Prevalence and Clinical Significance of Drug–Drug and Drug–Dietary Supplement Interactions among Patients Admitted for Cardiothoracic Surgery in Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Approval
2.2. Evaluation of Drug Interactions with Coadministered Medications and Interactions with Dietary Supplements
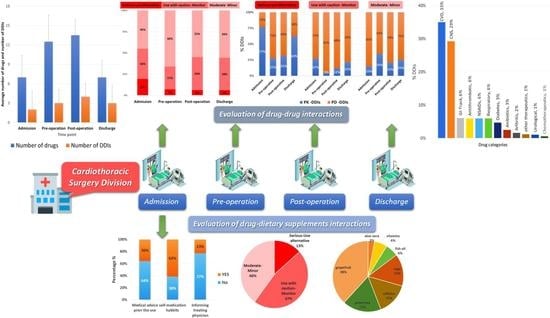
3. Results
3.1. Patients’ Demographics, CTS Diagnoses, Comorbidities, and Clinical Data during Hospitalization
3.2. Medications Administered at Different Time Points
3.3. DDIs Identified and Correlation with Administered Medications
3.4. Pharmacological Mechanisms and Clinical Significance of the Identified DDIs
3.5. Dietary Supplements, Reasons for Use, and Identified DDSIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohler, G.I.; Bode-Boger, S.M.; Busse, R.; Hoopmann, M.; Welte, T.; Boger, R.H. Drug-drug interactions in medical patients: Effects of in-hospital treatment and relation to multiple drug use. Int. J. Clin. Pharmacol. Ther. 2000, 38, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Dechanont, S.; Maphanta, S.; Butthum, B.; Kongkaew, C. Hospital admissions/visits associated with drug-drug interactions: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2014, 23, 489–497. [Google Scholar] [CrossRef]
- Aronson, J.K. Classifying drug interactions. Br. J. Clin. Pharmacol. 2004, 58, 343–344. [Google Scholar] [CrossRef] [Green Version]
- Bjerrum, L.; Lopez-Valcarcel, B.G.; Petersen, G. Risk factors for potential drug interactions in general practice. Eur. J. Gen. Pract. 2008, 14, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Piatkov, I.; Jones, T.; McLe, M. Drug Interactions, Pharmacogenomics and Cardiovascular Complication. In Drug Discovery; InTech: London, UK, 2013. [Google Scholar]
- Janković, S.M.; Pejčić, A.V.; Milosavljević, M.N.; Opančina, V.D.; Pešić, N.V.; Nedeljković, T.T.; Babić, G.M. Risk factors for potential drug-drug interactions in intensive care unit patients. J. Crit. Care 2018, 43, 1–6. [Google Scholar] [CrossRef]
- Murtaza, G.; Khan, M.Y.G.; Azhar, S.; Khan, S.A.; Khan, T.M. Assessment of potential drug-drug interactions and its associated factors in the hospitalized cardiac patients. Saudi Pharm. J. 2016, 24, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Spanakis, M.; Spanakis, E.G.; Kondylakis, H.; Sfakianakis, S.; Genitsaridi, I.; Sakkalis, V.; Tsiknakis, M.; Marias, K. Addressing drug-drug and drug-food interactions through personalized empowerment services for healthcare. In Proceedings of the Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Baniasadi, S.; Farzanegan, B.; Alehashem, M. Important drug classes associated with potential drug–drug interactions in critically ill patients: Highlights for cardiothoracic intensivists. Ann. Intensive Care 2015, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Clough, R.A.; Leavitt, B.J.; Morton, J.R.; Plume, S.K.; Hernandez, F.; Nugent, W.; Lahey, S.J.; Ross, C.S.; O’Connor, G.T. The effect of comorbid illness on mortality outcomes in cardiac surgery. Arch. Surg. 2002, 137, 428–433. [Google Scholar] [CrossRef]
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 14 May 2020).
