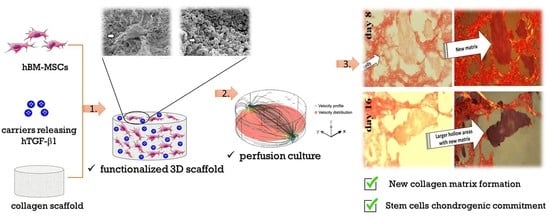
Chondrogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells in a Perfused Collagen Hydrogel Functionalized with hTGF-β1-Releasing PLGA Microcarrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. hBM-MSCs Isolation and Harvesting
2.2. PLGA-MCs Fabrication by Supercritical Emulsion Extraction Technology
2.3. Carrier Size Distribution and Morphological Analyses
2.4. hTGF-β1 Release Study
2.5. 3D Collagen Scaffolds Preparation and Characterization
2.5.1. Static and Dynamic Culture
2.5.2. Hematoxylin and Eosin and Sirius Red Staining
2.5.3. Immunofluorescence Assay
2.6. RNA Isolation and Gene Expression Profile
2.7. FEM Modeling
2.8. Statistical Analysis
3. Result and Discussion
3.1. PLGA Carriers Characterization and hTGF-β1 Release Profile
3.2. 3D Scaffold Environment Assembly and Characterization
3.3. Dynamic Culture by Perfusion Bioreactor
3.4. 3D Microenvironment and hBM-MSC Chondrogenic Commitment: Gene Expression and Immunofluorescence Assay
4. Conclusions and Perspectives
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| hTGF-β1 | Human transforming growth factor β1 |
| PLGA-MCs | Poly-lactic-co-glycolic acid microcarriers |
| hBM-MSCs | Human bone marrow mesenchymal stem cells |
| hASC | Human adipose stem cells |
| COL1A1 | Type I collagen |
| COL2A1 | Type II collagen |
| COL3A1 | Type III collagen |
| SOX9 | SRY-Box transcription factor 9 |
| ACAN | Aggrecan |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| q-IF | Semiquantitative immunofluorescence |
References
- Faust, H.J.; Guo, Q.; Elisseeff, J.H. Cartilage Tissue Engineering. In Principles of Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 937–952. ISBN 978-0-12-809880-6. [Google Scholar]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current Research on Pharmacologic and Regenerative Therapies for Osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal Stem Cell-Based Therapy of Osteoarthritis: Current Knowledge and Future Perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Rönn, K.; Reischl, N.; Gautier, E.; Jacobi, M. Current Surgical Treatment of Knee Osteoarthritis. Arthritis 2011, 2011, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastides, P.; Chimutengwende-Gordon, M.; Maffulli, N.; Khan, W. Stem Cell Therapy for Human Cartilage Defects: A Systematic Review. Osteoarthr. Cartil. 2013, 21, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Mollon, B.; Kandel, R.; Chahal, J.; Theodoropoulos, J. The Clinical Status of Cartilage Tissue Regeneration in Humans. Osteoarthr. Cartil. 2013, 21, 1824–1833. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.; Gilbert, T.; Myers-Irvin, J. The extracellular matrix as a biologic scaffold for tissue engineering. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2008; pp. 121–143. ISBN 978-0-12-370869-4. [Google Scholar]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Cipollaro, L.; Ciardulli, M.C.; Della Porta, G.; Peretti, G.M.; Maffulli, N. Biomechanical Issues of Tissue-Engineered Constructs for Articular Cartilage Regeneration: In Vitro and in Vivo Approaches. Br. Med. Bull. 2019, 132, 53–80. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef] [Green Version]
