An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery
Abstract
:1. Introduction and Historical Background
2. Nanotechnology-Based Vaccines, Vaccine Adjuvants and Delivery Systems
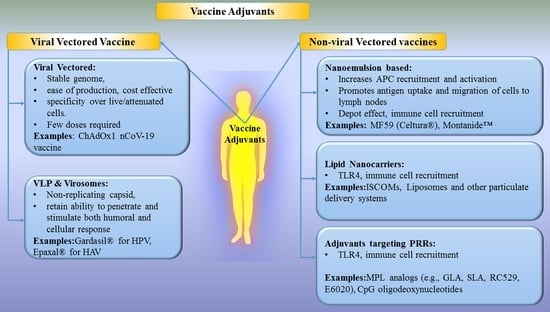
2.1. Viral Vectored Vaccines
2.2. Virus-Like Particles (VLPs) and Virosomes
2.3. Non-Viral Vectors
2.3.1. Nanoemulsion-Based Adjuvants
MF59
Montanide™
2.3.2. Lipid Nanocarriers
Immunostimulatory Complexes (ISCOMs)
Liposomes
Biodegradable Polymeric Nanoparticles
Non-Biodegradable NPs
Calcium Phosphate NPs (CPNPs)
Colloidally Stable Nanoparticles
Proteosomes
2.3.3. Adjuvants Targeting Pattern Recognition Receptors (PRRs)
C-Type Lectin Receptors (CLRs)
Toll-Like Receptor (TLRs)
3. Summary
4. Future Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tinkle, S.; Mcneil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.C.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Wang, R.; Nie, G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccines Immunother. 2014, 10, 2761–2774. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Wu, Z.; Liu, T.; Qian, R.; Wu, T.; Liu and Aizong Shen, Q. Polymeric Nanoparticles Engineered as a Vaccine Adjuvant-Delivery System. In Immunization-Vaccine Adjuvant Delivery System and Strategies; IntechOpen: London, UK, 2018. [Google Scholar]
- Immunization Basics|Vaccines and Immunizations|CDC. Available online: https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm (accessed on 12 March 2021).
- Plotkin, S.A. Vaccines: Past, present and future. Nat. Med. 2005, 11, S5. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Leke, R.; Adams, A.; Tangermann, R.H. Certification of polio eradication: Process and lessons learned. Bull. World Health Organ. 2004, 82, 24–30. [Google Scholar]
- Global Polio Eradication Initiative Applauds WHO African Region for Wild Polio-Free Certification. Available online: https://www.who.int/news/item/25-08-2020-global-polio-eradication-initiative-applauds-who-african-region-for-wild-polio-free-certification (accessed on 11 March 2021).
- Peek, L.J.; Middaugh, C.R.; Berkland, C. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 2008, 60, 915–928. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Allergy and Infectious Diseases. Understanding Vaccines: What They, How They Work; National Institute of Allergy and Infectious Diseases: North Bethesda, MD, USA, 2008. [Google Scholar]
- Kamboj, M.; Sepkowitz, K.A. Risk of Transmission Associated With Live Attenuated Vaccines Given to Healthy Persons Caring for or Residing With an Immunocompromised Patient. Infect. Control Hosp. Epidemiol. 2007, 28, 702–707. [Google Scholar] [CrossRef]
- Gomez, P.L.; Robinson, J.M. Vaccine Manufacturing. In s Vaccines, 6th ed.; Plotkin, S., Orenstien, W., Offit, P., Edwards, K., Eds.; WB Saunders Company: Londok, UK, 2013; pp. 44–57. [Google Scholar]
- Corradin, G.; Giudice, G. Novel Adjuvants for Vaccines. Curr. Med. Chem. Anti Inflamm. Anti Allergy Agents 2005, 4, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Kenney, R.; Cross, A. Adjuvants for the Future. In New Generation Vaccines, 4th ed.; Levine, M.M., Ed.; Informa Healthcare: New York, NY, USA, 2010; pp. 250–262. [Google Scholar]
- Miles, A.P.; McClellan, H.A.; Rausch, K.M.; Zhu, D.; Whitmore, M.D.; Singh, S.; Martin, L.B.; Wu, Y.; Giersing, B.K.; Stowers, A.W.; et al. Montanide® ISA 720 vaccines: Quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine 2005, 23, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- García, A.; De Sanctis, J.B. An overview of adjuvant formulations and delivery systems. APMIS 2014, 122, 257–267. [Google Scholar] [CrossRef]
- Tritto, E.; Mosca, F.; De Gregorio, E. Mechanism of action of licensed vaccine adjuvants. Vaccine 2009, 27, 3331–3334. [Google Scholar] [CrossRef]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, E.; Caproni, E.; Ulmer, J.B. Vaccine adjuvants: Mode of action. Front. Immunol. 2013, 4, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novartis Safety and Immunogenicity of MF59C.1 Adjuvanted Trivalent Subunit Influenza Vaccine in Elderly Subjects-NCT01162122 ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01162122 (accessed on 9 September 2020).
- Vaccines and Immunization. Available online: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1 (accessed on 6 February 2021).
- Matthews, Q.L.; Gu, L.; Krendelchtchikov, A.C.Z. Viral Vectors for Vaccine Development. In Novel Gene Therapy Approaches; InTechOpen: London, UK, 2013. [Google Scholar]
- Koudelka, K.J.; Pitek, A.S.; Manchester, M.; Steinmetz, N.F. Virus-Based Nanoparticles as Versatile Nanomachines. Annu. Rev. Virol. 2015, 2, 379–401. [Google Scholar] [CrossRef] [Green Version]
- Lauer, K.B.; Borrow, R.; Blanchard, T.J. Multivalent and multipathogen viral vector vaccines. Clin. Vaccine Immunol. 2017, 24, e00298-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ura, T.; Okuda, K.; Shimada, M. Developments in viral vector-based vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.D.; Tahara, K.; Maxwell, J.A.; Lalonde, R.; Fukuiwa, T.; Fujihashi, K.; Van Kampen, K.R.; Kong, F.K.; Tang, D.C.C.; Fukuchi, K.I. Nasal inoculation of an adenovirus vector encoding 11 tandem repeats of Aβ1-6 upregulates IL-10 expression and reduces amyloid load in a Mo/ Hu APPswe PS1dE9 mouse model of Alzheimer’s disease. J. Gene Med. 2007, 9, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Sjölander, A.; Cox, J.C.; Barr, I.G. ISCOMs: An adjuvant with multiple functions. J. Leukoc. Biol. 1998, 64, 713–723. [Google Scholar] [CrossRef] [PubMed]
- El-Sissi, A.F.; Mohamed, F.H.; Danial, N.M.; Gaballah, A.Q.; Ali, K.A. Chitosan and chitosan nanoparticles as adjuvant in local Rift Valley Fever inactivated vaccine. 3 Biotech 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Combining TLR9 Agonist With bNAbs for Reservoir Reduction and Immunological Control of HIV-NCT03837756-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03837756?cond=TLR&draw=3&rank=12 (accessed on 4 August 2020).