- Pagano, D.; Kappetein, A.P.; Sousa-Uva, M.; Beyersdorf, F.; Klautz, R.; Mohr, F.; Falk, V. EACTS clinical statement: Guidance for the provision of adult cardiac surgery. Eur. J. Cardio-thoracic Surg. 2016, 50, 1006–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravera, A.; Carubelli, V.; Sciatti, E.; Bonadei, I.; Gorga, E.; Cani, D.; Vizzardi, E.; Metra, M.; Lombardi, C. Nutrition and Cardiovascular Disease: Finding the Perfect Recipe for Cardiovascular Health. Nutrients 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Tachjian, A.; Maria, V.; Jahangir, A. Use of Herbal Products and Potential Interactions in Patients with Cardiovascular Diseases. J. Am. Coll. Cardiol. 2010, 55, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Bandaranayake, W.M. Quality Control, Screening, Toxicity, and Regulation of Herbal Drugs. In Modern Phytomedicine; Ahmad, I., Aqil, F., Owais, M., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Mateti, U.V.; Rajakannan, T.; Nekkanti, H.; Rajesh, V.; Mallaysamy, S.R.; Ramachandran, P. Drug-drug interactions in hospitalized cardiac patients. J. Young Pharm. 2011, 3, 329–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.; Jain, P.; Sharma, K.; Saraswat, P. A prospective analysis of drug interactions in patients of intensive cardiac care unit. J. Clin. Diagnostic Res. 2017, 11, FC01–FC04. [Google Scholar] [CrossRef]
- Wood, M.J.; Stewart, R.L.; Merry, H.; Johnstone, D.E.; Cox, J.L. Use of complementary and alternative medical therapies in patients with cardiovascular disease. Am Hear. J 2003, 145, 806–812. [Google Scholar] [CrossRef]
- Grant, S.J.; Bin, Y.S.; Kiat, H.; Chang, D.H.T. The use of complementary and alternative medicine by people with cardiovascular disease: A systematic review. BMC Public Health 2012, 12, 299. [Google Scholar] [CrossRef] [Green Version]
- Suroowan, S.; Mahomoodally, F. Common phyto-remedies used against cardiovascular diseases and their potential to induce adverse events in cardiovascular patients. Clin. Phytoscience 2015, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Chatsisvili, A.; Sapounidis, I.; Pavlidou, G.; Zoumpouridou, E.; Karakousis, V.A.; Spanakis, M.; Teperikidis, L.; Niopas, I. Potential drug-drug interactions in prescriptions dispensed in community pharmacies in Greece. Pharm. World Sci. 2010, 32, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; Von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef] [Green Version]
- Spanakis, M.; Sfakianakis, S.; Sakkalis, V.; Spanakis, E.G. PharmActa: Empowering Patients to Avoid Clinical Significant Drug(-)Herb Interactions. Medicines 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awortwe, C.; Makiwane, M.; Reuter, H.; Muller, C.; Louw, J.; Rosenkranz, B. Critical evaluation of causality assessment of herb-drug interactions in patients. Br J Clin Pharmacol 2018, 84, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Khan, Q.; Ismail, M.; Haider, I.; ul Haq, I.; Noor, S. QT interval prolongation in hospitalized patients on cardiology wards: A prospective observational study. Eur. J. Clin. Pharmacol. 2017, 73, 1511–1518. [Google Scholar] [CrossRef]
- Spanakis, M.; Roubedaki, M.; Tzanakis, I.; Zografakis-Sfakianakis, M.; Patelarou, E.; Patelarou, A. Impact of adverse drug reactions in patients with end stage renal disease in Greece. Int. J. Environ. Res. Public Health 2020, 17, 9101. [Google Scholar] [CrossRef]
