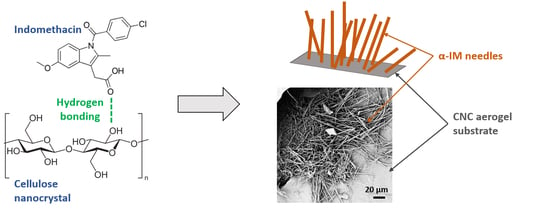
Stabilization of Metastable Indomethacin α in Cellulose Nanocrystal Aerogel Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Crystallization of α-IM within Cellulose Nanocrystal Aerogels
3.2. α-IM Stabilized in CNC Aerogels at High Temperature Thermal Holds
3.3. α-IM Stabilized in CNC Aerogels in the Presence of γ-IM Seed Crystals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Censi, R.; di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: Oxford, UK, 2010; ISBN 9780191707940. [Google Scholar]
- Saifee, M.; Inamdar, N.; Dhamecha, D.L.; Rathi, A.A. Drug Polymorphism: A Review. Int. J. Health Res. 2009, 2, 291–306. [Google Scholar] [CrossRef]
- Singhal, D.; Curatolo, W. Drug Polymorphism and Dosage Form Design: A Practical Perspective. Adv. Drug Deliv. Rev. 2004, 56, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Artusio, F.; Pisano, R. Surface-Induced Crystallization of Pharmaceuticals and Biopharmaceuticals: A Review. Int. J. Pharm. 2018, 547, 190–208. [Google Scholar] [CrossRef]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal Engineering of Active Pharmaceutical Ingredients to Improve Solubility and Dissolution Rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Atef, E.; Chauhan, H.; Prasad, D.; Kumari, D.; Pidgeon, C. Quantifying Solid-State Mixtures of Crystalline Indomethacin by Raman Spectroscopy Comparison with Thermal Analysis. ISRN Chromatogr. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Surwase, S.A.; Boetker, J.P.; Saville, D.; Boyd, B.J.; Gordon, K.C.; Peltonen, L.; Strachan, C.J. Indomethacin: New Polymorphs of an Old Drug. Mol. Pharm. 2013, 10, 4472–4480. [Google Scholar] [CrossRef]
- Andronis, V.; Zografi, G. Crystal Nucleation and Growth of Indomethacin Polymorphs from the Amorphous State. J. Non Cryst. Solids 2000, 271, 236–248. [Google Scholar] [CrossRef]
- Dubbini, A.; Censi, R.; Martena, V.; Hoti, E.; Ricciutelli, M.; Malaj, L.; di Martino, P. Influence of PH and Method of Crystallization on the Solid Physical Form of Indomethacin. Int. J. Pharm. 2014, 473, 536–544. [Google Scholar] [CrossRef]
- Aceves-Hernandez, J.M.; Nicolás-Vázquez, I.; Aceves, F.J.; Hinojosa-Torres, J.; Paz, M.; Castaño, V.M. Indomethacin Polymorphs: Experimental and Conformational Analysis. J. Pharm. Sci. 2009, 98, 2448–2463. [Google Scholar] [CrossRef]
- Kaneniwa, N.; Otsuka, M.; Hayashi, T. Physicochemical Characterization of Indomethacin Polymorphs and the Transformation Kinetics in Ethanol. Chem. Pharm. Bull. 1985, 33, 3447–3455. [Google Scholar] [CrossRef]
- Yokoyama, T.; Umeda, T.; Kuroda, K.; Nagafuku, T.; Yamamoto, T.; Asada, S. Studies on Drug Nonequivalence. IX. Relationship between Polymorphism and Rectal Absorption of Indomethacin. Yakugaku Zasshi 1979, 99, 837–842. [Google Scholar] [CrossRef]
- Chen, X.; Morris, K.R.; Griesser, U.J.; Byrn, S.R.; Stowell, J.G. Reactivity Differences of Indomethacin Solid Forms with Ammonia Gas. J. Am. Chem. Soc. 2002, 124, 15012–15019. [Google Scholar] [CrossRef] [PubMed]
- Nokhodchi, A.; Javadzadeh, Y.; Siahi-Shadbad, M.R.; Barzegar-Jalali, M. The Effect of Type and Concentration of Vehicles on the Dissolution Rate of a Poorly Soluble Drug (Indomethacin) from Liquisolid Compacts. J. Pharm. Pharm. Sci. 2005, 8, 18–25. [Google Scholar]
- van Duong, T.; Lüdeker, D.; van Bockstal, P.J.; de Beer, T.; van Humbeeck, J.; van den Mooter, G. Polymorphism of Indomethacin in Semicrystalline Dispersions: Formation, Transformation, and Segregation. Mol. Pharm. 2018, 15, 1037–1051. [Google Scholar] [CrossRef]