- Madry, H.; Rey-Rico, A.; Venkatesan, J.K.; Johnstone, B.; Cucchiarini, M. Transforming Growth Factor Beta-Releasing Scaffolds for Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2014, 20, 106–125. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863. [Google Scholar] [CrossRef] [Green Version]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Govoni, M.; Muscari, C.; Bonafè, F.; Morselli, P.G.; Cortesi, M.; Dallari, D.; Giordano, E. A Brief Very-Low Oxygen Tension Regimen Is Sufficient for the Early Chondrogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Adv. Med. Sci. 2021, 66, 98–104. [Google Scholar] [CrossRef]
- Citeroni, M.R.; Mauro, A.; Ciardulli, M.C.; Di Mattia, M.; El Khatib, M.; Russo, V.; Turriani, M.; Santer, M.; Della Porta, G.; Maffulli, N.; et al. Amnion-Derived Teno-Inductive Secretomes: A Novel Approach to Foster Tendon Differentiation and Regeneration in an Ovine Model. Front. Bioeng. Biotechnol. 2021, 9, 169. [Google Scholar] [CrossRef]
- Puetzer, J.L.; Petitte, J.N.; Loboa, E.G. Comparative Review of Growth Factors for Induction of Three-Dimensional In Vitro Chondrogenesis in Human Mesenchymal Stem Cells Isolated from Bone Marrow and Adipose Tissue. Tissue Eng. Part B Rev. 2010, 16, 435–444. [Google Scholar] [CrossRef]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The Chondrogenic Potential of Human Bone-Marrow-Derived Mesenchymal Progenitor Cells. J. Bone Jt. Surg. Am. 1998, 80, 1745–1757. [Google Scholar] [CrossRef]
- Huang, J.I.; Zuk, P.A.; Jones, N.F.; Zhu, M.; Lorenz, H.P.; Hedrick, M.H.; Benhaim, P. Chondrogenic Potential of Multipotential Cells from Human Adipose Tissue. Plast. Reconstr. Surg. 2004, 113, 585–594. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Marino, L.; Lovecchio, J.; Giordano, E.; Forsyth, N.R.; Selleri, C.; Maffulli, N.; Della Porta, G. Tendon and Cytokine Marker Expression by Human Bone Marrow Mesenchymal Stem Cells in a Hyaluronate/Poly-Lactic-Co-Glycolic Acid (PLGA)/Fibrin Three-Dimensional (3D) Scaffold. Cells 2020, 9, 1268. [Google Scholar] [CrossRef]
- Della Porta, G.; Campardelli, R.; Reverchon, E.; Fisher, J.P. Synergistic Effect of Sustained Release of Growth Factors and Dynamic Culture on Osteoblastic Differentiation of Mesenchymal Stem Cells: Sustained Growth Factor Release for Osteoblastic Differentiation. J. Biomed. Mater. Res. 2015, 103, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Della Porta, G.; Ciardulli, M.C.; Maffulli, N. Microcapsule Technology for Controlled Growth Factor Release in Musculoskeletal Tissue Engineering. Sports Med. Arthrosc. Rev. 2018, 26, e2–e9. [Google Scholar] [CrossRef]
- Della Porta, G.; Reverchon, E.; Maffulli, N.; Maffulli, N. Biomaterials and Supercritical Fluid Technologies: Which Perspectives to Fabricate Artificial Extracellular Matrix? CPD 2017, 23. [Google Scholar] [CrossRef]
- Govoni, M.; Lamparelli, E.P.; Ciardulli, M.C.; Santoro, A.; Oliviero, A.; Palazzo, I.; Reverchon, E.; Vivarelli, L.; Maso, A.; Storni, E.; et al. Demineralized Bone Matrix Paste Formulated with Biomimetic PLGA Microcarriers for the Vancomycin Hydrochloride Controlled Delivery: Release Profile, Citotoxicity and Efficacy against S. Aureus. Int. J. Pharm. 2020, 582, 119322. [Google Scholar] [CrossRef]