- Nalca, A.; Zumbrun, E.E. ACAM2000TM: The new smallpox vaccine for United States Strategic National Stockpile. Drug Des. Devel. Ther. 2010, 4, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Reynales, H.; Astudillo, P.; de Vallière, S.; Hatz, C.; Schlagenhauf, P.; Rath, B.; Velentgas, P.; Fariña, A.; Sales-Carmona, V.; Groth, N. A prospective observational safety study on MF59® adjuvanted cell culture-derived vaccine, Celtura® during the A/H1N1 (2009) influenza pandemic. Vaccine 2012, 30, 6436–6443. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Foged, C.; Korsholm, K.S.; Rades, T.; Christensen, D. Liposome-based adjuvants for subunit vaccines: Formulation strategies for subunit antigens and immunostimulators. Pharmaceutics 2016, 8, 7. [Google Scholar] [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhu, W.; Luo, Y.; Wang, B.Z. Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Gautam, S.K.; Kshirsagar, P.; Ross, K.A.; Spagnol, G.; Sorgen, P.; Wannemuehler, M.J.; Narasimhan, B.; Solheim, J.C.; Kumar, S.; et al. Amphiphilic polyanhydride-based recombinant MUC4β-nanovaccine activates dendritic cells. Genes Cancer 2019, 10, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable particles as vaccine antigen delivery systems for stimulating cellular immune responses. Hum. Vaccines Immunother. 2013, 9, 2584–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wafa, E.I.; Geary, S.M.; Goodman, J.T.; Narasimhan, B.; Salem, A.K. The effect of polyanhydride chemistry in particle-based cancer vaccines on the magnitude of the anti-tumor immune response. Acta Biomater. 2017, 50, 417–427. [Google Scholar] [CrossRef]
- Liu, L.; Kshirsagar, P.; Christiansen, J.; Gautam, S.K.; Aithal, A.; Gulati, M.; Kumar, S.; Solheim, J.C.; Batra, S.K.; Jain, M.; et al. Polyanhydride nanoparticles stabilize pancreatic cancer antigen MUC4β. J. Biomed. Mater. Res. Part A 2020. [Google Scholar] [CrossRef] [PubMed]
- Cell-Mediated Immunity-An Overview-Biology LibreTexts. Available online: https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Kaiser)/Unit_6%3A_Adaptive_Immunity/14%3A_Cell-Mediated_Immunity/14.1%3A_Cell-Mediated_Immunity_-_An_Overview (accessed on 12 March 2021).
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef]
- Wang, Z.B.; Xu, J. Better adjuvants for better vaccines: Progress in adjuvant delivery systems, modifications, and adjuvant–antigen codelivery. Vaccines 2020, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuniga, A.; Wang, Z.L.; Liniger, M.; Hangartner, L.; Caballero, M.; Pavlovic, J.; Wild, P.; Viret, J.F.; Glueck, R.; Billeter, M.A.; et al. Attenuated measles virus as a vaccine vector. Vaccine 2007, 25, 2974–2983. [Google Scholar] [CrossRef]
- García-Arriaza, J.; Marín, M.Q.; Merchán-Rubira, J.; Mascaraque, S.M.; Medina, M.; Ávila, J.; Hernández, F.; Esteban, M. Tauopathy Analysis in P301S Mouse Model of Alzheimer Disease Immunized with DNA and MVA Poxvirus-Based Vaccines Expressing Human Full-Length 4R2N or 3RC Tau Proteins. Vaccines 2020, 8, 127. [Google Scholar] [CrossRef] [Green Version]
- Anderholm, K.M.; Bierle, C.J.; Schleiss, M.R. Cytomegalovirus Vaccines: Current Status and Future Prospects. Drugs 2016, 76, 1625–1645. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Poxvirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [Green Version]
- Sandgren, K.J.; Truong, N.R.; Smith, J.B.; Bertram, K.; Cunningham, A.L. Vaccines for Herpes Simplex: Recent Progress Driven by Viral and Adjuvant Immunology. Methods Mol. Biol. 2020, 2060, 31–56. [Google Scholar] [CrossRef]
- Harrop, R.; John, J.; Carroll, M.W. Recombinant viral vectors: Cancer vaccines. Adv. Drug Deliv. Rev. 2006, 58, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Lambe, T. Clinical advances in viral-vectored influenza vaccines. Vaccines 2018, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Zeltins, A.; West, J.; Zabel, F.; El Turabi, A.; Balke, I.; Haas, S.; Maudrich, M.; Storni, F.; Engeroff, P.; Jennings, G.T.; et al. Incorporation of tetanus-epitope into virus-like particles achieves vaccine responses even in older recipients in models of psoriasis, Alzheimer’s and cat allergy. NPJ Vaccines 2017, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Ewer, K.J.; Sierra-Davidson, K.; Salman, A.M.; Illingworth, J.J.; Draper, S.J.; Biswas, S.; Hill, A.V.S. Progress with viral vectored malaria vaccines: A multi-stage approach involving “unnatural immunity”. Vaccine 2015, 33, 7444–7451. [Google Scholar] [CrossRef] [Green Version]
- Giel-Moloney, M.; Esteban, M.; Oakes, B.H.; Vaine, M.; Asbach, B.; Wagner, R.; Mize, G.J.; Spies, A.G.; McElrath, J.; Perreau, M.; et al. Recombinant HIV-1 vaccine candidates based on replication-defective flavivirus vector. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Tan, W.G.; Jin, H.-T.; West, E.E.; Penaloza-MacMaster, P.; Wieland, A.; Zilliox, M.J.; McElrath, M.J.; Barouch, D.H.; Ahmed, R. Comparative Analysis of Simian Immunodeficiency Virus Gag-Specific Effector and Memory CD8 + T Cells Induced by Different Adenovirus Vectors. J. Virol. 2013, 87, 1359–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, I.D.; Gibani, M.M.; Sewell, R.; Clutterbuck, E.A.; Campbell, D.; Plested, E.; Nuthall, E.; Voysey, M.; Silva-Reyes, L.; McElrath, M.J.; et al. Safety and immunogenicity of novel adenovirus type 26-and modified vaccinia Ankara-vectored Ebola vaccines: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2016, 315, 1610–1623. [Google Scholar] [CrossRef]
- Shukarev, G.; Callendret, B.; Luhn, K.; Douoguih, M. A two-dose heterologous prime-boost vaccine regimen eliciting sustained immune responses to Ebola Zaire could support a preventive strategy for future outbreaks. Hum. Vaccines Immunother. 2017, 13, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- AZD1222 Vaccine Against COVID-19 Developed by Oxford University and Astra Zeneca: Background Paper (Draft). Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-background-2021.1 (accessed on 11 March 2021).
- Guo, J.; Mondal, M.; Zhou, D. Development of novel vaccine vectors: Chimpanzee adenoviral vectors. Hum. Vaccines Immunother. 2018, 14, 1679–1685. [Google Scholar] [CrossRef] [Green Version]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Gallinaro, A.; Borghi, M.; Bona, R.; Grasso, F.; Calzoletti, L.; Palladino, L.; Cecchetti, S.; Vescio, M.F.; Macchia, D.; Morante, V.; et al. Integrase defective lentiviral vector as a vaccine platform for delivering influenza antigens. Front. Immunol. 2018, 9, 171. [Google Scholar] [CrossRef]
- Clinical Trials Safety and Immunity of Covid-19 aAPC Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT04299724 (accessed on 30 October 2020).