- Llinàs, A.; Goodman, J.M. Polymorph Control: Past, Present and Future. Drug Discov. Today 2008, 13, 198–210. [Google Scholar] [CrossRef]
- Nowak, M.; Gajda, M.; Baranowski, P.; Szymczyk, P.; Karolewicz, B.; Nartowski, K.P. Stabilisation and Growth of Metastable Form II of Fluconazole in Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Srirambhatla, V.K.; Guo, R.; Price, S.L.; Florence, A.J. Isomorphous Template Induced Crystallisation: A Robust Method for the Targeted Crystallisation of Computationally Predicted Metastable Polymorphs. Chem. Commun. 2016, 52, 7384–7386. [Google Scholar] [CrossRef] [PubMed]
- Telford, R.; Seaton, C.C.; Clout, A.; Buanz, A.; Gaisford, S.; Williams, G.R.; Prior, T.J.; Okoye, C.H.; Munshi, T.; Scowen, I.J. Stabilisation of Metastable Polymorphs: The Case of Paracetamol Form III. Chem. Commun. 2016, 52, 12028–12031. [Google Scholar] [CrossRef]
- Beckmann, W.; Nickisch, K.; Budde, U. Development of a Seeding Technique for the Crystallization of the Metastable A Modification of Abecarnil. Org. Process Res. Dev. 1998, 2, 298–304. [Google Scholar] [CrossRef]
- Chyall, L.J.; Tower, J.M.; Coates, D.A.; Houston, T.L.; Childs, S.L. Polymorph Generation in Capillary Spaces: The Preparation and Structural Analysis of a Metastable Polymorph of Nabumetone. Cryst. Growth Des. 2002, 2, 505–510. [Google Scholar] [CrossRef]
- Al-Ani, A.J.; Herdes, C.; Wilson, C.C.; Castro-Dominguez, B. Engineering a New Access Route to Metastable Polymorphs with Electrical Confinement. Cryst. Growth Des. 2020, 20, 1451–1457. [Google Scholar] [CrossRef]
- Joseph, S.; Rappolt, M.; Schoenitz, M.; Huzhalska, V.; Augustin, W.; Scholl, S.; Bunjes, H. Stability of the Metastable α-Polymorph in Solid Triglyceride Drug-Carrier Nanoparticles. Langmuir 2015, 31, 6663–6674. [Google Scholar] [CrossRef] [PubMed]
- Shakhtshneider, T.P. Phase Transformations and Stabilization of Metastable States of Molecular Crystals under Mechanical Activation. Solid State Ion. 1997, 101–103, 851–856. [Google Scholar] [CrossRef]
- Gu, C.H.; Chatterjee, K.; Young, V.; Grant, D.J.W. Stabilization of a Metastable Polymorph of Sulfamerazine by Structurally Related Additives. J. Cryst. Growth 2002, 235, 471–481. [Google Scholar] [CrossRef]
- Nartowski, K.P.; Tedder, J.; Braun, D.E.; Fábián, L.; Khimyak, Y.Z. Building Solids inside Nano-Space: From Confined Amorphous through Confined Solvate to Confined “metastable” Polymorph. Phys. Chem. Chem. Phys. 2015, 17, 24761–24773. [Google Scholar] [CrossRef]
- Ehmann, H.M.A.; Werzer, O. Surface Mediated Structures: Stabilization of Metastable Polymorphs on the Example of Paracetamol. Cryst. Growth Des. 2014, 14, 3680–3684. [Google Scholar] [CrossRef]
- Davey, R.J.; Blagden, N.; Potts, G.D.; Docherty, R. Polymorphism in Molecular Crystals: Stabilization of a Metastable Form by Conformational Mimicry. J. Am. Chem. Soc. 1997, 119, 1767–1772. [Google Scholar] [CrossRef]
- Eddleston, M.D.; Hejczyk, K.E.; Bithell, E.G.; Day, G.M.; Jones, W. Determination of the Crystal Structure of a New Polymorph of Theophylline. Chem. A Eur. J. 2013, 19, 7883–7888. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Ueda, K.; Moribe, K. Recent Progress of Structural Study of Polymorphic Pharmaceutical Drugs. Adv. Drug Deliv. Rev. 2017, 117, 71–85. [Google Scholar] [CrossRef]