- Tirado, D.F.; Palazzo, I.; Scognamiglio, M.; Calvo, L.; Della Porta, G.; Reverchon, E. Astaxanthin Encapsulation in Ethyl Cellulose Carriers by Continuous Supercritical Emulsions Extraction: A Study on Particle Size, Encapsulation Efficiency, Release Profile and Antioxidant Activity. J. Supercrit. Fluids 2019, 150, 128–136. [Google Scholar] [CrossRef]
- Trucillo, E.; Bisceglia, B.; Valdrè, G.; Giordano, E.; Reverchon, E.; Maffulli, N.; Della Porta, G. Growth Factor Sustained Delivery from Poly-lactic-co-glycolic Acid Microcarriers and Its Mass Transfer Modeling by Finite Element in a Dynamic and Static Three-dimensional Environment Bioengineered with Stem Cells. Biotechnol. Bioeng. 2019, 116, 1777–1794. [Google Scholar] [CrossRef]
- Govoni, M.; Berardi, A.C.; Muscari, C.; Campardelli, R.; Bonafè, F.; Guarnieri, C.; Reverchon, E.; Giordano, E.; Maffulli, N.; Della Porta, G. An Engineered Multiphase Three-Dimensional Microenvironment to Ensure the Controlled Delivery of Cyclic Strain and Human Growth Differentiation Factor 5 for the Tenogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells. Tissue Eng. Part A 2017, 23, 811–822. [Google Scholar] [CrossRef]
- Lovecchio, J.; Pannella, M.; Giardino, L.; Calzà, L.; Giordano, E. A Dynamic Culture Platform Enhances the Efficiency of the 3D HUVEC-based Tube Formation Assay. Biotechnol. Bioeng. 2020, 117, 789–797. [Google Scholar] [CrossRef]
- Alves da Silva, M.L.; Martins, A.; Costa-Pinto, A.R.; Correlo, V.M.; Sol, P.; Bhattacharya, M.; Faria, S.; Reis, R.L.; Neves, N.M. Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells in Chitosan-Based Scaffolds Using a Flow-Perfusion Bioreactor. J. Tissue Eng. Regen. Med. 2011, 5, 722–732. [Google Scholar] [CrossRef]
- Tığlı, R.S.; Cannizaro, C.; Gümüşderelioğlu, M.; Kaplan, D.L. Chondrogenesis in Perfusion Bioreactors Using Porous Silk Scaffolds and HESC-Derived MSCs. J. Biomed. Mater. Res. 2011, 96A, 21–28. [Google Scholar] [CrossRef]
- Fuentes-Mera, L.; Camacho, A.; Moncada-Saucedo, N.K.; Peña-Martínez, V. Current Applications of Mesenchymal Stem Cells for Cartilage Tissue Engineering. In Mesenchymal Stem Cells—Isolation, Characterization and Applications; Pham, P.V., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3615-6. [Google Scholar]
- Palazzo, I.; Lamparelli, E.P.; Ciardulli, M.C.; Scala, P.; Reverchon, E.; Forsyth, N.; Maffulli, N.; Santoro, A.; Della Porta, G. Supercritical Emulsion Extraction Fabricated PLA/PLGA Micro/Nano Carriers for Growth Factor Delivery: Release Profiles and Cytotoxicity. Int. J. Pharm. 2021, 592, 120108. [Google Scholar] [CrossRef]
- Giordano, R.; Canesi, M.; Isalberti, M.; Isaias, I.; Montemurro, T.; Viganò, M.; Montelatici, E.; Boldrin, V.; Benti, R.; Cortelezzi, A.; et al. Autologous Mesenchymal Stem Cell Therapy for Progressive Supranuclear Palsy: Translation into a Phase I Controlled, Randomized Clinical Study. J. Transl. Med. 2014, 12, 14. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Marino, L.; Lamparelli, E.P.; Guida, M.; Forsyth, N.R.; Selleri, C.; Della Porta, G.; Maffulli, N. Dose-Response Tendon-Specific Markers Induction by Growth Differentiation Factor-5 in Human Bone Marrow and Umbilical Cord Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 5905. [Google Scholar] [CrossRef]
