1. Introduction
Drug conjugates consisting of a cancer-cell-specific targeting domain coupled to a toxic molecule have been the focus of intense research and clinical development during the last few decades. Antibody–drug conjugates (ADCs), where a monoclonal antibody (mAb) is linked to a cytotoxic drug, have been the most successful for cancer therapy [
1]. By combining the specificity of an antibody and the cytotoxic activity of a drug agent, a broad therapeutic window can be obtained by a dramatic reduction in systemic toxicity compared to classical chemotherapy [
2]. An example of an ADC is trastuzumab emtansine (T-DM1), consisting of the monoclonal antibody trastuzumab targeting the human epidermal growth factor receptor 2 (HER2) linked with the maytansine derivate DM1 [
3]. HER2 is found over-expressed in 20 to 30% of patients with breast cancer, and to a lesser extent in patients with ovarian and gastric cancers [
4]. T-DM1 is approved for therapy of HER2-positive metastatic breast cancer by the US Food and Drug Administration. Its mode-of-action relies on binding to HER2 on the cancer cell surface, followed by endocytosis and transport to the lysosomes, where the mAb part is degraded [
5]. The cytotoxic drug DM1 subsequently diffuses to the cytosol and acts as an antimitotic agent that binds to tubulin and disrupts microtubule polymerization. This disruption leads to arrest in the G2/M phase of the cell cycle, typically followed by apoptosis [
6].
The general difficulties with ADCs include, for example, the high production cost and problems with site-specific drug attachment on the mAb, where the latter leads to a mixture of molecules with variations in drug attachment sites and the drug-to-antibody ratio (DAR). For example, Sun et al. evaluated the pharmacokinetics of T-DM1 with a DAR of up to ten and found that a DAR above six results in accelerated hepatic clearance due to high hydrophobicity [
7]. A high drug load has also been correlated to changes in the ADC’s binding properties and physical instability that may affect in vivo efficacy [
8]. Additionally, the antitumor potency of ADCs depends on the intracellular concentration of DM1; thus, ADCs with low DAR may suffer from insufficient delivery of the cytotoxic drug to cells with low target-expression [
5,
9]. The production of ADCs with an optimal DAR is still challenging despite advances in site-specific conjugation chemistries [
2]. Besides this, the large size of the mAb-carrier limits tumor penetration, which may result in insufficient delivery to the tumor mass’s interior [
10].
As an alternative to mAbs, engineered affinity proteins (EAPs) have been evaluated as carriers of cytotoxic drugs [
11]. One class of EAPs is the affibody molecules, which are small (6.5 kDa) scaffold proteins. They are generated by protein engineering techniques, typically resulting in variants with strong affinities and specific binding to selected molecular targets [
12]. The small size enables rapid penetration into solid tumor mass, and the typically strong affinity permits good tumor retention [
13]. Since naturally occurring cysteine amino acids are lacking in the affibody scaffold [
12], cysteines may be inserted at desired positions. They allow for the site-specific conjugation of drug molecules using thiol-directed chemistry, thus achieving a well-controlled DAR and a well-controlled spatial positioning of the drugs. Moreover, the production of affibody molecules can be carried out in relatively simple procaryotic host cells at a high yield [
14].
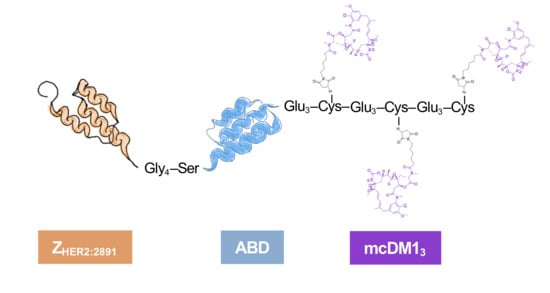
We have previously investigated the properties and therapeutic potential of anti-HER2 affibody molecules equipped with a single mcDM1 drug molecule, so-called AffiDCs [
15,
16,
17]. Given the results on ADCs described above, it is attractive to evaluate the possibility of increasing the drug load for AffiDCs. The impact of an increased number of drugs per affibody targeting moiety has not been explored before to any significant extent. In this study, the protein carrier consisted of an anti-HER2 affibody molecule (Z
HER2:2891) with a strong affinity to HER2 (equilibrium dissociation constant, K
D = 66 pM) [
18]. In the tested constructs, Z
HER2:2891 was fused to an albumin-binding domain (ABD). The ABD is a small protein domain that can form a complex with serum albumin and can be utilized for prolongation of the plasma half-life in vivo [
15,
16,
17,
19].