- Juurlink, D.N.; Mamdani, M.; Kopp, A.; Laupacis, A.; Redelmeier, D.A. Drug-Drug Interactions among Elderly Patients Hospitalized for Drug Toxicity. J. Am. Med. Assoc. 2003, 289, 1652–1658. [Google Scholar] [CrossRef] [Green Version]
- Zwart-Van Rijkom, J.E.F.; Uijtendaal, E.V.; Ten Berg, M.J.; Van Solinge, W.W.; Egberts, A.C.G. Frequency and nature of drug-drug interactions in a Dutch university hospital. Br. J. Clin. Pharmacol. 2009, 68, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, D.; Suthar, J.; Malhotra, S.; Patel, V.; Patel, P. A study of potential adverse drug-drug interactions among prescribed drugs in medicine outpatient department of a tertiary care teaching hospital. J. Basic Clin. Pharm. 2014, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Bucşa, C.; Farcaş, A.; Cazacu, I.; Leucuta, D.; Achimas-Cadariu, A.; Mogosan, C.; Bojita, M. How many potential drug-drug interactions cause adverse drug reactions in hospitalized patients? Eur. J. Intern. Med. 2013, 24, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Adhimoolam, M.; Kannan, S. Study of drug–Drug interactions among the hypertensive patients in a tertiary care teaching hospital. Perspect. Clin. Res. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Drug-induced hyperkalemia: Old culprits and new offenders. Am. J. Med. 2000, 109, 307–314. [Google Scholar] [CrossRef]
- Zack, J.; Berg, J.; Juan, A.; Pannacciulli, N.; Allard, M.; Gottwald, M.; Zhang, H.; Shao, Y.; Ben-Yehuda, O.; Jochelson, P. Pharmacokinetic drug-drug interaction study of ranolazine and metformin in subjects with type 2 diabetes mellitus. Clin. Pharmacol. Drug Dev. 2015, 4, 121–129. [Google Scholar] [CrossRef]
- Wiggins, B.S.; Saseen, J.J.; Page, R.L.; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B. Recommendations for Management of Clinically Significant Drug-Drug Interactions with Statins and Select Agents Used in Patients with Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyanasundaram, A.; Lincoff, A.M. Managing adverse effects and drug-drug interactions of antiplatelet agents. Nat. Rev. Cardiol. 2011, 8, 592–600. [Google Scholar] [CrossRef]
- Vazquez, S.R. Drug-drug interactions in an era of multiple anticoagulants: A focus on clinically relevant drug interactions. Blood 2018, 132, 2230–2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizirianakis, I.S.; Spanakis, M.; Termentzi, A.; Niopas, I.; Kokkalou, E. Clinical and pharmacogenomic assessment of herb-drug interactions to improve drug delivery and pharmacovigilance. In Plants in Traditional and Modern Medicine: Chemistry and Activity; Kokkalou, E., Ed.; Transworld Research Network: Kerala, India, 2010; ISBN 978-81-7895-432-5. [Google Scholar]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Cirillo, C.; Capasso, R. Constipation and Botanical Medicines: An Overview. Phytother. Res. 2015, 29, 1488–1493. [Google Scholar] [CrossRef]
- Vazquez Cisneros, L.C.; Lopez-Uriarte, P.; Lopez-Espinoza, A.; Navarro Meza, M.; Espinoza-Gallardo, A.C.; Guzman Aburto, M.B. Effects of green tea and its epigallocatechin (EGCG) content on body weight and fat mass in humans: a systematic review. Nutr. Hosp. 2017, 34, 731–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujioka, K.; Greenway, F.; Sheard, J.; Ying, Y. The Effects of Grapefruit on Weight and Insulin Resistance: Relationship to the Metabolic Syndrome. J. Med. Food 2006, 9, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Omeish, A.F.; Abbadi, W.; Ghanma, I.M.; Drabaa, Z.; Botoosh, F.A.; Seif, A.; Massadeh, M. Hospital-based study on the use of herbal medicine in patients with coronary artery disease in Jordan. J. Pak. Med. Assoc. 2011, 61, 683–687. [Google Scholar] [PubMed]
