Assay. Microvasc. Res. 2020, 128, 103961. [Google Scholar] [CrossRef] [PubMed]
- Çulcu, Ö.; Tunçel, E.; Tamer, S.İ.; Tirnaksiz, F.F. Characterization of Thermosensitive Gels for the Sustained Delivery of Dexketoprofen Trometamol for Dermal Applications. J. Gazi Univ. Health Sci. Inst. 2020, 2, 1–12. [Google Scholar]
- Wang, B.; Wu, X.; Li, J.; Hao, X.; Lin, J.; Cheng, D.; Lu, Y. Thermosensitive Behavior and Antibacterial Activity of Cotton Fabric Modified with a Chitosan-Poly(N-Isopropylacrylamide) Interpenetrating Polymer Network Hydrogel. Polymers 2016, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- United States Pharmacopoeia. USP 42—NF 37. Chapter 724 Drug Release. In Proceedings of the United States Pharmacopoeia Convention, Rockville, MD, USA, 20 November 2019.
- Moeini, A.; Cimmino, A.; Dal Poggetto, G.; Di Biase, M.; Evidente, A.; Masi, M.; Lavermicocca, P.; Valerio, F.; Leone, A.; Santagata, G.; et al. Effect of PH and TPP Concentration on Chemico-Physical Properties, Release Kinetics and Antifungal Activity of Chitosan-TPP-Ungeremine Microbeads. Carbohydr. Polym. 2018, 195, 631–641. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-Printed Chitosan-Based Scaffolds: An in Vitro Study of Human Skin Cell Growth and an in-Vivo Wound Healing Evaluation in Experimental Diabetes in Rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales—Pharmaceutical Press: Chicago, IL, USA, 2009; ISBN 978-1-58212-135-2. [Google Scholar]
- Chang, Z.; Chen, Y.; Tang, S.; Yang, J.; Chen, Y.; Chen, S.; Li, P.; Yang, Z. Construction of Chitosan/Polyacrylate/Graphene Oxide Composite Physical Hydrogel by Semi-Dissolution/Acidification/Sol-Gel Transition Method and Its Simultaneous Cationic and Anionic Dye Adsorption Properties. Carbohydr. Polym. 2020, 229, 115431. [Google Scholar] [CrossRef]
- Halima, N.B. Poly(Vinyl Alcohol): Review of Its Promising Applications and Insights into Biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Mosleh, Y.; de Zeeuw, W.; Nijemeisland, M.; Bijleveld, J.C.; van Duin, P.; Poulis, J.A. The Structure–Property Correlations in Dry Gelatin Adhesive Films. Adv. Eng. Mater. 2021, 23, 2000716. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Comparative Study of Chitosan and Chitosan–Gelatin Scaffold for Tissue Engineering. Int. Nano Lett. 2017, 7, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Gutschke, E.; Bracht, S.; Nagel, S.; Weitschies, W. Adhesion Testing of Transdermal Matrix Patches with a Probe Tack Test—In Vitro and in Vivo Evaluation. Eur. J. Pharm. Biopharm. 2010, 75, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Flores-Arriaga, J.C.; Chavarría-Bolaños, D.; Pozos-Guillén, A.D.J.; Escobar-Barrios, V.A.; Cerda-Cristerna, B.I. Synthesis of a PVA Drug Delivery System for Controlled Release of a Tramadol–Dexketoprofen Combination. J. Mater. Sci. Mater. Med. 2021, 32, 56. [Google Scholar] [CrossRef] [PubMed]
- Khouri, J.; Penlidis, A.; Moresoli, C. Viscoelastic Properties of Crosslinked Chitosan Films. Processes 2019, 7, 157. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, A.A.; Banderas, L.M.; Otero, M.D.C.; Yenilmez, E.; Şenel, B.; Yazan, Y. Dexketoprofen Trometamol-Loaded Poly-Lactic-Co-Glycolic Acid (PLGA) Nanoparticles: Preparation, in Vitro Characterization and Cyctotoxity. Trop. J. Pharm. Res. 2019, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pieklarz, K.; Galita, G.; Tylman, M.; Maniukiewicz, W.; Kucharska, E.; Majsterek, I.; Modrzejewska, Z. Physico-Chemical Properties and Biocompatibility of Thermosensitive Chitosan Lactate and Chitosan Chloride Hydrogels Developed for Tissue Engineering Application. J. Funct. Biomater. 2021, 12, 37. [Google Scholar] [CrossRef]
- Ibrahim, M.; Mahmoud, A.A.; Osman, O.; Abd El-Aal, M.; Eid, M. Molecular Spectroscopic Analyses of Gelatin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 81, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Rajak, P.; Nath, L.K.; Bhuyan, B. Liquid Crystals: An Approach in Drug Delivery. Indian J. Pharm. Sci. 2019, 81, 11–21. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive Polymers with Lower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound Healing and Antimicrobial Effect of Active Secondary Metabolites in Chitosan-Based Wound Dressings: A Review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef]
- Martínez-Martínez, M.; Rodríguez-Berna, G.; Bermejo, M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Merino, V. Covalently Crosslinked Organophosphorous Derivatives-Chitosan Hydrogel as a Drug Delivery System for Oral Administration of Camptothecin. Eur. J. Pharm. Biopharm. 2019, 136, 174–183. [Google Scholar] [CrossRef]