The cytotoxic drug mcDM1 consists of the tubulin polymerization inhibitor DM1 and the non-cleavable maleimidocaproyl (mc) linker. It is relatively hydrophobic, which may drive clearance by the liver. A linker with the amino acid sequence Glu–Glu–Glu has previously been shown to reduce the hydrophobicity and liver uptake of anti-HER2 AffiDCs, when placed next to a C-terminal cysteine where mcDM1 is attached [
16]. Therefore, glutamic acids were also added to the constructs in this study with the aim of gaining shielding from the hydrophobic effect of mcDM1 to minimize uptake in the liver. The conjugate with one mcDM1 molecule had a C-terminal ending with the amino acid sequence Glu
3–Cys, and the conjugate with three mcDM1 drug molecules had the C-terminal end Glu
3–Cys–Glu
3–Cys–Glu
3–Cys. In both cases, mcDM1 molecules were attached to the cysteines. The biophysical properties were investigated, followed by the determination of cytotoxic potential on cell lines with different levels of HER2 expression. The tumor-targeting ability and non-specific uptake in normal organs were investigated in mice carrying HER2 overexpressing ovarian cancer xenografts. This study aimed to examine the effect of drug loading on biochemical characteristics, in vitro cytotoxic efficacy, cellular processing, and in vivo biodistribution by comparing AffiDCs with one or three mcDM1 molecules.
2. Materials and Methods
2.1. General
The chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Merck (Darmstadt, Germany), except where otherwise indicated. Restriction enzymes were purchased from New England Biolabs (Ipswitch, MA, USA). Oligonucleotides were synthesized by Integrated DNA Technologies (Leuven, Belgium).
2.2. Construction of Genes Encoding Affibody Constructs
The HER2-binding affibody molecule used for targeting was Z
HER2:2891 [
18], herein referred to as Z
HER2. The gene encoding Z
HER2–ABD–(Glu
3Cys)
3 was PCR-amplified from a plasmid encoding Z
HER2–ABD–Glu
3Cys [
17] using Phusion polymerase (New England Biolabs). During PCR amplification, NdeI and BamHI restriction enzyme sites were added and a DNA sequence was placed at the N terminus encoding a peptide tag with the sequence His–Glu–His–Glu–His–Glu allowing for radionuclide labeling. The gene was cloned into the pET-21a(+) plasmid vector (Novagen, Madison, WI, USA) using NdeI and BamHI restriction enzymes. Gene integrity was confirmed by DNA sequencing.
2.3. Expression and Purification of Affibody Constructs
The affibody/ABD fusion proteins were expressed in
Escherichia coli BL21 Star (DE3) (New England Biolabs), grown in tryptic soy broth supplemented with 5 g/L yeast extract and 100 μg/mL ampicillin. The cells were grown at 37 °C in shake flasks. Expression was induced by adding 1 mM of isopropyl β-
d-1-thiogalactopyranoside (Appolo Scientific, Stockport, UK) when the OD
600 was between 0.6 and 1.0. The cells were cultured for another 3 h at 37 °C. The cell suspension was centrifuged and the cytoplasmic fraction was released by sonication. The cell lysate was clarified by passage through a 0.45 µm Acrodisc syringe filter (Pall, Port Washington, NY, USA). Human serum albumin-based affinity chromatography on a HiTrap NHS sepharose column (GE Healthcare, Uppsala, Sweden) was performed to isolate the ABD fused affibody constructs. The purification was carried out on an ÄKTA system (GE Healthcare Life Sciences, Uppsala, Sweden), essentially as previously described [
20]. Elution was carried out with 500 mM acetic acid (pH = 2.6). The fractions containing affibody fusion proteins were pooled and lyophilized.