- Shakeel, M.; Bruce, J.; Jehan, S.; McAdam, T.K.; Bruce, D.M. Use of complementary and alternative medicine by patients admitted to a surgical unit in Scotland. Ann. R. Coll. Surg. Engl. 2008, 90, 571–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasuski, R.A.; Michaelis, K.; Eckart, R.E. The cardiovascular patient’s perceptions of complementary and alternative medicine. Clin. Cardiol. 2006, 29, 161–164. [Google Scholar] [CrossRef]
- Gallo, E.; Pugi, A.; Lucenteforte, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Pharmacovigilance of herb-drug interactions among preoperative patients. Altern. Ther. Heal. Med. 2014, 20, 13–17. [Google Scholar]
- Bailey, D.G.; Dresser, G.; Arnold, J.M. Grapefruit-medication interactions: Forbidden fruit or avoidable consequences? CMAJ 2013, 185, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, M.J.; Cancalon, P.; Widmer, W.W.; Greenblatt, D.J. The effect of grapefruit juice on drug disposition. Expert Opin. Drug Metab. Toxicol. 2011, 7, 267–286. [Google Scholar] [CrossRef]
- Kirby, B.J.; Unadkat, J.D. Grapefruit juice, a glass full of drug interactions? Clin. Pharmacol. Ther. 2007, 81, 631–633. [Google Scholar] [CrossRef]
- Bailey, D.G. Grapefruit-medication interactions. CMAJ 2013, 185, 507–508. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.G. Better to Avoid Grapefruit with Certain Statins. Am. J. Med. 2016, 129, e301. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.Q.; Yamazoe, Y. Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines. Acta Pharmacol. Sin. 2004, 25, 129–136. [Google Scholar]
- Alemdaroglu, N.C.; Dietz, U.; Wolffram, S.; Spahn-Langguth, H.; Langguth, P. Influence of green and black tea on folic acid pharmacokinetics in healthy volunteers: Potential risk of diminished folic acid bioavailability. Biopharm. Drug Dispos. 2008, 29, 335–348. [Google Scholar] [CrossRef]
- Werba, J.P.; Misaka, S.; Giroli, M.G.; Shimomura, K.; Amato, M.; Simonelli, N.; Vigo, L.; Tremoli, E. Update of green tea interactions with cardiovascular drugs and putative mechanisms. J. Food Drug. Anal. 2018, 26, S72–S77. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.U.; Choi, M.S.; Choe, K.; Yoo, H.H. Interactions between herbs and antidiabetics: An overview of the mechanisms, evidence, importance, and management. Arch. Pharm. Res. 2015, 38, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Kochhar, A.; Sangha, J. Hypoglycemic and hypolipidemic effect of Aloe vera L. in non-insulin dependent diabetics. J. Food Sci. Technol. 2014, 51, 90–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, Y.; Tatsuno, I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: Present, past and future. Expert Rev. Clin. Pharmacol. 2017, 10, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Block, R.C.; Abdolahi, A.; Smith, B.; Meednu, N.; Thevenet-Morrison, K.; Cai, X.; Cui, H.; Mousa, S.; Brenna, J.T.; Georas, S. Effects of low-dose aspirin and fish oil on platelet function and NF-kappaB in adults with diabetes mellitus. Prostaglandins Leukot Essent Fat. Acids 2013, 89, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Zhou, S.Y.; Fabriaga, E.; Zhang, P.H.; Zhou, Q. Food-drug interactions precipitated by fruit juices other than grapefruit juice: An update review. J. Food Drug Anal. 2018, 26, S61–S71. [Google Scholar] [CrossRef]