- Nicoud, L.; Licordari, F.; Myerson, A.S. Estimation of the Solubility of Metastable Polymorphs: A Critical Review. Cryst. Growth Des. 2018, 18, 7228–7237. [Google Scholar] [CrossRef]
- Lee, E.H. A Practical Guide to Pharmaceutical Polymorph Screening & Selection. Asian J. Pharm. Sci. 2014, 9, 163–175. [Google Scholar] [CrossRef]
- Peterson, M.L.; Morissette, S.L.; McNulty, C.; Goldsweig, A.; Shaw, P.; LeQuesne, M.; Monagle, J.; Encina, N.; Marchionna, J.; Johnson, A.; et al. Iterative High-Throughput Polymorphism Studies on Acetaminophen and an Experimentally Derived Structure for Form III. J. Am. Chem. Soc. 2002, 124, 10958–10959. [Google Scholar] [CrossRef]
- Nath, N.K.; Nangia, A. Novel Form V of Tolbutamide and a High Z′ Crystal Structure of Form III. CrystEngComm 2011, 13, 47–51. [Google Scholar] [CrossRef]
- Desprez, S.; Descamps, M. Transformations of Glassy Indomethacin Induced by Ball-Milling. J. Non Cryst. Solids 2006, 352, 4480–4485. [Google Scholar] [CrossRef]
- Xu, T.; Nahar, K.; Dave, R.; Bates, S.; Morris, K. Polymorphic Transformation of Indomethacin during Hot Melt Extrusion Granulation: Process and Dissolution Control. Pharm. Res. 2018. [Google Scholar] [CrossRef]
- Slavin, P.A.; Sheen, D.B.; Shepherd, E.E.A.; Sherwood, J.N.; Feeder, N.; Docherty, R.; Milojevic, S. Morphological Evaluation of the γ-Polymorph of Indomethacin. J. Cryst. Growth 2002, 237–239, 300–305. [Google Scholar] [CrossRef]
- Yoshioka, M.; Hancock, B.C.; Zografi, G. Crystallization of Indomethacin from the Amorphous State below and above Its Glass Transition Temperature. J. Pharm. Sci. 1994, 83, 1700–1705. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical Significance of Cellulose: A Review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Ali, J.; Saigal, N.; Baboota, S.; Ahuja, A. Microcrystalline Cellulose as a Versatile Excipient in Drug Research. J. Young Pharm. 2009, 1, 6. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an Agricultural Waste to a Valuable Pharmaceutical Ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.K.; Letchford, K.; Wasserman, B.Z.; Ye, L.; Hamad, W.Y.; Burt, H.M. The Use of Nanocrystalline Cellulose for the Binding and Controlled Release of Drugs. Int. J. Nanomed. 2011, 6, 321–330. [Google Scholar] [CrossRef]
- Plackett, D.V.; Letchford, K.; Jackson, J.K.; Burt, H.M. A Review of Nanocellulose as a Novel Vehicle for Drug Delivery. Nord. Pulp. Pap. Res. J. 2014, 29, 105–118. [Google Scholar] [CrossRef]
- de France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-Based Aerogel Microspheres for Oral Drug Delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef]
- Hees, T.; Zhong, F.; Rudolph, T.; Walther, A.; Mülhaupt, R. Nanocellulose Aerogels for Supporting Iron Catalysts and In Situ Formation of Polyethylene Nanocomposites. Adv. Funct. Mater. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Dong, H.; Snyder, J.F.; Tran, D.T.; Leadore, J.L. Hydrogel, Aerogel and Film of Cellulose Nanofibrils Functionalized with Silver Nanoparticles. Carbohydr. Polym. 2013, 95, 760–767. [Google Scholar] [CrossRef]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug Release from Nanoparticles Embedded in Four Different Nanofibrillar Cellulose Aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and Chemically Cross-Linked Cellulose Cryogels: Structure, Properties and Application for Controlled Release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef]