2.4. Conjugation with mcDM1
The lyophilized affibody fusion proteins were dissolved to a final concentration of 0.1 mM in 100 mM Tris-HCl buffer (pH = 7.85). To prevent the formation of disulfide bonds between the C-terminal cysteines of the constructs, tris (2-carboxyethyl) phosphine (TCEP) was added to a final concentration of 5 mM, followed by incubation for 30 min at 37 °C. Before mcDM1 was added, the protein solution’s pH was adjusted to 6.5 using 1 M HCl. A freshly prepared solution of mcDM1 (Levena Biopharma, San Diego, CA, USA), dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 20 mM, was mixed with the affibody constructs at a molar ratio of 4:3, followed by overnight incubation at room temperature. The conjugation mixture was prepared for reversed-phase high-performance liquid chromatography (RP-HPLC) purification by dilution with 0.1% trifluoroacetic acid in water at 1:1 (v/v). The acidified mixture was loaded on a Zorbax C18 SB column (Agilent, Santa Clara, CA, USA) and bound material was eluted by a gradient from 30 to 70% buffer B (0.1% trifluoroacetic acid in acetonitrile) over 40 min at a flow rate of 3 mL/min. The eluted mcDM1-coupled conjugates were lyophilized. 2-iodoacetamide (IAA) was used to cap the three free cysteines in ZHER2–ABD–(Glu3Cys)3 to create the non-toxic control ZHER2–ABD–AA3. Lyophilized ZHER2–ABD–(Glu3Cys)3 was dissolved in alkylation buffer (0.2 M NH4HCO3, pH 8.0). TCEP was added to a final concentration of 5 mM, followed by incubation for 30 min at 37 °C. 2-iodoacetamide was added to a final concentration of 10 mM, and the mixture was incubated at room temperature for 30 min in the dark to alkylate the cysteines. The capped protein was purified by RP-HPLC as described above for the affibody–mcDM1 conjugates, followed by lyophilization. The lyophilized protein was dissolved in phosphate-buffered saline (PBS; 10 mM Na-phosphate, 2.7 mM KCl, 137 mM NaCl, pH 7.4) and stored at −80 °C until use.
The concentration of conjugates was determined using the BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
Purified conjugates were analyzed by SDS-PAGE (Biorad, Hercules, CA, USA) under reducing conditions. A total of 5 µg was loaded into each lane. The conjugates’ oligomeric state was determined by size-exclusion chromatography by passing the conjugates through a Superdex 75 5/150 column (GE Healthcare, Uppsala, Sweden) at a flow rate of 0.45 mL/min with PBS as a running buffer. The molecular weight of the purified conjugates was measured by electrospray ionization time-of-flight (ESI-TOF) mass spectrometry (Agilent, Santa Clara, CA, USA). Analysis of purity was performed by RP-HPLC (Zorbax 300SB-C18; Agilent) using a gradient from 30 to 60% of 0.1% trifluoroacetic acid in acetonitrile for 30 min, at a flow rate of 1 mL/min.
2.5. Affinity Determination
The affinity of the affibody-based conjugates to HER2, human serum albumin (HSA) and mouse serum albumin (MSA) was measured by surface plasmon resonance on a Biacore 3000 instrument (GE Healthcare). All three ligands, HER2-Fc chimera (R & D Systems, Minneapolis, MN, USA), HSA (Novozymes, Bagsvaerd, Denmark) and MSA (Sigma-Aldrich, St. Louis, MO, USA) were coupled to individual flow cells on CM5 chips via amine groups. HBS-EP (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% v/v surfactant P20, pH 7.4) was prepared as the running buffer and also for the dilution of analytes. The flow rate was 50 µL/min and the temperature was 25 °C. To regenerate the chips, 10 mM HCl was injected for 30 s between each cycle. The binding kinetics were fitted using a 1:1 kinetics model in the Biacore evaluation software.
2.6. Cell Culture
AU565, SKBR3, SKOV3, MCF7, BT474, and A549 cell lines were obtained from the American Type Culture Collection (ATCC via LGC Promochem, Borås, Sweden). They were grown in McCoy’s 5A (SKOV3, SKBR3), RPMI-1640 (AU565, BT474), or Dulbecco’s modified Eagle medium (MCF7, A549) (Cytiva Hyclone, Uppsala, Sweden) in a humidified incubator at 37 °C in 5% CO2 atmosphere. The media were supplemented with 10% fetal bovine serum (FBS) (20% for BT474) (Sigma-Aldrich) and a mixture of penicillin 100 IU/mL and 100 µg/mL streptomycin.