- An, G.; Mukker, J.K.; Derendorf, H.; Frye, R.F. Enzyme- and transporter-mediated beverage-drug interactions: An update on fruit juices and green tea. J. Clin. Pharmacol. 2015, 55, 1313–1331. [Google Scholar] [CrossRef]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef]
- Skelin, M.; Lucijanic, T.; Amidzic Klaric, D.; Resic, A.; Bakula, M.; Liberati-Cizmek, A.M.; Gharib, H.; Rahelic, D. Factors Affecting Gastrointestinal Absorption of Levothyroxine: A Review. Clin. Ther. 2017, 39, 378–403. [Google Scholar] [CrossRef] [PubMed]
- van Oijen, M.G.; Laheij, R.J.; Peters, W.H.; Jansen, J.B.; Verheugt, F.W. Association of aspirin use with vitamin B12 deficiency (results of the BACH study). Am. J. Cardiol. 2004, 94, 975–977. [Google Scholar] [CrossRef]
- Noor, S.; Ismail, M.; Ali, Z. Potential drug-drug interactions among pneumonia patients: Do these matter in clinical perspectives? BMC Pharmacol. Toxicol. 2019, 20, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ommen, S.R.; Odell, J.A.; Stanton, M.S. Atrial Arrhythmias after Cardiothoracic Surgery. N. Engl. J. Med. 1997, 336, 1429–1434. [Google Scholar] [CrossRef]
- Baeza-Herrera, L.A.; Rojas-Velasco, G.; Márquez-Murillo, M.F.; Portillo-Romero, A.D.R.; Medina-Paz, L.; Álvarez-álvarez, R.; Ramos-Enríquez, Á.; Baranda-Tovar, F.M. Atrial fibrillation in cardiac surgery. Arch. Cardiol. Mex. 2019, 89, 348–359. [Google Scholar] [CrossRef] [Green Version]
- Sabzi, F.; Faraji, R. Liver Function Tests Following Open Cardiac Surgery. J. Cardiovasc. Thorac. Res. 2015, 7, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turgeon, J.; Michaud, V. Clinical decision support systems: Great promises for better management of patients’ drug therapy. Expert Opin. Drug Metab. Toxicol. 2016, 12, 993–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spanakis, M.; Sfakianakis, S.; Kallergis, G.; Spanakis, E.G.; Sakkalis, V. PharmActa: Personalized pharmaceutical care eHealth platform for patients and pharmacists. J. Biomed. Inform. 2019, 100, 103336. [Google Scholar] [CrossRef]
- Manias, E.; Kusljic, S.; Wu, A. Interventions to reduce medication errors in adult medical and surgical settings: A systematic review. Ther. Adv. Drug Saf. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Sancar, M.; Kaşik, A.; Okuyan, B.; Batuhan, S.; İzzettin, F.V. Determination of potential drug–drug interactions using various software programs in a community pharmacy setting. Turkish J. Pharm. Sci. 2019, 16, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Trifirò, G.; Patadia, V.; Schuemie, M.J.; Coloma, P.M.; Gini, R.; Herings, R.; Hippisley-Cox, J.; Mazzaglia, G.; Giaquinto, C.; Scotti, L.; et al. EU-ADR healthcare database network vs. spontaneous reporting system database: Preliminary comparison of signal detection. Stud. Health Technol. Inform. 2011, 166, 25–30. [Google Scholar]






| Methods | |
|---|---|
| Study design | Analysis of DDIs 1 and DDSIs 2 in patients admitted to CTS 3 clinic in Greece |
| Setting | Cardiothoracic surgery of University Hospital of Heraklion in Greece |
| Participants | Patients that need cardiothoracic surgery (CTS) due to progressed CVD 4 |
| Variables | Record of demographic characteristics, clinical values, comorbidities, medication regimens, and dietary supplement usage Analyze DDIs and DDSIs, their clinical significance, and pharmacological mechanisms |
| Data sources/measurement | DDIs, DDSIs and their clinical significance based on literature search and relative databases (Medscape, Drugs.com) |
| Study size | Target population: patients admitted for CTS Study population: signed informed consent form to participate |