- Yan, G.; Chen, B.; Zeng, X.; Sun, Y.; Tang, X.; Lin, L. Recent Advances on Sustainable Cellulosic Materials for Pharmaceutical Carrier Applications. Carbohydr. Polym. 2020, 244, 116492. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, C.; He, X.; Zhang, X.; Zhang, W.; Zhang, X. Polyethylenimine-Grafted Cellulose Nanofibril Aerogels as Versatile Vehicles for Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Zeglinski, J.; Hudson, S.; Davern, P.; Hodnett, B.K. Dependence of Heterogeneous Nucleation on Hydrogen Bonding Lifetime and Complementarity. Cryst. Growth Des. 2018, 18, 7158–7172. [Google Scholar] [CrossRef]
- Banerjee, M.; Saraswatula, S.; Williams, A.; Brettmann, B. Effect of Purification Methods on Commercially Available Cellulose Nanocrystal Properties and TEMPO Oxidation. Processes 2020, 8, 698. [Google Scholar] [CrossRef]
- Vranić, E.; Uzunović, A. Dissolution Studies of Physical Mixtures of Indomethacin with Alpha- and Gamma-Cyclodextrins. Bosn. J. Basic Med. Sci. 2010, 10, 197–203. [Google Scholar] [CrossRef]
- Wada, S.; Kudo, S.; Takiyama, H. Development of Simultaneous Control of Polymorphism and Morphology in Indomethacin Crystallization. J. Cryst. Growth 2016, 435, 37–41. [Google Scholar] [CrossRef]
- Xiang, T.-X.; Anderson, B.D. Molecular Dynamics Simulation of Amorphous Indomethacin. Mol. Pharm. 2013, 10, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Khomane, K.S.; More, P.K.; Raghavendra, G.; Bansal, A.K. Molecular Understanding of the Compaction Behavior of Indomethacin Polymorphs. Mol. Pharm. 2013, 10, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Lohani, S.; Nesmelova, I.V.; Suryanarayanan, R.; Grant, D.J.W. Spectroscopic Characterization of Molecular Aggregates in Solutions: Impact on Crystallization of Indomethacin Polymorphs from Acetonitrile and Ethanol. Cryst. Growth Des. 2011, 11, 2368–2378. [Google Scholar] [CrossRef]
- Gao, J.; Li, Q.; Chen, W.; Liu, Y.; Yu, H. Self-Assembly of Nanocellulose and Indomethacin into Hierarchically Ordered Structures with High Encapsulation Efficiency for Sustained Release Applications. ChemPlusChem 2014, 79, 725–731. [Google Scholar] [CrossRef]









| IM Polymorphic Form | Melting Temperature | Space Group | Unit Cell Dimensions | Z, Z’ | Solubility in Water at 25 °C |
|---|---|---|---|---|---|
| α | 152–154 °C | P21 | a = 5.4616 ± 0.0016 Å b = 25.31 ± 0.009 Å c = 18.152 ± 0.007 Å α = 90° β = 94.38 ± 0.03° γ = 90° | 6, 3 | 0.8 ± 0.01 mg/mL |
| γ | 160–161 °C | P | a = 0.295 ± 0.002 Å b = 10.969 ± 0.001 Å c = 9.742 ± 0.001 Å α = 69.38 ± 0.01° β = 110.79 ± 0.01° γ = 92.78 ± 0.01° | 2, 1 | 0.4 mg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, M.; Brettmann, B. Stabilization of Metastable Indomethacin α in Cellulose Nanocrystal Aerogel Scaffolds. Pharmaceutics 2021, 13, 441. https://doi.org/10.3390/pharmaceutics13040441
Banerjee M, Brettmann B. Stabilization of Metastable Indomethacin α in Cellulose Nanocrystal Aerogel Scaffolds. Pharmaceutics. 2021; 13(4):441. https://doi.org/10.3390/pharmaceutics13040441
Chicago/Turabian StyleBanerjee, Manali, and Blair Brettmann. 2021. "Stabilization of Metastable Indomethacin α in Cellulose Nanocrystal Aerogel Scaffolds" Pharmaceutics 13, no. 4: 441. https://doi.org/10.3390/pharmaceutics13040441
APA StyleBanerjee, M., & Brettmann, B. (2021). Stabilization of Metastable Indomethacin α in Cellulose Nanocrystal Aerogel Scaffolds. Pharmaceutics, 13(4), 441. https://doi.org/10.3390/pharmaceutics13040441