- NCT02054286 Safety, Tolerability and Immunogenicity Induced by the THV01 Treatment in Patients Infected with HIV-1 Clade B and Treated With Highly Active Antiretroviral Therapy (HAART). 2014. Available online: https://clinicaltrials.gov/show/NCT02054286 (accessed on 15 March 2021).
- Howles, S.; Guimarães-Walker, A.; Yang, H.; Hancock, G.; di Gleria, K.; Tarragona-Fiol, T.; Hayes, P.; Gilmour, J.; Bridgeman, A.; Hanke, T.; et al. Vaccination with a modified vaccinia virus Ankara (MVA)-vectored HIV-1 immunogen induces modest vector-specific T cell responses in human subjects. Vaccine 2010, 28, 7306–7312. [Google Scholar] [CrossRef]
- Study to Evaluate the Dosage and Safety of Two Intramuscular Injections of an Investigational Clade B HIV Vaccine-NCT01320176. Available online: https://clinicaltrials.gov/ct2/show/NCT01320176 (accessed on 17 June 2020).
- Ramsauer, K.; Schwameis, M.; Firbas, C.; Müllner, M.; Putnak, R.J.; Thomas, S.J.; Desprès, P.; Tauber, E.; Jilma, B.; Tangy, F. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: A randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect. Dis. 2015, 15, 519–527. [Google Scholar] [CrossRef]
- Phase II Study to Evaluate Safety and Immunogenicity of a Chikungunya Vaccine-NCT02861586. Available online: https://clinicaltrials.gov/ct2/show/NCT02861586 (accessed on 17 June 2020).
- Donaldson, B.; Lateef, Z.; Walker, G.F.; Young, S.L.; Ward, V.K. Virus-like particle vaccines: Immunology and formulation for clinical translation. Expert Rev. Vaccines 2018, 17, 833–849. [Google Scholar] [CrossRef]
- Garcea, R.L.; Gissmann, L. Virus-like particles as vaccines and vessels for the delivery of small molecules. Curr. Opin. Biotechnol. 2004, 15, 513–517. [Google Scholar] [CrossRef]
- Salvador, A.; Igartua, M.; Hernández, R.M.; Pedraz, J.L. An Overview on the Field of Micro- and Nanotechnologies for Synthetic Peptide-Based Vaccines. J. Drug Deliv. 2011, 2011, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Moser, C.; Amacker, M.; Zurbriggen, R. Influenza virosomes as a vaccine adjuvant and carrier system. Expert Rev. Vaccines 2011, 10, 437–446. [Google Scholar] [CrossRef]
- Plummer, E.M.; Manchester, M. Viral nanoparticles and virus-like particles: Platforms for contemporary vaccine design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 174–196. [Google Scholar] [CrossRef]
- Qian, C.; Liu, X.; Xu, Q.; Wang, Z.; Chen, J.; Li, T.; Zheng, Q.; Yu, H.; Gu, Y.; Li, S.; et al. Recent progress on the versatility of virus-like particles. Vaccines 2020, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Alon, D.; Stein, G.Y.; Hadas-Golan, V.; Tau, L.; Brosh, T.; Turner, D. Immunogenicity of Sci-B-Vac (a Third-Generation Hepatitis B Vaccine) in HIV-Positive Adults. Isr. Med. Assoc. J. 2017, 19, 143–146. [Google Scholar]
- VBI Vaccines Announces Initiation of Phase 3 Clinical Program for Sci-B-Vac® Hepatitis B Vaccine|VBI Vaccines. Available online: https://www.vbivaccines.com/press-releases/sci-b-vac-phase-3-initiation/ (accessed on 14 March 2021).
- HEPLISAV-B|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/heplisav-b (accessed on 12 March 2021).
- FDA Approves Merck’s GARDASIL 9 for the Prevention of Certain HPV-Related Head and Neck Cancers-Merck.com. Available online: https://www.merck.com/news/fda-approves-mercks-gardasil-9-for-the-prevention-of-certain-hpv-related-head-and-neck-cancers/ (accessed on 12 March 2021).
- FDA Approves Cervarix, GlaxoSmithKline’s Cervical Cancer Vaccine|GSK. Available online: https://www.gsk.com/en-gb/media/press-releases/fda-approves-cervarix-glaxosmithkline-s-cervical-cancer-vaccine/ (accessed on 21 July 2020).
- Chabeda, A.; van Zyl, A.R.; Rybicki, E.P.; Hitzeroth, I.I. Substitution of Human Papillomavirus Type 16 L2 Neutralizing Epitopes Into L1 Surface Loops: The Effect on Virus-Like Particle Assembly and Immunogenicity. Front. Plant Sci. 2019, 10, 779. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Bovier, P.A. Epaxal®: A virosomal vaccine to prevent hepatitis A infection. Expert Rev. Vaccines 2008, 7, 1141–1150. [Google Scholar] [CrossRef]
- Development of vaccines candidates-Mymetics Corp. Available online: https://www.mymetics.com/vaccine-pipeline/ (accessed on 22 June 2020).