| Bias | Diligence in informing the purpose and objectives of the study Diligence in recording the medication regimens in correct time periods Recording demographics and medication regiments Analysis of data regarding significance Dietary supplement and self-medication habits feedback |
| Results | |
| Participants | 76 patients signed informed consent form (95% of total patients in the clinic) |
| Descriptive data | 60.5% male and 39.5 female average age 66 years (min 37, max 85) Average comorbidities: 5 Average hospitalization: 10 days Admittance diagnosis 5: CABG (50%), SAVR 24%, CABG/SAVR/MVPL/CEA/BENTALL 16%, AAA 2% |
| Outcome data | Comorbidities: hypertension, hyperlipidemias and diabetes were most frequent 166 unique DDIs PK-DDIs 6: 53 unique combinations PD-DDIs 7: 113 unique combinations PK-DDSIs: 18 unique combinations PD-DDSIs: 12 unique combinations |
| Main results | 12% of the identified DDIs were characterized as serious and an alternative should have been used Positive trend between number of medications and prevalence of DDIs regardless the time point PK-DDIs were highly prevalent during admission and discharge whereas PD-DDIs recorded mostly during pre- and postoperation periods. 60% of patients use DS products 60% of the DDSIs were related to PK processes and 36% of the identified PK-DDSIs were due to the consumption of grapefruit juice Patients avoid or neglect seeking medical advice regarding DS usage |
| Demographics 1 | Mean (±S.D.) | Min/Max |
| Age (y) | 66 (±10.20) | 37/85 |
| Height (m) | 1.65 (±0.1) | 1.40/1.84 |
| Weight (kg) | 81.1 (±15.26) | 52.6/122.7 |
| BMI (kg/m2) | 29.9 (±5.05) | 17.1/45.4 |
| Comorbidities | 5 | 1/10 |
| Days hospitalization (d) | 10 | 2/18 |
| Preoperative hospitalization (d) | 2 | 1/8 |
| Postoperative hospitalization (d) | 8 | 2/12 |
| Diagnosis 2 | Number of Patients (%) | |
| CABG | 38 (50%) | |
| SAVR | 18 (24%) | |
| MVP/CEA/Bentall/CABG-SAVR | 12(16%) | |
| AAA | 2 (2%) | |
| Other | 6 (8%) | |
| Comorbidities | ||
| Hypertension | 64 (84%) | |
| Diabetes | 40 (52%) | |
| Hyperlipidemias | 61 (80%) | |
| Thyroid | 23 (30%) | |
| Central Nervous System | 18 (24%) | |
| Gastrointestinal | 4 (6%) | |
| Respiratory | 5 (4%) | |
| Other | 8 (10%) | |
| Social Habits (Smoking & Alcohol) | Number of Patients (%) | |
| Smoker | 25 (32%) | |
| Ex-smoker | 32 (42%) | |
| Nonsmoker | 20 (26%) | |
| Alcoholic | 6 (8%) | |
| Social drinker | 34 (45%) | |
| Nondrinker | 36 (47%) |
| Drug A | Drug B | Drug Categories | Pharmacological Outcome | Number of Cases | |
|---|---|---|---|---|---|
| Pharmacokinetic drug interactions: Serious-use alternative | |||||
| amiodarone | acenocoumarol | antiarrhythmic | anticoagulant | CYP * metabolism inhibition acenocoumarol levels | 4 |
| amlodipine | simvastatin | Ca2+-blocker | antilipidemic | CYP3A4 inhibition (statin-rhabdomyolysis) | 4 |
| aspirin | methotrexate | NSAIDs * | rheumatoid arthritis | PK-Renal clearance (methotrexate toxicity) | 2 |
| esomeprazole | cilostazol | PPI * | antiplatelet | PK-CYP2C19 inhibition of cilostazol | 2 |
| esomeprazole | clopidogrel | PPI | antiplatelet | Reduced antiplatelet activity -CYP2C9 metabolism | 11 |
| esomeprazole | escitalopram | PPI | SSRI * | PK-CYP2C19 metabolism inhibition | 1 |
| haloperidol | amiodarone | antipsychotic | antiarrhythmic | PK-CYP2D6 inhibition | 1 |
| ranolazine | carvedilol | angina | β-blocker | PK-CYP2D6 metabolism (carvedilol) | 3 |