- Gentile, P.; Nandagiri, V.K.; Daly, J.; Chiono, V.; Mattu, C.; Tonda-Turo, C.; Ciardelli, G.; Ramtoola, Z. Localised Controlled Release of Simvastatin from Porous Chitosan–Gelatin Scaffolds Engrafted with Simvastatin Loaded PLGA-Microparticles for Bone Tissue Engineering Application. Mater. Sci. Eng. C 2016, 59, 249–257. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Castro-Alpízar, J.; Lopretti-Correa, M.; Vega-Baudrit, J. Exploration of Bioengineered Scaffolds Composed of Thermo-Responsive Polymers for Drug Delivery in Wound Healing. Int. J. Mol. Sci. 2021, 22, 1408. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Bae, Y.C. Thermodynamic Framework for Switching the Lower Critical Solution Temperature of Thermo-Sensitive Particle Gels in Aqueous Solvent. Polymer 2020, 195, 122428. [Google Scholar] [CrossRef]
- Kathe, K.; Kathpalia, H. Film Forming Systems for Topical and Transdermal Drug Delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Portet, S. A Primer on Model Selection Using the Akaike Information Criterion. Infect. Dis. Model. 2020, 5, 111–128. [Google Scholar] [CrossRef]
- Rehman, Q.; Akash, M.S.H.; Rasool, M.F.; Rehman, K. Role of Kinetic Models in Drug Stability. In Drug Stability and Chemical Kinetics; Akash, M.S.H., Rehman, K., Eds.; Springer: Singapore, 2020; pp. 155–165. ISBN 9789811564260. [Google Scholar]
- Dima, C.; Pătraşcu, L.; Cantaragiu, A.; Alexe, P.; Dima, Ş. The Kinetics of the Swelling Process and the Release Mechanisms of Coriandrum Sativum L. Essential Oil from Chitosan/Alginate/Inulin Microcapsules. Food Chem. 2016, 195, 39–48. [Google Scholar] [CrossRef] [PubMed]






| Composition (w/v %) | F1 | F2 | F3 |
|---|---|---|---|
| DKT | 0.44 | 0.44 | 0.44 |
| Chitosan | 4 | 4 | 4 |
| PVA | - | 2 | - |
| Gelatin | - | - | 4 |
| Citric acid | 5 | 5 | 5 |
| Benzoic acid | 0.2 | 0.2 | 0.2 |
| Distilled water | q.s 25.0 mL | q.s 25.0 mL | q.s 25.0 mL |
| Model | Equation | Terms | |
|---|---|---|---|
| Zero-order | (2) | : amount of drug dissolved in time : zero-order release constant in mg·h−1 | |
| First-order | (3) | : change in concentration with respect to change on time; : first-order release constant in h−1 | |
| Hixson–Crowell | (4) | : Hixson–Crowell constant in mg1/3 h−1 | |
| Higuchi | (5) | : Higuchi constant in h0.5 | |
| Korsmeyer–Peppas | (6) | : for non-Fickian diffusion | |
| Model | 32 °C | 38 °C | ||||
|---|---|---|---|---|---|---|
| Release Rate | R2 | AIC | Release Rate | R2 | AIC | |
| Zero-order | 2.70 ± 0.25 | 0.9661 | 29.81 | 5.70 ± 0.43 | 0.9886 | 15.00 |
| First-order | 0.0557 ± 0.0016 | 0.9968 | −29.01 | 0.167 ± 0.016 | 0.9827 | −9.53 |
| Hixson–Crowell | 0.0669 ± 0.0028 | 0.9931 | −24.18 | 0.1759 ± 0.0074 | 0.9965 | −17.52 |
| Higuchi | 16.51 ± 0.41 | 0.9976 | 14.08 | 26.1 ± 1.1 | 0.9965 | 10.34 |
| Korsmeyer–Peppas | 0.181 ± 0.023 | 0.9979 | −32.48 | 0.279 ± 0.040 | 0.9941 | −20.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Henríquez, L.; Sanabria-Espinoza, P.; Murillo-Castillo, B.; Montes de Oca-Vásquez, G.; Batista-Menezes, D.; Calvo-Guzmán, B.; Ramírez-Arguedas, N.; Vega-Baudrit, J. Topical Chitosan-Based Thermo-Responsive Scaffold Provides Dexketoprofen Trometamol Controlled Release for 24 h Use. Pharmaceutics 2021, 13, 2100. https://doi.org/10.3390/pharmaceutics13122100
Castillo-Henríquez L, Sanabria-Espinoza P, Murillo-Castillo B, Montes de Oca-Vásquez G, Batista-Menezes D, Calvo-Guzmán B, Ramírez-Arguedas N, Vega-Baudrit J. Topical Chitosan-Based Thermo-Responsive Scaffold Provides Dexketoprofen Trometamol Controlled Release for 24 h Use. Pharmaceutics. 2021; 13(12):2100. https://doi.org/10.3390/pharmaceutics13122100
Chicago/Turabian StyleCastillo-Henríquez, Luis, Pablo Sanabria-Espinoza, Brayan Murillo-Castillo, Gabriela Montes de Oca-Vásquez, Diego Batista-Menezes, Briner Calvo-Guzmán, Nils Ramírez-Arguedas, and José Vega-Baudrit. 2021. "Topical Chitosan-Based Thermo-Responsive Scaffold Provides Dexketoprofen Trometamol Controlled Release for 24 h Use" Pharmaceutics 13, no. 12: 2100. https://doi.org/10.3390/pharmaceutics13122100
APA StyleCastillo-Henríquez, L., Sanabria-Espinoza, P., Murillo-Castillo, B., Montes de Oca-Vásquez, G., Batista-Menezes, D., Calvo-Guzmán, B., Ramírez-Arguedas, N., & Vega-Baudrit, J. (2021). Topical Chitosan-Based Thermo-Responsive Scaffold Provides Dexketoprofen Trometamol Controlled Release for 24 h Use. Pharmaceutics, 13(12), 2100. https://doi.org/10.3390/pharmaceutics13122100