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy-An overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Hardee, C.L.; Arévalo-Soliz, L.M.; Hornstein, B.D.; Zechiedrich, L. Advances in non-viral DNA vectors for gene therapy. Genes 2017, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.R.; Dodd, S.; Schaefer, M.; Ugozzoli, M.; Singh, M.; Otten, G.R.; Amiji, M.M.; O’Hagan, D.T.; Brito, L.A. The Development of Self-Emulsifying Oil-in-Water Emulsion Adjuvant and an Evaluation of the Impact of Droplet Size on Performance. J. Pharm. Sci. 2015, 104, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Ott, G.S.; Van Nest, G.; Rappuoli, R.; Del Giudice, G. The history of MF59® adjuvant: A phoenix that arose from the ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Friedland, L.R.; Hanon, E.; Didierlaurent, A.M. Towards an evidence based approach for the development of adjuvanted vaccines. Curr. Opin. Immunol. 2017, 47, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Della Cioppa, G.; Nicolay, U.; Lindert, K.; Leroux-Roels, G.; Clement, F.; Castellino, F.; Galli, G.; Groth, N.; Levin, Y.; Del Giudice, G. A dose-range study in older adults to compare the safety and immunogenicity profiles of MF59®-adjuvanted and non-adjuvanted seasonal influenza vaccines following intradermal and intramuscular administration. Hum. Vaccines Immunother. 2014, 10, 1701–1710. [Google Scholar] [CrossRef]
- Yang, A.Q.; Yang, H.Y.; Guo, S.J.; Xie, Y.E. MF59 adjuvant enhances the immunogenicity and protective immunity of the OmpK/Omp22 fusion protein from Acineterbacter baumannii through intratracheal inoculation in mice. Scand. J. Immunol. 2019, 90. [Google Scholar] [CrossRef]
- Chang, H.; Duan, J.; Zhou, P.; Su, L.; Zheng, D.; Zhang, F.; Fang, F.; Li, X.; Chen, Z. Single immunization with MF59-adjuvanted inactivated whole-virion H7N9 influenza vaccine provides early protection against H7N9 virus challenge in mice. Microbes Infect. 2017, 19, 616–625. [Google Scholar] [CrossRef]
- Jakopin, Z. Murabutide Revisited: A Review of its Pleiotropic Biological Effects. Curr. Med. Chem. 2013, 20, 2068–2079. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, S. Th1 Cells. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Oxford, UK, 2016; pp. 287–293. [Google Scholar]
- Kantipakala, R.; Bonam, S.R.; Vemireddy, S.; Miryala, S.; Halmuthur, M.S.K. Squalane-based emulsion vaccine delivery system: Composition with murabutide activate Th1 response. Pharm. Dev. Technol. 2019, 24, 269–275. [Google Scholar] [CrossRef]
- Feinen, B.; Petrovsky, N.; Verma, A.; Merkel, T.J. Advax-adjuvanted recombinant protective antigen provides protection against inhalational anthrax that is further enhanced by addition of murabutide adjuvant. Clin. Vaccine Immunol. 2014, 21, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaurav, M.; Madan, J.; Sudheesh, M.S.; Pandey, R.S. Combined adjuvant-delivery system for new generation vaccine antigens: Alliance has its own advantage. Artif. Cells Nanomed. Biotechnol. 2018, 46, S818–S831. [Google Scholar] [CrossRef] [PubMed]
- Ascarateil, S.; Puget, A.; Koziol, M.-E. Safety data of Montanide ISA 51 VG and Montanide ISA 720 VG, two adjuvants dedicated to human therapeutic vaccines. J. Immunother. Cancer 2015, 3, P428. [Google Scholar] [CrossRef] [Green Version]
- Koyasu, S.; Moro, K. Type 2 innate immune responses and the natural helper cell. Immunology 2011, 132, 475–481. [Google Scholar] [CrossRef]
- Savoji, M.A.; Haghighat, S.; Mirzaee, M.; Golkaran, B.; Mirzaee, R.; Esfandiari, B.; Mahdavi, M. Formulation of HBs antigen in Montanide ISA266 shows superiority to commercial HBsAg vaccine in the induction of humoral immune responses. Gastroenterol. Hepatol. Bed Bench 2019, 12, 292–300. [Google Scholar] [PubMed]
- Khabazzadeh Tehrani, N.; Mahdavi, M.; Maleki, F.; Zarrati, S.; Tabatabaie, F. The role of Montanide ISA 70 as an adjuvant in immune responses against Leishmania major induced by thiol-specific antioxidant-based protein vaccine. J. Parasit. Dis. 2016, 40, 760–767. [Google Scholar] [CrossRef] [Green Version]
- Van Doorn, E.; Liu, H.; Huckriede, A.; Hak, E. Safety and tolerability evaluation of the use of Montanide ISATM51 as vaccine adjuvant: A systematic review. Hum. Vaccines Immunother. 2016, 12, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Rhee, J.H. Current and new approaches for mucosal vaccine delivery. In Mucosal Vaccines, 2nd ed.; Kiyono, H., Pascual, D.W., Eds.; Academic Press: Oxfod, UK, 2020. [Google Scholar]
- Comber, J.D.; Philip, R. MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines. Ther. Adv. Vaccines 2014, 2, 77–89. [Google Scholar] [CrossRef]
- Morelli, A.B.; Becher, D.; Koernig, S.; Silva, A.; Drane, D.; Maraskovsky, E. ISCOMATRIX: A novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J. Med. Microbiol. 2012, 61, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Pabreja, S.; Garg, T.; Rath, G.; Goyal, A.K. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 532–539. [Google Scholar] [CrossRef]
- Cibulski, S.P.; Mourglia-Ettlin, G.; Teixeira, T.F.; Quirici, L.; Roehe, P.M.; Ferreira, F.; Silveira, F. Novel ISCOMs from Quillaja brasiliensis saponins induce mucosal and systemic antibody production, T-cell responses and improved antigen uptake. Vaccine 2016, 34, 1162–1171. [Google Scholar] [CrossRef]
- Sivakumar, S.M.; Safhi, M.M.; Kannadasan, M.; Sukumaran, N. Vaccine adjuvants-Current status and prospects on controlled release adjuvancity. Saudi Pharm. J. 2011, 19, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Alving, C.R.; Beck, Z.; Matyas, G.R.; Rao, M. Liposomal Adjuvants for Human Vaccines. Expert Opin. Drug Deliv. 2016, 13, 807–816. [Google Scholar] [CrossRef]
- Perrie, Y.; Kastner, E.; Khadke, S.; Roces, C.B.; Stone, P. Manufacturing methods for liposome adjuvants. Methods Mol. Biol. 2017, 1494, 127–144. [Google Scholar]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Khademi, F.; Taheri, R.A.; Momtazi-Borojeni, A.A.; Farnoosh, G.; Johnston, T.P.; Sahebkar, A. Potential of cationic liposomes as adjuvants/delivery systems for tuberculosis subunit vaccines. Rev. Physol. Biochem. Pharmacol. 2018, 175, 47–69. [Google Scholar]
- Varypataki, E.M.; van der Maaden, K.; Bouwstra, J.; Ossendorp, F.; Jiskoot, W. Cationic Liposomes Loaded with a Synthetic Long Peptide and Poly(I:C): A Defined Adjuvanted Vaccine for Induction of Antigen-Specific T Cell Cytotoxicity. AAPS J. 2015, 17, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.; Korsholm, K.S.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 513–521. [Google Scholar] [CrossRef]
- Moyo, N.; Vogel, A.B.; Buus, S.; Erbar, S.; Wee, E.G.; Sahin, U.; Hanke, T. Efficient Induction of T Cells against Conserved HIV-1 Regions by Mosaic Vaccines Delivered as Self-Amplifying mRNA. Mol. Ther. Methods Clin. Dev. 2019, 12, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.C.; Yang, J.; Hussein, W.M.; Zhao, L.; Wang, X.; Khalil, Z.G.; Capon, R.J.; Toth, I.; Stephenson, R.J. Polyethylenimine: An Intranasal Adjuvant for Liposomal Peptide-Based Subunit Vaccine against Group A Streptococcus. ACS Infect. Dis. 2020, 6, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Deng, B.; Seffouh, A.; Ortega, J.; Long, C.A.; Suresh, R.V.; He, X.; Miura, K.; Lee, S.-M.; Wu, Y.; et al. Antibody response of a particle-inducing, liposome vaccine adjuvant admixed with a Pfs230 fragment. NPJ Vaccines 2020, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Moderna COVID-19 Vaccine|FDA. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine (accessed on 16 February 2021).
- Pfizer-BioNTech COVID-19 Vaccine|FDA. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine (accessed on 16 February 2021).