| ranolazine | metformin | angina | diabetes II | PK-renal clearance (metformin) OCT2 | 2 |
| ranolazine | simvastatin | angina | antilipidemic | CYP3A4 inhibition (statin-rhabdomyolysis) | 3 |
| Pharmacokinetic drug interactions: Use with caution-Monitor | |||||
| haloperidol | metoprolol | antipsychotic | β-blocker | PK CYP2D6 metabolism inhibition (metoprolol) | 6 |
| atorvastatin | valsartan | antilipidemic | ARBs * | PK-OATB1 * transporter | 4 |
| amiodarone | metoprolol | antiarrhythmic | β-blocker | PK-CYP2D6 inhibition for metoprolol (bradycardia) | 3 |
| omeprazole | clopidogrel | PPI | antiplatelet | PK CYP2C9 metabolism (clopidogrel) | 3 |
| ciprofloxacin | acenocoumarol | antibiotic | anticoagulant | PK-CYP1A2 inhibition acenocoumarol levels | 2 |
| Pharmacokinetic drug interactions: Moderate-Minor | |||||
| budesonide | acenocoumarol | corticosteroid | anticoagulant | PK-CYP3A4 induction metabolism of acenocoumarol | 13 |
| amiodarone | codeine | antiarrhythmic | analgesic | PK-CYP2D6 (codeine) | 2 |
| carvedilol | haloperidol | β-blocker | antipsychotic | PK-CYP2D6 inhibition | 2 |
| ciprofloxacin | alprazolam | antibiotic | anxiolytics | PK CYP3A4 metabolism inhibition (alprazolam) | 3 |
| ferrous (gluconate, sulfate etc.) | levothyroxine | anemia | thyroid | PK-T4 GI absorption | 4 |
| Pharmacodynamic drug interactions: Serious-use alternative | |||||
| alprazolam | haloperidol | anxiolytics | antipsychotic | synergism sedation | 2 |
| amiloride | potassium chloride | diuretic | hypokalemia | synergism hyperkalemia | 3 |
| citalopram | duloxetine | SSRI * | SNRI * | synergism (serotonin syndrome) | 1 |
| fenofibrate | pitavastatin | antilipidemic | antilipidemic | synergism | 2 |
| haloperidol | amiodarone | antipsychotic | antiarrhythmic | QT prolongation | 1 |
| morphine | escitalopram | analgesic | SSRI | serotonin syndrome | 1 |
| quetiapine | haloperidol | antipsychotic | antipsychotic | enhance antidopaminergic effect, QT prolongation | 6 |
| tramadol | pethidine | analgesic | analgesic | synergism sedation | 2 |
| Pharmacodynamic drug interactions: Use with caution-Monitor | |||||
| alprazolam | morphine | anxiolytics | analgesic | PD-synergism sedation | 16 |
| aspirin | acenocoumarol | NSAIDS | antiplatelet | PD-synergism risk of bleeding | 11 |
| carvedilol | furosemide | β-blocker | diuretic | PD-antagonism and serum potassium | 9 |
| ciprofloxacin | haloperidol | antibiotic | antipsychotic | PD-QT prolongation | 3 |
| quetiapine | ipratropium | antipsychotic | anticholinergic | PD-synergism anticholinergic effects, hypoglycemia, QT-prolongation | 8 |
| Pharmacodynamic drug interactions: Moderate-Minor | |||||
| aspirin | perindopril | NSAIDs | ACE * | PD-antagonism kidney (decrease in renal function) | 11 |
| bisoprolol | furosemide | β-blocker | diuretic | PD-antagonism (serum potassium) | 17 |
| bromazepam | morphine | anxiolytics | analgesic | PD-synergism sedation | 25 |
| ceftriaxone | furosemide | antibiotic | diuretic | nephrotoxicity | 35 |
| perindopril | enoxaparin | ACE | antiplatelet | PD-hyperkalemia | 7 |
| Drugs | DS | PK-PD Mechanism | Drug Category | Clin. Sign. | Potential Clinical Outcome | No Cases |
|---|---|---|---|---|---|---|
| metformin | aloe vera | PD | diabetes II | 2 | hypoglycemia | 2 |
| levothyroxine | caffeine | PK-GI absorption | thyroid | 2 | decreased T4 levels | 5 |
| aspirin | fish oil | PD | anticoagulate | 2 | bleeding | 2 |
| clopidogrel | fish oil | PD | anticoagulate | 2 | bleeding | 1 |
