Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
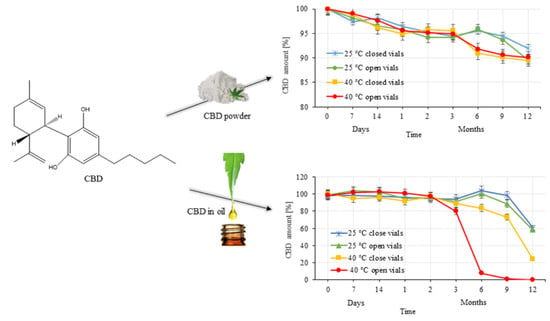
2.2. Stability Study
2.3. Preparation of Stability Samples and Reference Standard Samples before LC-MS
2.4. LC-UV-MS Analysis of Stability Samples
2.5. Statistical Analysis
3. Results
3.1. HPLC-MS Analysis
3.2. Evaluation of CBD Stability Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, G.; Grovey, B.; Furnish, T.; Wallace, M. Medical Cannabis for Neuropathic Pain. Curr. Pain Headache Rep. 2018, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Tomida, I.; Pertwee, R.G.; Azuara-Blanco, A. Cannabinoids and glaucoma. Br. J. Ophthalmol. 2004, 88, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.A.; Sansone, L.A. Marijuana and body weight. Innov. Clin. Neurosci. 2014, 11, 50–54. [Google Scholar] [PubMed]
- Parker, L.A.; Rock, E.M.; Limebeer, C.L. Regulation of nausea and vomiting by cannabinoids. Br. J. Pharmacol. 2011, 163, 1411–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardo, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Beardsley, P.M.; Brands, B.; Ekwere, I.T.; Elliott, S.; Gysling, K.; Jain, R.; Kaduri, P.; Kitanaka, J.; Rahimi-Movaghar, A.; Nudmamud-Thanoi, S.; et al. WHO Expert Committee on Drug Dependance: No. 1013, Cannabidiol; WHO Press: Geneva, Switzerland, 2018; pp. 1–27. [Google Scholar]
- Szaflarski, J.P.; Bebin, E.M.; Comi, A.M.; Patel, A.D.; Joshi, C.; Checketts, D.; Beal, J.C.; Laux, L.C.; De Boer, L.M.; Wong, M.H.; et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results. Epilepsia 2018, 59, 1540–1548. [Google Scholar] [CrossRef] [Green Version]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkotter, J.; Hellmich, M.R.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, 94. [Google Scholar] [CrossRef] [Green Version]
- Sulé-Suso, J.; Watson, N.A.; Van Pittius, D.G.; Jegannathen, A. Striking lung cancer response to self-administration of cannabidiol: A case report and literature review. SAGE Open Med. Case Rep. 2019, 7, 2050313X19832160. [Google Scholar] [CrossRef]
- Martinez-Martinez, E.; Martin-Ruiz, A.; Martin, P.; Calvo, V.; Provencio, M.; Garcia, J.M. CB2 cannabinoid receptor activation promotes colon cancer progression via AKT/GSK3beta signaling pathway. Oncotarget 2016, 7, 68781–68791. [Google Scholar] [CrossRef] [Green Version]
- Lindholst, C. Long term stability of cannabis resin and cannabis extracts. Aust. J. Forensic Sci. 2010, 42, 181–190. [Google Scholar] [CrossRef]
- Carbone, M.; Castelluccio, F.; Daniele, A.; Sutton, A.; Ligresti, A.; Di Marzo, V.; Gavagnin, M. Chemical characterisation of oxidative degradation products of Δ9-THC. Tetrahedron 2010, 66, 9497–9501. [Google Scholar] [CrossRef]
- Layton, C.; Runco, J.; Aubin, A. Forced Degradation of Cannabidiol; Waters Corporation: Milford, MA, USA, 2016; Volume 6, p. 720005766EN. [Google Scholar]
- International Conference on Harmonization Guideline: Impurities: Guideline for Residual Solvents Q3C (R5); ICH Steering Committee: London, UK, 2005; Volume 4, pp. 1–25. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-33.pdf (accessed on 25 July 2020).