- Petkar, K.C.; Chavhan, S.S.; Agatonovik-Kustrin, S.; Sawant, K.K. Nanostructured materials in drug and gene delivery: A review of the state of the art. Crit. Rev. Ther. Drug Carrier Syst. 2011, 28, 101–164. [Google Scholar] [CrossRef]
- Gutjahr, A.; Phelip, C.; Coolen, A.L.; Monge, C.; Boisgard, A.S.; Paul, S.; Verrier, B. Biodegradable polymeric nanoparticles-based vaccine adjuvants for lymph nodes targeting. Vaccines 2016, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.A.; Lyod, H.; Wu, W.; Huntimer, L.; Ahmed, S.; Sambol, A.; Broderick, S.; Flickinger, Z.; Rajan, K.; Bronich, T.; et al. Hemagglutinin-based polyanhydride nanovaccines against H5N1 infuenza elicit protective virus neutralizing titers and cell-mediated immunity. Int. J. Nanomed. 2015, 10, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Thukral, A.; Ross, K.; Hansen, C.; Phanse, Y.; Narasimhan, B.; Steinberg, H.; Talaat, A.M. A single dose polyanhydride-based nanovaccine against paratuberculosis infection. NPJ Vaccines 2020, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Kunda, N.K.; Alfagih, I.M.; Miyaji, E.N.; Figueiredo, D.B.; Gonçalves, V.M.; Ferreira, D.M.; Dennison, S.R.; Somavarapu, S.; Hutcheon, G.A.; Saleem, I.Y. Pulmonary dry powder vaccine of pneumococcal antigen loaded nanoparticles. Int. J. Pharm. 2015, 495, 903–912. [Google Scholar] [CrossRef]
- Rodrigues, T.C.; Oliveira, M.L.S.; Soares-Schanoski, A.; Chavez-Rico, S.L.; Figueiredo, D.B.; Gonçalves, V.M.; Ferreira, D.M.; Kunda, N.K.; Saleem, I.Y.; Miyaji, E.N. Mucosal immunization with PspA (Pneumococcal surface protein A)-adsorbed nanoparticles targeting the lungs for protection against pneumococcal infection. PLoS ONE 2018, 13, e0191692. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Song, H.; Zhang, H.; Yang, P.; Zhang, C.; Huang, P.; Kong, D.; Wang, W. Engineering biodegradable guanidyl-decorated PEG-PCL nanoparticles as robust exogenous activators of DCs and antigen cross-presentation. Nanoscale 2017, 9, 13413–13418. [Google Scholar] [CrossRef]
- Gu, P.; Wusiman, A.; Wang, S.; Zhang, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wang, D. Polyethylenimine-coated PLGA nanoparticles-encapsulated Angelica sinensis polysaccharide as an adjuvant to enhance immune responses. Carbohydr. Polym. 2019, 223. [Google Scholar] [CrossRef]
- Khademi, F.; Sahebkar, A.; Fasihi-Ramandi, M.; Taheri, R.A. Induction of strong immune response against a multicomponent antigen of Mycobacterium tuberculosis in BALB/c mice using PLGA and DOTAP adjuvant. APMIS 2018, 126, 509–514. [Google Scholar] [CrossRef]
- Jesus, S.; Soares, E.; Borchard, G.; Borges, O. Poly-ϵ-caprolactone/chitosan nanoparticles provide strong adjuvant effect for hepatitis B antigen. Nanomedicine 2017, 12, 2335–2348. [Google Scholar] [CrossRef]
- Chuang, C.C.; Tsai, M.H.; Yen, H.J.; Shyu, H.F.; Cheng, K.M.; Chen, X.A.; Chen, C.C.; Young, J.J.; Kau, J.H. A fucoidan-quaternary chitosan nanoparticle adjuvant for anthrax vaccine as an alternative to CpG oligodeoxynucleotides. Carbohydr. Polym. 2020, 229, 115403. [Google Scholar] [CrossRef]
- Heegaard, P.M.H.; Boas, U.; Sorensen, N.S. Dendrimers for vaccine and immunostimulatory uses. A review. Bioconjug. Chem. 2010, 21, 405–418. [Google Scholar] [CrossRef]
- Asgary, V.; Shoari, A.; Afshar Moayad, M.; Shafiee Ardestani, M.; Bigdeli, R.; Ghazizadeh, L.; Khosravy, M.S.; Panahnejad, E.; Janani, A.; Bashar, R.; et al. Evaluation of G2 Citric Acid-Based Dendrimer as an Adjuvant in Veterinary Rabies Vaccine. Viral Immunol. 2018, 31, 47–54. [Google Scholar] [CrossRef]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.A. Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Rev. Vaccines 2020, 19, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.A.F.M.; Diebold, S.S.; Smyth, L.A.; Walters, A.A.; Lombardi, G.; Al-Jamal, K.T. Application of carbon nanotubes in cancer vaccines: Achievements, challenges and chances. J. Control Release 2019, 297, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Tovar, G.; Palestino, G.; Rosales-Mendoza, S. An overview on the role of silica-based materials in vaccine development. Expert Rev. Vaccines 2016, 15, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Sen, D.; Deerinck, T.J.; Ellisman, M.H.; Parker, I.; Cahalan, M.D. Quantum Dots for Tracking Dendritic Cells and Priming an Immune Response In Vitro and In Vivo. PLoS ONE 2008, 3, e3290. [Google Scholar] [CrossRef]
- Lambkin, I.; Pinilla, C.; Hamashin, C.; Spindler, L.; Russell, S.; Schink, A.; Moya-Castro, R.; Allicotti, G.; Higgins, L.; Smith, M.; et al. Toward targeted oral vaccine delivery systems: Selection of lectin mimetics from combinatorial libraries. Pharm. Res. 2003, 20, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Combadière, B.; Mahé, B. Particle-based vaccines for transcutaneous vaccination. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 293–315. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Liu, Y.; Wu, X.; Ji, Y.; Zhao, Y.; et al. Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012, 12, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Sawutdeechaikul, P.; Jiangchareon, B.; Wanichwecharungruang, S.; Palaga, T. Oxidized carbon nanoparticles as an effective protein antigen delivery system targeting the cell-mediated immune response. Int. J. Nanomed. 2019, 14, 4867–4880. [Google Scholar] [CrossRef] [Green Version]
- Tosic, J.; Stanojevic, Z.; Vidicevic, S.; Isakovic, A.; Ciric, D.; Martinovic, T.; Kravic-Stevovic, T.; Bumbasirevic, V.; Paunovic, V.; Jovanovic, S.; et al. Graphene quantum dots inhibit T cell-mediated neuroinflammation in rats. Neuropharmacology 2019, 146, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, X.; Huang, X.; Zhang, J.; Xia, N.; Zhao, Q. Calcium phosphate nanoparticles as a new generation vaccine adjuvant. Expert Rev. Vaccines 2017, 16, 895–906. [Google Scholar] [CrossRef]
- Masson, J.D.; Thibaudon, M.; Bélec, L.; Crépeaux, G. Calcium phosphate: A substitute for aluminum adjuvants? Expert Rev. Vaccines 2017, 16, 289–299. [Google Scholar] [CrossRef]