2.7. In Vitro Cytotoxicity Analysis
Serial dilutions of affibody-based conjugates were tested on 5 cell lines, AU565, SKBR3, SKOV3, A549 and MCF7. At 24 h before incubation with conjugates, 5000 cells/well (2000 cells/well for SKOV3) were seeded in a 96-well plate. The cells were then incubated with media containing different concentrations of the conjugates. After 3 days, a colorimetric assay was performed (Cell Counting Kit-8, Sigma-Aldrich) for the determination of cell viability. According to the manufacturer’s protocol, a measurement of A450 was carried out to represent cell viability and the acquired absorbance values were plotted against the corresponding conjugate concentrations. The half maximal inhibitory concentration (IC50) was determined by Prism (version 9.0.0.) using a log (inhibitor) vs. response-variable slope (four parameters) model (GraphPad Software, La Jolla, CA, USA).
2.8. Radiolabeling
Radiolabeling of the conjugates with [
99mTc(CO)
3(H
2O)
3]
+ was performed as described earlier [
16]. In brief, technetium-99m was eluted as pertechnetate in 500 μL 0.9% NaCl from a commercial
99Mo/
99mTc generator. It was incubated in a sealed vial containing CRS kit (PSI, Villigen, Switzerland) at 100 °C for 30 min to generate the [
99mTc(CO)
3(H
2O)
3]
+ (tricarbonyl technetium) precursor. After incubation, 50 μL of the tricarbonyl technetium solution was mixed with 50 μL 0.1 M HCl and 60 μg protein in 50 μL PBS solution. The mixture was incubated at 60 °C for 60 min. The mixture was incubated with a 5000-fold molar excess of histidine at 60 °C for 10 min to remove unbound radioactivity. Subsequently, the mixture was passed through a NAP-5 desalting column (GE Healthcare), pre-equilibrated, and eluted with 1% BSA in PBS to isolate the radiolabeled conjugates. The radiochemical yield and purity were determined by radio-iTLC analysis in PBS and measured using the Cyclone Storage Phosphor System (PerkinElmer, Waltham, MA, USA).
2.9. In Vitro Specificity and Cellular Processing
The SKOV3 and BT474 cell lines were seeded in 3 cm Petri dishes (ca. 5 × 105 cells per dish), and a set of three dishes was used for each group. To determine binding specificity, 2 nM of radiolabeled conjugates were incubated with the cells at 37 °C in a 5% CO2 atmosphere for 60 min. For the saturation of HER2 receptors on the cells, an additional set of dishes were pre-incubated with 1000 nM of non-radiolabeled conjugates at room temperature for 15 min before incubation with the radiolabeled compound. The medium was discarded, and the cells were washed with PBS and detached by trypsin. The cell suspension was collected, and radioactivity was measured using an automatic gamma spectrometer equipped with a 3 inch NaI (Tl) well detector (1480 Wizard, Wallac, Finland). An unpaired two-tailed t-test was used to analyze the data in Prism (version 9.0.0.).
The radiolabeled conjugates’ internalization rate and cellular processing were studied using a continuous incubation method described earlier [
21]. In brief, the radiolabeled conjugates (2 nM) were added to the cells, and the dishes were incubated at 37 °C in a 5% CO
2 atmosphere. At different time points (1, 2, 4, 6, and 24 h after addition of the conjugates), the medium was collected from one set of dishes, and the cells were washed with PBS. The membrane-bound fraction was collected by incubating the cells in 0.5 mL of glycine buffer containing 4 M urea (pH 2.0) on ice for 5 min. The buffer was collected, and the cells were washed once with the same buffer (0.5 mL) and once with PBS solution (1 mL). The internalized fraction was collected by incubating the cells with 1 M NaOH solution (0.5 mL) at 37 °C for 30 min. The cell lysates were collected and washed once with the same buffer (0.5 mL) and once with PBS solution (1 mL). The radioactivity in each fraction was measured and calculated for the percentage of cell-associated radioactivity. Data were normalized by taking the maximum value of cell-associated radioactivity in each dataset as 100%.
