Performance of Choline Geranate Deep Eutectic Solvent as Transdermal Permeation Enhancer: An In Vitro Skin Histological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cell Lineages
2.1.2. Porcine Ear Skin
2.1.3. Chemicals
2.2. Experimental Procedures
2.2.1. Synthesis and Characterization of DES
Synthesis of Choline Geranate Deep Eutectic Solvent (CG-DES)
Assessment of the Cytotoxicity Potential of Plain CG-DES via Mitochondrial Activity (MTT) Assay
Assessment of the Genotoxicity Potential of Plain CG-DES, via the Comet™ Assay
2.2.2. The Effect of DES on Skin Permeability
Porcine Ear Skin Preparation
Preparation of DES Suspensions
In Vitro Skin Permeation Testing
Skin Conservation for Histological Preparations
2.2.3. Preparation of Porcine Ear Skin Histological Cuts
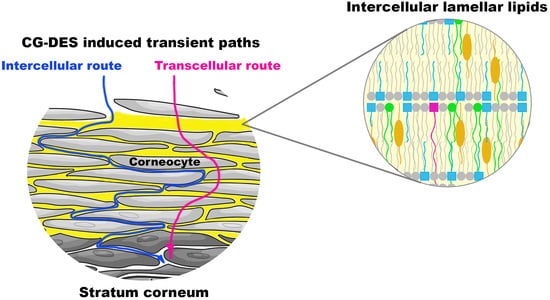
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neupane, R.; Boddu, S.H.S.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers—Chemical Methods in Penetration Enhancement—Drug Manipulation Strategies and Vehicle Effects; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-45013-0. [Google Scholar]
- Jung, E.C.; Maibach, H.I. Animal models for percutaneous absorption. J. Appl. Toxicol. 2015, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.S.; Morishita, M.; Onuki, Y.; Takayama, K. Current challenges in non-invasive insulin delivery systems: A comparative review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. Int. J. Pharm. Sci. Res. 2016, 7, 2274–2290. [Google Scholar] [CrossRef]
- Lassmann-Vague, V.; Raccah, D. Alternatives routes of insulin delivery. Diabetes Metab. 2006, 32, 513–522. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Gooris, G.S.; Groen, D.; Barlow, D.J.; Lawrence, M.J.; Demé, B.; Bouwstra, J.A. Stratum corneum lipid matrix: Location of acyl ceramide and cholesterol in the unit cell of the long periodicity phase. Biochim. Biophys. Acta 2016, 1858, 1926–1934. [Google Scholar] [CrossRef] [Green Version]
- Kessner, D.; Kiselev, M.; Dante, S.; Hauß, T.; Lersch, P.; Wartewig, S.; Neubert, R.H.H. Arrangement of ceramide [EOS] in a stratum corneum lipid model matrix: New aspects revealed by neutron diffraction studies. Eur. Biophys. J. 2008, 37, 989–999. [Google Scholar] [CrossRef]
- Schröter, A.; Kessner, D.; Kiselev, M.A.; Hauß, T.; Dante, S.; Neubert, R.H.H. Basic Nanostructure of Stratum Corneum Lipid Matrices Based on Ceramides [EOS] and [AP]: A Neutron Diffraction Study. Biophys. J. 2009, 97, 1104–1114. [Google Scholar] [CrossRef] [Green Version]
- Hinder, A.; Schmelzer, C.E.H.; Rawlings, A.V.; Neubert, R.H.H. Investigation of the Molecular Structure of the Human Stratum Corneum Ceramides [NP] and [EOS] by Mass Spectrometry. Skin Pharmacol. Physiol. 2011, 24, 127–135. [Google Scholar] [CrossRef]
- Ochalek, M. Barrier Properties of Stratum Corneum Lipid Model Membranes Based on Ceramide [AP] and [EOS]. Ph.D. Thesis, Martin-Luther Universität, Halle, Germany, 2012. [Google Scholar]
- Das, C.; Olmsted, P.D. The physics of stratum corneum lipid membranes. Phil. Trans. R. Soc. A 2016, 374, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Abd, E.; Yousuf, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Z.; Quan, Y.S.; Zang, L.; Jin, M.N.; Kamiyama, F.; Katsumi, H.; Yamamoto, A.; Tsutsumi, S. Transdermal delivery of insulin using trypsin as a biochemical enhancer. Biol. Pharm. Bull. 2008, 31, 1574–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monti, D.; Egiziano, E.; Burgalassi, S.; Chetoni, P.; Chiappe, C.; Sanzone, A.; Tampucci, S. Ionic liquids as potential enhancers for transdermal drug delivery. Int. J. Pharm. 2017, 516, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Agrawal, M.; Chaudhary, S.; Tirkey, V.; Dhwaj, A.; Mishra, N. Review article on permeation enhancers: A major breakthrough in drug delivery technology. Int. J. Pharm. Sci. Res. 2017, 8, 1001–1011. [Google Scholar]
- Jorge, L.R.; Harada, L.K.; Silva, E.C.; Campos, W.F.; Moreli, F.C.; Shimamoto, G.; Pereira, J.F.B.; Oliveira Jr., J. M.; Tubino, M.; Vila, M.M.D.C.; et al. Non-invasive Transdermal Delivery of Human Insulin Using Ionic Liquids: In vitro Studies. Front. Pharmacol. 2020, 11, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, W.F.; Silva, E.C.; Oliveira, T.J.; Oliveira, J.M., Jr.; Tubino, M.; Pereira, C.; Vila, M.M.D.C.; Balcão, V.M. Transdermal permeation of bacteriophage particles by choline oleate: Potential for treatment of soft-tissue infections. Future Microbiol. 2020, 15. [Google Scholar] [CrossRef]