| eplerenone | grapefruit | PK-CYP3A4 inhibition | diuretic | 2 | hyperkaliemia | 1 |
| amlodipine | grapefruit | PK-CYP3A4 inhibition | Ca2+-blocker | 3 | mlodipine-ADRs | 2 |
| clopidogrel | grapefruit | PK-CYP3A4 inhibition | antiplatelet | 2 | reduced bioactivation | 6 |
| simvastatin | grapefruit | PK-CYP3A4 inhibition | antilipidemic | 1 | statin-ADRs | 1 |
| ranolazine | grapefruit | PK-CYP3A4 inhibition | chronic angina | 1 | QT prolongation | 1 |
| atorvastatin | grapefruit | PK-CYP3A4 inhibition | antilipidemic | 1 | statin-ADRs | 7 |
| tamsulosin | grapefruit | PK-CYP3A4 inhibition | prostatic hyperplasia | 2 | tamsulosin-ADRs | 1 |
| alfuzosin | grapefruit | PK-CYP3A4 inhibition | a1-antagonist-prostate | 3 | alfuzosin-ADRs | 1 |
| finasteride | grapefruit | PK-CYP3A4 inhibition | prostatic hyperplasia | 3 | finasteride-ADRs | 1 |
| alprazolam | grapefruit | PK-CYP3A4 inhibition | anxiety | 2 | cilostazol-ADRs | 1 |
| cilostazol | grapefruit | PK-CYP3A4 inhibition | antiplatelet | 2 | risk for bleeding | 1 |
| ivabradine | grapefruit | PK-CYP3A4 inhibition | angina | 1 | ivabradine-ADRs | 1 |
| ticagrelor | grapefruit | PK-CYP3A4 inhibition | antiplatelet | 1 | risk for bleeding | 1 |
| prasugrel | green tea | PD | antiplatelet | 3 | Increased drug action | 1 |
| aspirin | green tea | PD | antiplatelet | 3 | bleeding | 7 |
| clopidogrel | green tea | PD | antiplatelet | 3 | bleeding | 5 |
| cilostazol | green tea | PD | antiplatelet | 3 | risk for bleeding | 1 |
| ferrous sulfate | green tea | PK-GI absorption | iron deficiency | 2 | reduced Fe absorption | 1 |
| folic acid (FA) | green tea | PK-GI absorption | iron deficiency | 2 | reduced FA absorption | 1 |
| metformin | sage | PD | antidiabetic | 3 | hypoglycemia | 4 |
| alprazolam | sage | PD | anxiety | 3 | increased sedation | 2 |
| gabapentin | sage | PD | anticonvulsant | 3 | convulsions | 1 |
| insulin | sage | PD | diabetes I | 3 | hypoglycemia | 1 |
| sigagliptin | sage | PD | diabetes II | 3 | hypoglycemia | 1 |
| aspirin | B12 | PK-GI absorption | B12-deficiency | 2 | B12-deficiency | 1 |
| ensomeprazole | B12 | PK-GI absorption | B12-deficiency | 3 | B12-deficiency | 1 |
| Total | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanakis, M.; Melissourgaki, M.; Lazopoulos, G.; Patelarou, A.E.; Patelarou, E. Prevalence and Clinical Significance of Drug–Drug and Drug–Dietary Supplement Interactions among Patients Admitted for Cardiothoracic Surgery in Greece. Pharmaceutics 2021, 13, 239. https://doi.org/10.3390/pharmaceutics13020239
Spanakis M, Melissourgaki M, Lazopoulos G, Patelarou AE, Patelarou E. Prevalence and Clinical Significance of Drug–Drug and Drug–Dietary Supplement Interactions among Patients Admitted for Cardiothoracic Surgery in Greece. Pharmaceutics. 2021; 13(2):239. https://doi.org/10.3390/pharmaceutics13020239
Chicago/Turabian StyleSpanakis, Marios, Maria Melissourgaki, George Lazopoulos, Athina E. Patelarou, and Evridiki Patelarou. 2021. "Prevalence and Clinical Significance of Drug–Drug and Drug–Dietary Supplement Interactions among Patients Admitted for Cardiothoracic Surgery in Greece" Pharmaceutics 13, no. 2: 239. https://doi.org/10.3390/pharmaceutics13020239
APA StyleSpanakis, M., Melissourgaki, M., Lazopoulos, G., Patelarou, A. E., & Patelarou, E. (2021). Prevalence and Clinical Significance of Drug–Drug and Drug–Dietary Supplement Interactions among Patients Admitted for Cardiothoracic Surgery in Greece. Pharmaceutics, 13(2), 239. https://doi.org/10.3390/pharmaceutics13020239