- Gimenez-Rota, C.; Palazzo, I.; Scognamiglio, M.R.; Mainar, A.; Reverchon, E.; Della Porta, G. β-Carotene, α-Tocoferol and Rosmarinic Acid Encapsulated within PLA/PLGA Microcarriers by Supercritical Emulsion Extraction: Encapsulation Efficiency, Drugs Shelf-Life and Antioxidant Activity. J. Supercrit. Fluids 2019, 146, 199–207. [Google Scholar] [CrossRef]
- Nihant, N.; Schugens, C.; Grandfils, C.; Jerome, R.; Teyssie, P. Polylactide Microparticles Prepared by Double Emulsion-Evaporation. J. Colloid Interface Sci. 1995, 173, 55–65. [Google Scholar] [CrossRef]
- Della Porta, G.; Falco, N.; Giordano, E.; Reverchon, E. PLGA Microspheres by Supercritical Emulsion Extraction: A Study on Insulin Release in Myoblast Culture. J. Biomater. Sci. Polym. Ed. 2013, 24, 1831–1847. [Google Scholar] [CrossRef]
- Samavedi, S.; Poindexter, L.K.; Van Dyke, M.; Goldstein, A.S. Synthetic Biomaterials for Regenerative Medicine Applications. In Regenerative Medicine Applications in Organ Transplantation; Elsevier: Amsterdam, The Netherland, 2014; pp. 81–99. ISBN 978-0-12-398523-1. [Google Scholar]
- Pasini, A.; Lovecchio, J.; Ferretti, G.; Giordano, E. Medium Perfusion Flow Improves Osteogenic Commitment of Human Stromal Cells. Stem Cells Int. 2019, 2019, 1304194. [Google Scholar] [CrossRef]
- Suchorska, W.M.; Lach, M.S.; Richter, M.; Kaczmarczyk, J.; Trzeciak, T. Bioimaging: An Useful Tool to Monitor Differentiation of Human Embryonic Stem Cells into Chondrocytes. Ann. Biomed. Eng. 2016, 44, 1845–1859. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [Green Version]
- Knapp, D.M.; Barocas, V.H.; Moon, A.G.; Yoo, K.; Petzold, L.R.; Tranquillo, R.T. Rheology of Reconstituted Type I Collagen Gel in Confined Compression. J. Rheol. 1997, 41, 971–993. [Google Scholar] [CrossRef] [Green Version]
- de Winter, J.C.F. Using the Student’s t-Test with Extremely Small Sample Sizes. Pract. Assess. Res. Eval. 2013, 18, 10. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical Modeling of Drug Delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Li, H.; Tan, C.; Li, L. Review of 3D Printable Hydrogels and Constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Yeatts, A.B.; Fisher, J.P. Tubular Perfusion System for the Long-Term Dynamic Culture of Human Mesenchymal Stem Cells. Tissue Eng. Part C Methods 2011, 17, 337–348. [Google Scholar] [CrossRef]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The Control of Chondrogenesis. J. Cell. Biochem. 2006, 97, 33–44. [Google Scholar] [CrossRef]
- Li, H.; Haudenschild, D.R.; Posey, K.L.; Hecht, J.T.; Di Cesare, P.E.; Yik, J.H.N. Comparative Analysis with Collagen Type II Distinguishes Cartilage Oligomeric Matrix Protein as a Primary TGFβ-Responsive Gene. Osteoarthr. Cartil. 2011, 19, 1246–1253. [Google Scholar] [CrossRef] [Green Version]