- Sokolova, V.; Knuschke, T.; Kovtun, A.; Buer, J.; Epple, M.; Westendorf, A.M. The use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials 2010, 31, 5627–5633. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Mitchell, A.R.; Johnson, S.L.; Wagner-Bartak, C.; Morcol, T.; Bell, S.J.D. Calcium phosphate nanoparticle adjuvant. Clin. Diagn. Lab. Immunol. 2000, 7, 899–903. [Google Scholar] [CrossRef] [Green Version]
- MorçÖl, T.; Hurst, B.L.; Tarbet, E.B. Calcium phosphate nanoparticle (CaPNP) for dose-sparing of inactivated whole virus pandemic influenza A (H1N1) 2009 vaccine in mice. Vaccine 2017, 35, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.; Aufderhorst, U.W.; Alt, M.; Dittmer, U.; Eis-Hübinger, A.M.; Giebel, B.; Roggendorf, M.; Epple, M.; Krawczyk, A. Induction of herpes simplex virus type 1 cell-to-cell spread inhibiting antibodies by a calcium phosphate nanoparticle-based vaccine. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 138–148. [Google Scholar] [CrossRef]
- Sun, B.; Yu, S.; Zhao, D.; Guo, S.; Wang, X.; Zhao, K. Polysaccharides as vaccine adjuvants. Vaccine 2018, 36, 5226–5234. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, X.; Kwak, M.; Xu, L.; Zhang, L.J.; Yu, Q.; Jin, J.O. Maturation of dendritic cells by pullulan promotes anti-cancer effect. Oncotarget 2016, 7, 44644–44659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagatomo, D.; Taniai, M.; Ariyasu, H.; Taniguchi, M.; Aga, M.; Ariyasu, T.; Ohta, T.; Fukuda, S. Cholesteryl Pullulan Encapsulated TNF-α Nanoparticles Are an Effective Mucosal Vaccine Adjuvant against Influenza Virus. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.M.; Levine, M.M.; Dougan, G.; Good, M.F.; Nabel, G.J.; Nataro, J.P.; Rappuoli, R. New Generation Vaccines, 4th ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 1040. [Google Scholar]
- Burt, D.; Mallett, C.; Plante, M.; Zimmermann, J.; Torossian, K.; Fries, L. Proteosome-adjuvanted intranasal influenza vaccines: Advantages, progress and future considerations. Expert Rev. Vaccines 2011, 10, 365–375. [Google Scholar] [CrossRef]
- Fries, L.; Lambkin, R.; Gelder, C.; White, G.; Burt, D.; Lowell, G.; Oxford, J. FluINsureTM, an inactivated trivalent influenza vaccine for intranasal administration, is protective in human challenge with A/Panama/2007/99 (H3N2) virus. Int. Congr. Ser. 2004, 1263, 661–665. [Google Scholar] [CrossRef]
- FDA Approves Use of Menactra® Vaccine for Booster Immunization against Potentially Deadly Disease. 8 September 2014. Available online: http://www.news.sanofi.us/press-releases?item=137127 (accessed on 8 July 2020).
- Lambkin-Williams, R.; Gelder, C.; Broughton, R.; Mallett, C.P.; Gilbert, A.S.; Mann, A.; He, D.; Oxford, J.S.; Burt, D. An intranasal Proteosome-adjuvanted trivalent influenza vaccine is safe, immunogenic & efficacious in the human viral influenza challenge model. Serum IgG & mucosal IgA are important correlates of protection against illness associated with infection. PLoS ONE 2016, 11, e163089. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasou, A.; Sultanoglu, N.; Goodbourn, S.; Randall, R.E.; Kostrikis, L.G. Targeting pattern recognition receptors (PRR) for vaccine adjuvantation: From synthetic PRR agonists to the potential of defective interfering particles of viruses. Viruses 2017, 9, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghparast, A.; Zakeri, A.; Ebrahimian, M.; Ramezani, M. Targeting Pattern Recognition Receptors (PRRs) in Nano- Adjuvants: Current Perspectives. Curr. Bionanotechnol. 2016, 2, 47–59. [Google Scholar] [CrossRef]
- Bermejo-Jambrina, M.; Eder, J.; Helgers, L.C.; Hertoghs, N.; Nijmeijer, B.M.; Stunnenberg, M.; Geijtenbeek, T.B.H. C-type lectin receptors in antiviral immunity and viral escape. Front. Immunol. 2018, 9, 590. [Google Scholar] [CrossRef]
- Sharma, R.; Mody, N.; Kushwah, V.; Jain, S.; Vyas, S. C-Type lectin receptor(s)-targeted nanoliposomes: An intelligent approach for effective cancer immunotherapy. Nanomedicine 2017, 12, 1945–1959. [Google Scholar] [CrossRef]
- Haro, M.A.; Dyevoich, A.M.; Phipps, J.P.; Haas, K.M. Activation of B-1 cells promotes tumor cell killing in the peritoneal cavity. Cancer Res. 2019, 79, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Phipps, J.P.; Haas, K.M. An Adjuvant That Increases Protective Antibody Responses to Polysaccharide Antigens and Enables Recall Responses. J. Infect. Dis. 2019, 219, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Awuah, D.; Alobaid, M.; Latif, A.; Salazar, F.; Emes, R.D.; Ghaemmaghami, A.M. The Cross-Talk between miR-511-3p and C-Type Lectin Receptors on Dendritic Cells Affects Dendritic Cell Function. J. Immunol. 2019, 203, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Vandergriff, A.; Huang, K.; Shen, D.; Hu, S.; Hensley, M.T.; Caranasos, T.G.; Qian, L.; Cheng, K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 2018, 8, 1869–1878. [Google Scholar] [CrossRef]
- Chen, J.Q.; Szodoray, P.; Zeher, M. Toll-Like Receptor Pathways in Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2016, 50, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.A.; Shah, M.; Kim, J.; Choi, S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med. Res. Rev. 2019, 39, 1053–1090. [Google Scholar] [CrossRef] [Green Version]
- Casanova, J.L.; Abel, L.; Quintana-Murci, L. Human TLRs and IL-1Rs in host defense: Natural insights from evolutionary, epidemiological, and clinical genetics. Annu. Rev. Immunol. 2011, 29, 447–491. [Google Scholar] [CrossRef]
- Ruysschaert, J.M.; Lonez, C. Role of lipid microdomains in TLR-mediated signalling. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1860–1867. [Google Scholar] [CrossRef]
- Köberlin, M.S.; Heinz, L.X.; Superti-Furga, G. Functional crosstalk between membrane lipids and TLR biology. Curr. Opin. Cell Biol. 2016, 39, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Cluff, C.W. Monophosphoryl lipid a (MPL) as an adjuvant for anti-cancer vaccines: Clinical results. Adv. Exp. Med. Biol. 2009, 667, 111–123. [Google Scholar] [CrossRef]
- Shetab Boushehri, M.A.; Lamprecht, A. TLR4-Based Immunotherapeutics in Cancer: A Review of the Achievements and Shortcomings. Mol. Pharm. 2018, 15, 4777–4800. [Google Scholar] [CrossRef]
- Fendrix (Hepatitis B vaccine)|GSK Pharma UK|Public Site. Available online: https://public.gsk.co.uk/products/fendrix.html (accessed on 5 September 2020).