2.10. LigandTracer Analysis and Interaction Map Generation
The binding affinity against the HER2-expressing cells of the radiolabeled conjugates was assessed in real-time using a LigandTracer Yellow instrument (Ridgeview Diagnostics, Uppsala, Sweden) as described previously [
17]. Increasing concentrations of radiolabeled conjugates (1 nM and 2 nM) were added to the cell culture medium. The binding phase at each concentration was measured for 90 min, followed by over-night incubation with medium only, to measure the dissociation phase. The signal was corrected for nuclide decay, and the binding curves were fitted using TraceDrawer (Ridgeview Instruments, Uppsala, Sweden). Interaction map analysis (Ridgeview Diagnostics, Uppsala, Sweden) was performed to estimate the interaction heterogeneity as described by Altschuh et al. [
22].
2.11. Biodistribution in Tumor-Bearing Mice
Animal studies were planned in agreement with EU Directive 2010/63/EU for animal experiments and Swedish national legislation concerning laboratory animals’ protection, and were approved by the Ethics Committee for Animal Research in Uppsala, Sweden (animal permission C86/15, approved 28 August 2015).
To study the biodistribution of radiolabeled ZHER2–ABD–mcDM1 and ZHER2–ABD–mcDM13, 15 female BALB/c nu/nu mice xenografted with SKOV3 cells in the right hind leg were injected with 6 μg of 99mTc-labeled conjugates in 100 μL of 2% BSA in PBS intravenously (i.v.). The injected radioactivity was calculated to give 60 kBq per mouse at the dissection time point. A group of animals (n = 3 or 4) was euthanized at 4 and 24 h by intraperitoneal (i.p.) injection of ketamine–xylazine solution (30 μL of solution per gram body weight; ketamine 10 mg/mL; xylazine 1 mg/mL). The organs and tissues were collected, weighed, and measured for radioactivity using an automatic gamma spectrometer. For organs, the percentage of injected dose per gram of sample (%ID/g) was calculated. An unpaired two-tailed t-test was used to analyze the data in Prism (version 9.0.0.).
4. Discussion
This study investigated the impact of drug loading on HER2-specific drug conjugates based on an affibody scaffold protein (AffiDCs). Affibody molecules possess favorable characteristics as carriers of cytotoxic drugs since they typically interact with their intended target in a highly selective manner and with high affinity [
12]. A previous study by our group showed that the HER2-specific affibody–drug conjugate Z
HER2–ABD–mcDM1, carrying one cytotoxic mcDM1 drug molecule, was able to significantly reduce the volume of xenografted SKOV3 tumors in mice, leading to significantly increased survival [
17]. However, for antibody–drug conjugates (ADCs), it has been shown that the cytotoxic effect of tubulin polymerization inhibitors, such as DM1, is dependent on the intracellular concentration [
5]. From this perspective, a high DAR is desirable. Studies with ADCs have shown that an increase in DAR leads to increased cytotoxic potential but ultimately affects both the clearance rate and affinity for their target negatively [
7]. A critical issue is thus to find the optimal DAR [
7]. For drug conjugates based on scaffold proteins, the balance between drug loading and in vivo behavior has not been investigated, which motivated us to perform the current study. We hypothesized that increasing the DAR would result in the delivery of a higher dose of the drug to the tumor cells.
A benefit stemming from the cysteine-free affibody backbone is that the DAR and coupling-site specificity of AffiDCs can be precisely regulated by inserting cysteines at desired positions to which the drug molecules can be attached. A previous attempt by Serwotka-Suszczak et al. to load affibody molecules with multiple drug molecules has shown that the separation of partially and fully loaded AffiDCs was difficult [
23]. In that study, the tubulin polymerization inhibitor MMAE was conjugated to an HER2-binding affibody carrier, and final purification after conjugation was carried out with hydrophobic interaction chromatography. In contrast, it was straightforward in the present study to isolate the fully loaded AffiDC via an RP-HPLC chromatographic step. A difference between the study by Serwotka-Suszczak et al. and the present study is that non-identical drugs were used, which imparted a different level of hydrophobicity on the AffiDCs, which may have resulted in a more difficult separation process after drug attachment. Differences in the resolution during separation might also have played a role since purification in the HPLC mode in the present study typically results in better resolution in the chromatographic separation step.