- Kawato, Y.; Hirao, M.; Ebina, K.; Shi, K.; Hashimoto, J.; Honjo, Y.; Yoshikawa, H.; Myoui, A. Nkx3.2 Promotes Primary Chondrogenic Differentiation by Upregulating Col2a1 Transcription. PLoS ONE 2012, 7, e34703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, H.; Chaboissier, M.-C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The Transcription Factor Sox9 Has Essential Roles in Successive Steps of the Chondrocyte Differentiation Pathway and Is Required for Expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef] [Green Version]
- Buxton, A.N.; Bahney, C.S.; Yoo, J.U.; Johnstone, B. Temporal Exposure to Chondrogenic Factors Modulates Human Mesenchymal Stem Cell Chondrogenesis in Hydrogels. Tissue Eng. Part A 2011, 17, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Tamaddon, M.; Burrows, M.; Ferreira, S.A.; Dazzi, F.; Apperley, J.F.; Bradshaw, A.; Brand, D.D.; Czernuszka, J.; Gentleman, E. Monomeric, Porous Type II Collagen Scaffolds Promote Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells in Vitro. Sci. Rep. 2017, 7, 43519. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, G.; Venkateswaran, S.; López-Ruiz, E.; Perán, M.; Pernagallo, S.; Díaz-Monchón, J.J.; Canadas, R.F.; Antich, C.; Oliveira, J.M.; Callanan, A.; et al. A Soft 3D Polyacrylate Hydrogel Recapitulates the Cartilage Niche and Allows Growth-Factor Free Tissue Engineering of Human Articular Cartilage. Acta Biomater. 2019, 90, 146–156. [Google Scholar] [CrossRef]








| FEM Modeling Parameters | Values | Units |
|---|---|---|
| Glucose at time zero | 0 | mol/m3 |
| Waste at time zero | 0 | mol/m3 |
| Nutrient consumption | 4.74·10−12 | mol/s |
| Waste production | 3.4·10−12 | mol/s |
| D nutrients coefficient | 3.9·10−9 | m2/s |
| D waste coefficient | 1.6·10−9 | m2/s |
| κ collagen hydrogel | 2·10−9 | m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamparelli, E.P.; Lovecchio, J.; Ciardulli, M.C.; Giudice, V.; Dale, T.P.; Selleri, C.; Forsyth, N.; Giordano, E.; Maffulli, N.; Della Porta, G. Chondrogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells in a Perfused Collagen Hydrogel Functionalized with hTGF-β1-Releasing PLGA Microcarrier. Pharmaceutics 2021, 13, 399. https://doi.org/10.3390/pharmaceutics13030399
Lamparelli EP, Lovecchio J, Ciardulli MC, Giudice V, Dale TP, Selleri C, Forsyth N, Giordano E, Maffulli N, Della Porta G. Chondrogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells in a Perfused Collagen Hydrogel Functionalized with hTGF-β1-Releasing PLGA Microcarrier. Pharmaceutics. 2021; 13(3):399. https://doi.org/10.3390/pharmaceutics13030399
Chicago/Turabian StyleLamparelli, Erwin Pavel, Joseph Lovecchio, Maria Camilla Ciardulli, Valentina Giudice, Tina P. Dale, Carmine Selleri, Nicholas Forsyth, Emanuele Giordano, Nicola Maffulli, and Giovanna Della Porta. 2021. "Chondrogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells in a Perfused Collagen Hydrogel Functionalized with hTGF-β1-Releasing PLGA Microcarrier" Pharmaceutics 13, no. 3: 399. https://doi.org/10.3390/pharmaceutics13030399
APA StyleLamparelli, E. P., Lovecchio, J., Ciardulli, M. C., Giudice, V., Dale, T. P., Selleri, C., Forsyth, N., Giordano, E., Maffulli, N., & Della Porta, G. (2021). Chondrogenic Commitment of Human Bone Marrow Mesenchymal Stem Cells in a Perfused Collagen Hydrogel Functionalized with hTGF-β1-Releasing PLGA Microcarrier. Pharmaceutics, 13(3), 399. https://doi.org/10.3390/pharmaceutics13030399