- Tian, M.; Zhou, Z.; Tan, S.; Fan, X.; Li, L.; Ullah, N. Formulation in DDA-MPLA-TDB liposome enhances the immunogenicity and protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Front. Immunol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Dowling, D.J.; Scott, E.A.; Scheid, A.; Bergelson, I.; Joshi, S.; Pietrasanta, C.; Brightman, S.; Sanchez-Schmitz, G.; Van Haren, S.D.; Ninković, J.; et al. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 2017, 140, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Buonsanti, C.; Balocchi, C.; Harfouche, C.; Corrente, F.; Galli Stampino, L.; Mancini, F.; Tontini, M.; Malyala, P.; Bufali, S.; Baudner, B.; et al. Novel adjuvant Alum-TLR7 significantly potentiates immune response to glycoconjugate vaccines. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Hanagata, N. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int. J. Nanomed. 2017, 12, 515–531. [Google Scholar] [CrossRef] [Green Version]
- Krieg, A.M.; Yi, A.K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995, 374, 546–549. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Goldinger, S.M.; Dummer, R.; Baumgaertner, P.; Mihic-Probst, D.; Schwarz, K.; Hammann-Haenni, A.; Willers, J.; Geldhof, C.; Prior, J.O.; Kündig, T.M.; et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8 + T-cell responses in melanoma patients. Eur. J. Immunol. 2012, 42, 3049–3061. [Google Scholar] [CrossRef] [Green Version]
- Naito, Y.; Hamaoka, S.; Kinoshita, M.; Kainuma, A.; Shimizu, M.; Katoh, H.; Moriyama, K.; Ishii, K.J.; Sawa, T. The protective effects of nasal PcrV-CpG oligonucleotide vaccination against Pseudomonas aeruginosa pneumonia. Microbiol. Immunol. 2018, 62, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Fujihashi, K.; Yamamoto, N.; Hasegawa, H.; Ainai, A.; Sato, K.; Iho, S.; Yamamoto, S.; Maeyama, J.I.; Odagiri, T.; et al. CpG ODN G9.1 as a novel nasal ODN adjuvant elicits complete protection from influenza virus infection without causing inflammatory immune responses. Vaccine 2019, 37, 5382–5389. [Google Scholar] [CrossRef]
- Nikitczuk, K.P.; Schloss, R.S.; Yarmush, M.L.; Lattime, E.C. PLGA-Polymer Encapsulating Tumor Antigen and CpG DNA Administered into the Tumor Microenvironment Elicits a Systemic Antigen-Specific IFN-γ Response and Enhances Survival. J. Cancer Ther. 2013, 4, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Alizadeh, D.; Zhang, L.; Liu, W.; Farrukh, O.; Manuel, E.; Diamond, D.J.; Badie, B. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin. Cancer Res. 2011, 17, 771–782. [Google Scholar] [CrossRef] [Green Version]
- COVID-19 Vaccine Tracker|RAPS. Available online: https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker (accessed on 11 March 2021).
- ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant)-COVISHIELDTM. Available online: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/en/Smpcserum.pdf (accessed on 11 March 2021).
- Vellanki, R. COVID-19 Vaccines: Roll-Out and Updated Studies, Part 2. February 2021. Lawrence, Evans & Co., LLC. Available online: http://www.lawrenceevans.com/wp-content/uploads/2021/02/COVID-19-Vaccine-Series-Part-2-Feb-2021.pdf (accessed on 11 March 2021).
- FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine (accessed on 11 March 2021).
- Frey, S.E.; Reyes, M.R.A.D.L.; Reynales, H.; Bermal, N.N.; Nicolay, U.; Narasimhan, V.; Forleo-Neto, E.; Arora, A.K. Comparison of the safety and immunogenicity of an MF59®-adjuvanted with a non-adjuvanted seasonal influenza vaccine in elderly subjects. Vaccine 2014, 32, 5027–5034. [Google Scholar] [CrossRef]
- Gasparini, R.; Amicizia, D.; Lai, P.L.; Panatto, D. Aflunov®: A prepandemic influenza vaccine. Expert Rev. Vaccines 2012, 11, 145–157. [Google Scholar] [CrossRef] [PubMed]
- A Comparison of Matured Dendritic Cells and Montanide® in Study Subjects with High Risk of Melanoma Recurrence-NCT02334735-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02334735 (accessed on 9 September 2020).
- Alderson, M.R.; McGowan, P.; Baldridge, J.R.; Probst, P. TLR4 agonists as immunomodulatory agents. J. Endotoxin Res. 2006, 12, 313–319. [Google Scholar] [CrossRef] [PubMed]
- DeGregorio, M.; Soe, L.; Wolf, M. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small cell lung cancer (START): A randomized, double-blind, phase III trial. J. Thorac. Dis. 2014, 6, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Wyeth Study To Evaluate a 13-valent Pneumococcal Conjugate Vaccine in Infants-NCT00464945-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00464945?recrs=e&cond=prevnar&draw=2&rank=7 (accessed on 9 September 2020).