A common approach to creating ADCs is to reduce the four interchain disulfide bridges in the antibody, followed by conjugation of the drugs to the reduced cysteines via a thiol–maleimide reaction, analogous to the conjugation reaction used in the present study. This approach can achieve a maximum DAR of 8 in the ADC [
24]. However, the purification of the fully loaded antibody from other side products with lower DAR is often carried out by hydrophobic interaction chromatography or size-exclusion chromatography, which usually results in incomplete separation [
24,
25]. The utilization of a high-resolution RP-HPLC step, as used in the current study, is not available since it would denaturate most antibodies.
The investigation of the interactions between Z
HER2–ABD–mcDM1
3 (DAR = 3) and HER2 (
Figure 3A), as well as with HER2-overexpressing SKOV3 cells (
Figure 6), showed a decreased binding affinity compared to the same interactions involving Z
HER2–ABD–mcDM1 (DAR = 1) and the non-toxic control Z
HER2–ABD–AA
3. In both assays, the on-rate for Z
HER2–ABD–mcDM1
3 was slower. This observation could be partially explained by the increase in mass after the conjugation of three mcDM1 molecules, since larger analytes often have a slower on-rate than smaller ones. However, a decrease in target-affinity has been reported in studies on ADCs upon an increase in the drug load [
7,
24]. In those studies, the percental increase in molecular mass for the ADCs is only minor. Therefore, it is likely that the decrease in affinity is partially a consequence of increasing the drug load of the AffiDCs in this study. The conjugation sites for mcDM1 were placed at the C-terminus of the AffiDC, and it is thus not likely that the mcDM1 is sterically hindering the interaction between the affibody and HER2, since mcDM1 and the affibody are separated by an ABD. Another possible explanation is that the hydrophobic nature of mcDM1 leads to micro-aggregates forming, which would decrease the functional concentration of Z
HER2–ABD–mcDM1
3. Such behavior would manifest as a slower association rate in
Figure 3A and
Figure 6, since the association rate is dependent on the concentration, and an identical dissociation rate, since the dissociation rate is concentration-independent. However, according to
Figure 2B, where Z
HER2–ABD–mcDM1
3 is analyzed by separation under native conditions, no multimers were formed, and the area under the curve is virtually identical for the three conjugates, thus strongly speaking against the formation of micro-aggregates. At the moment, the mechanism by which multiple mcDM1 drugs influence the interactions involving Z
HER2 is unclear. It was also observed that the binding affinity of Z
HER2–ABD–mcDM1
3 to both serum albumins was decreased five-fold, compared to the other two.
The cytotoxic potency of the DAR = 3 and DAR = 1 AffiDCs was compared on HER2-overexpressing cell lines. On AU565 and SKBR3 cell lines, the IC
50 values were sub-nanomolar with no significant difference between the high and low DAR variants. For some antibody–drug conjugates, the in vitro potency has been reported to be correlated with the DAR [
7]. However, the linker has also sometimes been found to influence the efficacy of ADCs with similar DARs [
26]. A reason for the lack of a higher efficiency in Z
HER2–ABD–mcDM1
3 could be attributed to the decrease in affinity to HER2. However, a more than 10-fold improved potency of Z
HER2–ABD–mcDM1
3 compared to Z
HER2–ABD–mcDM1 was observed on the SKOV3 cell line, so the difference in potency appears to be cell line-dependent. It is notable that SKOV3 is more resistant to AffiDC poisoning than AU565 and SKBR3, as shown in
Figure 4 and an earlier study [
17].
The binding specificity test (
Figure 5A) demonstrated a significantly higher level of unspecific binding (after HER2 blocking) for Z
HER2–ABD–mcDM1
3 compared to other conjugates. This is most likely associated with the higher lipophilicity of Z
HER2–ABD–mcDM1
3. A higher lipophilicity typically correlates with a higher unspecific binding, as has been shown, for example, for a series of anti-EGFR affibody molecules conjugated to dyes with different hydrophobicity values [
27].
We found that Z
HER2–ABD–AA
3 could recognize an additional binding site on SKOV3 cells with lower affinity (
Figure 6). The binding of affibody molecules and antibodies to receptors of the HER family receptors with different affinities has been described previously [
28]. The formation of altered binding sites was ascribed to the formation of homo- and heterodimers of the receptors on the cell surface, with accompanying conformation changes [
28]. The non-toxic control may be capable of recognizing such low-affinity sites, while the mcDM1-conjugated AffiDCs are not.