- Guidance for Industry Q1A(R2): Stability Testing of New Drug Substances and Products; US. Food and Drug Administration: Rockville, MD, USA, 2003. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q1ar2-stability-testing-new-drug-substances-and-products (accessed on 25 July 2020).
- Palazzoli, F.; Citti, C.; Licata, M.; Vilella, A.; Manca, L.; Zoli, M.; Vandelli, M.A.; Forni, F.; Cannazza, G. Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS) method for the determination of cannabidiol (CBD), Δ9-tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J. Pharm. Biomed. Anal. 2018, 150, 25–32. [Google Scholar] [PubMed]
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J. Pharm. Biomed. Anal. 2016, 128, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Deidda, R.; Avohou, H.T.; Baronti, R.; Davolio, P.L.; Pasquini, B.; Del Bubba, M.; Hubert, C.; Hubert, P.; Orlandini, S.; Furlanetto, S. Analytical quality by design: Development and control strategy for a LC method to evaluate the cannabinoids content in cannabis olive oil extracts. J. Pharm. Biomed. Anal. 2019, 166, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Nemeškalová, A.; Hajkova, K.; Mikulu, L.; Sykora, D.; Kuchar, M. Combination of UV and MS/MS detection for the LC analysis of cannabidiol-rich products. Talanta 2020, 219, 121250. [Google Scholar] [CrossRef]
- Zgair, A.; Wong, J.C.; Sabri, A.; Fischer, P.M.; Barrett, D.A.; Constantinescu, C.S.; Gershkovich, P. Development of a simple and sensitive HPLC-UV method for the simultaneous determination of cannabidiol and Delta(9)-tetrahydrocannabinol in rat plasma. J. Pharm. Biomed. Anal. 2015, 114, 145–151. [Google Scholar] [CrossRef]
- Turner, C.E.; Hadley, K.W.; Fetterman, P.S.; Doorenbos, N.J.; Quimby, M.W.; Waller, C. Constituents of Cannabis sativa L. IV: Stability of cannabinoids in stored plant material. J. Pharm. Sci. 1973, 62, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Trofin, I.; Dabija, G.; Vaireanu, D.I.; Laurentiu, F. The influence of long-term storage conditions on the stability of cannabinoids derived from cannabis resin. Rev. Chim. 2012, 63, 422–427. [Google Scholar]
- Maskan, M. Change in colour and rheological behaviour of sunflower seed oil during frying after adsorbent treatment of used oil. Eur. Food Res. Technol. 2003, 218, 20–25. [Google Scholar] [CrossRef]
- Tan, Y.A.; Ong, S.H.; Berger, K.G.; Oon, H.H.; Poh, L.B. A study of the cause of rapid color development of heated refined palm oil. J. Am. Oil Chem. Soc. 1985, 62, 999–1006. [Google Scholar] [CrossRef]
- Stevenson, S.G.; Vaisey-Genser, M.; Eskin, N.A.M. Quality control in the use of deep frying oils. J. Am. Oil Chem. Soc. 1984, 61, 1102–1108. [Google Scholar] [CrossRef]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality traits of “Cannabidiol oils”: Cannabinoids tontent, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairbairn, J.W.; Liebmann, J.A.; Rowan, M.G. The stability of cannabis and its preparations on storage. J. Pharm. Pharmacol. 1976, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Henry, J.T. Constituents of Cannabis sativa L. IX: Stability of synthetic and naturally occurring cannabinoids in chloroform. J. Pharm. Sci. 1975, 64, 357–359. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosović, E.; Sýkora, D.; Kuchař, M. Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics 2021, 13, 412. https://doi.org/10.3390/pharmaceutics13030412
Kosović E, Sýkora D, Kuchař M. Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics. 2021; 13(3):412. https://doi.org/10.3390/pharmaceutics13030412
Chicago/Turabian StyleKosović, Ema, David Sýkora, and Martin Kuchař. 2021. "Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution" Pharmaceutics 13, no. 3: 412. https://doi.org/10.3390/pharmaceutics13030412
APA StyleKosović, E., Sýkora, D., & Kuchař, M. (2021). Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics, 13(3), 412. https://doi.org/10.3390/pharmaceutics13030412