| Type of Mechanism | Representative Materials * | Proposed Mechanism | References | |
|---|---|---|---|---|
| Antigen Examples | Adjuvant Examples | |||
| Depot formation at the site of injection | Diphtheria toxoid, Hepatitis A, B | Alum, w/o emulsion, MPLA, biodegradable particles | Slow release, enhanced antigen uptake and presentation by APCs | [19,20,21,22,23,24,25,26,27,28,29] |
| Recruitment of innate immune cell | Diphtheria toxoid, Hepatitis A, HBsAg, HPV | Alum, MF59, w/o emulsion, CpG-ODN, particulate adjuvants | Upregulation of CTK and CMK, cellular recruitment at the site of injection | [19,20,22,23,24,25,26,27,28,30,31] |
| Antigen presentation/targeting | Diphtheria toxoid, Hepatitis A, HBsAg, HPV | Alum, MF59, w/o emulsion, CpG-ODN, particulate adjuvants (polymer-PLGA) | Targeting antigen to APC, uptake of antigen through PRRs on surface (TLRs and CLRs) and intracellularly (NLRs and RLRs), Dendritic cell activation, MHC class II expression | [19,20,22,24,25,26,27,29,30] |
| Activation of inflammasomes | Diphtheria toxoid | Alum, LPS, particulate adjuvants, DAP, MDP, MF59 | Activation of PRRs-NLRs (NODs) and MHC II transactivator | [19,20,22,23,24,25,26,27,28,32,33] |
| Activation and maturation of APCs | Hepatitis A vaccines, influenza vaccine | LPS, liposomes, DOTAP, CpG-ODN, MF59, AS04, α-GAL, TDM, TDB | Maturation of DC’s-upregulation of CD40, CD54, CD80, CD83, CD86 and MHC class II molecules | [19,20,21,23,24,25,26,27,34,35] |
| Immunomodulation/CTL induction | Hepatitis A vaccines, influenza vaccine | LPS, liposomes, DOTAP, CpG-ODN, MF59, AS04, α-GAL, TDM, TDB | enhanced ability of APCs to induce T lymphocyte activation and differentiation, B cell (Humoral) and CD8+ cell responses (adaptive) immunity | [19,20,23,24,25,26,27,36,37,38,39] |
| Product | Application | Adjuvants Used | Approval Year, Company, Status of Research | Ref |
|---|---|---|---|---|
| Viral Vectored Vaccines | ||||
| ACAM2000 | Smallpox | MVA-BN | 2007, Sanofi Pasteur Biologics Co., Cambridge, MA, USA | [31] |
| Chimpanzee adenovirus vector (ChAdOx1) | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Coronavirus disease (COVID-19) | Chimpanzee Adenoviral vector | 2020, University of Oxford in collaboration with AstraZeneca, Cambridge, UK | [59] |
| Sputnik V (Gam-Covid-Vac) | SARS-CoV-2, COVID-19 | Replication-deficient Ad types 5 and 26 vectors | 2020, Gamaleya Research Insitute, Acellena Contract Drug Research and Development, Moscow, Russia | [188] |
| COVISHIELD™ (ChAdOx1) | SARS-CoV-2, COVID-19 | Chimpanzee Adenoviral vector | 2020, Serum Institute of India Pvt. Ltd., Pune, Maharashtra, India | [189] |
| Convidicea (Ad5nCoV) | SARS-CoV-2, COVID-19 | Recombinant Adenoviral vector, Ad5 | 2020, CanSino Biologics, Tianjin China (approved for use in Mexico, China) | [190] |
| Janssen COVID‑19 Vaccine (Ad26) | SARS-CoV-2, COVID-19 | Adenoviral vector, Ad 26 | 2021, Janssen Biotech, Inc., Horsham, PA, USA (Emergency use authorization by US FDA) | [191] |
| Virus Like Particles | ||||
| Recombivax HB® | Hepatitis B Virus (HBV) | Amorphous aluminum hydroxyphosphate sulfate | 1986, Merck and Co. Inc., Kenilworth, NJ, USA | [75] |
| Engerix-B | HBV | Aluminum hydroxide | 1989, Glaxo Smithkline (GSK), Middlesex, UK | [75] |
| Gardasil® | Human papillomavirus (HPV), cervical cancer and genital warts | Hydroxyphosphate sulphate | 2006, Merck and Co. Inc., Kenilworth, NJ, USA | [75] |
| Cervarix | HPV | AS04 (aluminum hydroxide and MPLA) | 2009, Glaxo Smithkline Biologicals SA, Rixensart, Belgium | [75] |
| Hecolin | Hepatitis E Virus (HEV) | Aluminum hydroxide | 2011, Xiamen Innovax Biotech, Xiamen, Fujian, China | [75] |
| Gardasil-9® | HPV | Hydroxyphosphate sulphate | 2014, Merck and Co. Inc., Kenilworth, NJ, USA | [75] |
| Heplisav-B | HBV | 1018 ISS CpG ODN | 2017, Dynavax Technologies Corporation, Emeryville, CA, USA | [75] |
| Sci-B-Vac® | HBV | Aluminum hydroxide | 2020 (under regulatory approval process) VBI Vaccines Inc., Cambridge, MA, USA | [75] |
| Mosquirixs | Malaria and HBV | AS01 (MPL and Quillaja saponaria 21 (QS21)) | 2015, GlaxoSmithKline Biologicals S.A., Rixensart, Belgium | [75] |
| Virosome-based vaccine | ||||
| Epaxal™ | Hepatitis A virus (HAV) | IRIV | 1994, Berna Biotech Ltd., Berne, Switzerland | [73] |
| Inflexal®V | Influenza vaccine | IRIV | 1997, Berna Biotech Ltd., Berne, Switzerland | [73] |
| Invivac® | Influenza vaccine | IRIV | 2004, Solvay Pharmaceuticals B.V., DA Weesp, The Netherlands | [73] |
| NasalFlu® | Influenza vaccine | IRIV | 2001, Berna Biotech Ltd., Berne, Switzerland | [73] |
| Epaxal Junior™ | Novel pandemic A influenza virus (H1N1) | IRIV | 1994, Berna Biotech Ltd., Berne, Switzerland. | [73] |
| Non-viral vectored Vaccines | ||||
| Celtura® | H1N1 | MF59 | 2009, Novartis AG, Basel, Switzerland | [32] |
| Fluad® | Seasonal influenza in infants and young children | MF59 | 1997, Novartis AG, Basel, Switzerland Phase III Trials Completed 2010-11 | [21,192] |
| Aflunov® | Pre-pandemic influenza (H5N1) | MF59 | 2010, Seqirus S.R.L., Monteriggioni, SI, Italy | [193] |
| Montanide | Malaria, HIV, cancer | MF59 | Under clinical trial | [194] |
| FENDRIX | HBV | Aluminum phosphate and MPLA | 2005, GlaxoSmithKline Biologicals., Rixensart, Belgium | [176,195] |
| Stimuvax® | Lung, breast, prostate and colorectal cancer | Liposome, MPLA | Merck KGaA, Darmstadt, Germany, Phase III Clinical Trial Completed | [196] |
| mRNA-1273 | COVID-19 | Liposome | 2020, Moderna, Cambridge, MA, USA | [54] |
| BNT162b2 | COVID-19 | Liposome | 2020, Pfizer, New York, NY, USA and BioNTech, Mainz, Rhineland-Palatinate, Germany | [122] |
| Prevnar® | Invasive Pneumococcal disease | Aluminum phosphate | 2000, Wyeth Pharmaceuuticals, Madison, NJ, USA | [197] |
| Menactra® | Meningococcal disease | Aluminum | 2005, Sanofi Pasteur, Lyon, France | [158] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkar, K.C.; Patil, S.M.; Chavhan, S.S.; Kaneko, K.; Sawant, K.K.; Kunda, N.K.; Saleem, I.Y. An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery. Pharmaceutics 2021, 13, 455. https://doi.org/10.3390/pharmaceutics13040455
Petkar KC, Patil SM, Chavhan SS, Kaneko K, Sawant KK, Kunda NK, Saleem IY. An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery. Pharmaceutics. 2021; 13(4):455. https://doi.org/10.3390/pharmaceutics13040455
Chicago/Turabian StylePetkar, Kailash C., Suyash M. Patil, Sandip S. Chavhan, Kan Kaneko, Krutika K. Sawant, Nitesh K. Kunda, and Imran Y. Saleem. 2021. "An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery" Pharmaceutics 13, no. 4: 455. https://doi.org/10.3390/pharmaceutics13040455
APA StylePetkar, K. C., Patil, S. M., Chavhan, S. S., Kaneko, K., Sawant, K. K., Kunda, N. K., & Saleem, I. Y. (2021). An Overview of Nanocarrier-Based Adjuvants for Vaccine Delivery. Pharmaceutics, 13(4), 455. https://doi.org/10.3390/pharmaceutics13040455