To achieve a higher in vivo bioavailability of the AffiDCs, an albumin-binding domain (ABD) was included that can bind to serum albumin in blood and prevent kidney filtration. The biodistribution (
Figure 7) revealed that the uptake in blood was higher for both AffiDCs than previously reported for AffiDCs lacking the ABD domain [
15], thus showing a clear prolongation of the plasma half-life imparted by the ABD. A comparison of the uptake in blood of Z
HER2–ABD–mcDM1 and Z
HER2–ABD–mcDM1
3 at both 4 h and 24 h p.i. showed the more rapid clearance of Z
HER2–ABD–mcDM1
3. The difference in kidney uptake between Z
HER2–ABD–mcDM1 and Z
HER2–ABD–mcDM1
3 was not significant. However, there was a significantly higher liver uptake of Z
HER2–ABD–mcDM1
3 than Z
HER2–ABD–mcDM1, suggesting an increased hepatic clearance. In the RP-HPLC analysis (
Figure 2C), Z
HER2–ABD–mcDM1
3 was eluted later than Z
HER2–ABD–mcDM1, showing a more hydrophobic character. These observations are consistent with the results derived by Hamblett et al., which showed that an increased DAR was accompanied by an increasing hydrophobicity, which resulted in accelerated plasma clearance [
24]. The observations are also consistent with the study by Lyon et al., which showed that rapid clearance was correlated with selective uptake by the liver [
29]. Previous studies on affibody molecules and other proteins have shown that hydrophobic patches or positive charges in the proteins promote liver uptake [
30]. It is thus likely that mcDM1 promotes liver uptake, and that the incorporation of the glutamic acids near the sites of mcDM1 attachment does not completely shield this effect. In a previous study by our group, a hexa-glutamate spacer next to mcDM1 was analyzed [
16]. Even though it did not decrease liver uptake in vivo compared to the same construct with three glutamic acids, it still shortened the elution volume in an RP-HPLC analysis, suggesting a better shielding of the hydrophobic character of mcDM1. It is possible that liver uptake may be alleviated by increasing the number of glutamic acids between the mcDM1 molecules in Z
HER2–ABD–mcDM1
3. Another approach might be to attach the mcDM1 molecules further apart on the Z
HER2–ABD carrier.
Considering the uptake in normal organs, Z
HER2–ABD–mcDM1
3 showed a significantly higher (
p < 0.01) uptake in liver, spleen, and bone in comparison to Z
HER2–ABD–mcDM1. The receptor-mediated specificity of uptake has previously been tested for
177Lu-labeled Z
HER2–ABD–DOTA [
31]. In that study, the conjugate’s design was very similar to the design in this study, with an identical targeting Z
HER2 moiety and ABD, but with a
177Lu-labeled DOTA chelator at the C-terminus instead of mcDM1. For the
177Lu-labeled Z
HER2–ABD–DOTA, the pre-injection of a large excess of non-labeled compound resulted in a significant (more than three-fold) reduction in tumor uptake, but the uptake in normal tissues was not changed. Thus, there was no measurable cross-reactivity of Z
HER2 with murine HER2. In the present study, the difference in uptake in normal organs therefore resulted from the increase in DAR, and not from the differences in affinity to HER2.
The uptake in tumors increased over time, and at 24 h p.i. the uptake reached 6.8% ID/g for ZHER2–ABD–mcDM1 and 3.3% ID/g for ZHER2–ABD–mcDM13. Since ZHER2–ABD–mcDM13 carried three times as much mcDM1, the delivery of drug molecules to the tumor was 1.45-fold higher for this variant. However, the enhanced delivery of drug molecules to the tumors was compromised by increased uptake in the liver. An important lesson from this study is thus that the development of novel targeted drugs should include the evaluation of their distribution in normal tissues. Such extensive structure–properties relationship studies are necessary to identify molecular design features, which reduce the uptake of high-DAR AffiDC in normal tissues, first and foremost in the liver. The possibility of producing constructs with a well-defined structure and, therefore, with reproducible biodistribution is a favorable feature for AffiDC to have in such studies. The use of radioactive labels, as in this study, facilitates quantitative biodistribution measurements.