- Silva, E.C.; Oliveira, T.J.; Moreli, F.C.; Harada, L.K.; Vila, M.M.D.C.; Balcão, V.M. Newly isolated lytic bacteriophages for Staphylococcus intermedius, structurally and functionally stabilized in a hydroxyethylcellulose gel containing choline geranate: Potential for transdermal permeation in veterinary phage therapy. Res. Vet. Sci. 2020. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Mota, J.P.; Rosado, C.; Almeida, T.S. Applicability of Ionic Liquids in Topical Drug Delivery Systems: A Mini Review. J. Pharmacol. Clin. Res. 2018, 4, 555649. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Baby, A.R.; Araújo, M.E.M.; Fernandes, A.S.; Costa, J.G.; de Almeida, T.S. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Harada, L.K.; Pereira, J.F.B.; Campos, W.F.; Silva, E.C.; Moutinho, C.G.; Vila, M.M.D.C.; Oliveira, J.M.; Teixeira, J.A.; Balcão, V.M.; Tubino, M. Insights into protein-ionic liquid interactions aiming at macromolecule delivery systems. J. Braz. Chem. Soc. 2018, 29, 1983–1998. [Google Scholar] [CrossRef]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Takemori, S.; Tahara, Y. Lactic Acid Permeation through Deep Eutectic Solvents-Based Polymer Inclusion Membranes. Membranes 2020, 10, 244. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Banerjee, A.; Apte, S.; Kern, T.L.; Jones, M.R.; Sesto, R.E.; Koppisch, A.T.; Fox, D.T.; Mitragotri, S. Choline and geranate deep eutectic solvent as a broad-spectrum antiseptic agent for preventive and therapeutic applications. Adv. Healthc. Matherials 2016, 5, 1282–1289. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; Del Sesto, R.E.; Koppisch, A.T.; et al. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todo, H. Transdermal Permeation of Drugs in Various Animal Species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, L.K.H.; Favaro, L.I.L.; Rios, A.C.; Silva, E.C.; Silva, W.F.; Stigliani, T.P.; Guilger, M.; Lima, R.; Oliveira, J.M.; Aranha, N.; et al. Sericin from Bombyx mori cocoons. Part I: Extraction and physicochemical-biological characterization for biopharmaceutical applications. Process Biochem. 2017, 61, 163–177. [Google Scholar] [CrossRef]
- Salerno, C.; Carlucci, A.M.; Bregni, C. Study of in vitro drug release and percutaneous absorption of fluconazole from topical dosage forms. AAPS PharmSciTech 2010, 11, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Sato, K. The presence of food-derived collagen peptides in human body-structure and biological activity. Food Funct. 2017, 8, 4325–4330. [Google Scholar] [CrossRef]
- Wildfeuer, A.; Faergemann, J.; Laufen, H.; Pfaff, G.; Zimmermann, T.; Seidl, H.P.; Lach, P. Bioavailability of fluconazole in the skin after oral medication. Mycoses 1994, 37, 127–130. [Google Scholar] [CrossRef]
- Sedlák, E.; Stagg, L.; Wittung-Stafshede, P. Effect of Hofmeister ions on protein thermal stability: Roles of ion hydration and peptide groups? Arch. Biochem. Biophys. 2008, 479, 69–73. [Google Scholar] [CrossRef]
- Alturkistani, H.A.; Tashkandi, F.M.; Mohammedsaleh, Z.M. Histological Stains: A Literature Review and Case Study. Glob. J. Health Sci. 2016, 8, 72–79. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Cook, H.C.; Turner, D.R. Manual of Histological Techniques and Their Diagnostic Application, 2nd ed.; Churchill Livingstone: London, UK, 1994; ISBN 978-0443045349. [Google Scholar]
- Chemical, C. A Role for ω-Hydroxy VLCFA Ceramides in the Skin’s Lipid Matrix. Available online: https://www.caymanchem.com/news/eos (accessed on 15 January 2021).
- Schmitt, T. Investigation of the Influence of Asymmetric Long Chain Ceramides [NP] and [AP] on Stratum Corneum Lipid Matrix Architecture with Neutron Diffraction. Ph.D. Thesis, Ludwig-Maximilians—University of Munich, Munich, Germany, 2018. [Google Scholar]
- Dutta, S.; Chawla, S.; Kumar, S. Psoriasis: A review of existing therapies and recent advances in treatment. J. Ration. Pharmacother. Res. 2018, 1, 1–22. [Google Scholar]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; SchäkeL, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H.; Bolla, S.R. A novel self-nanoemulsifying drug delivery system for curcumin used in the treatment of wound healing and inflammation. 3 Biotech. 2019, 9, 360. [Google Scholar] [CrossRef]
- Nardo, V.; Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Barygina, V.; Lotti, J.; Daaboul, F.; Lotti, T. Use of curcumin in psoriasis. Maced. J. Med. Sci. 2018, 6, 218–220. [Google Scholar] [CrossRef] [Green Version]
- Vigato, A.A.; Querobino, S.M.; Faria, N.C.; Candidi, A.C.B.B.; Magalhães, L.G.; Cereda, C.M.S.; Tófoli, G.R.; Campos, E.V.R.; Machado, I.P.; Fraceto, L.F.; et al. Physico-chemical characterization and biopharmaceutical evaluation of lipid-poloxamer-based organogels for curcumin skin delivery. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Peram, M.R.; Jalalpure, S.; Kumbar, V.; Patil, S.; Joshi, S.; Bhat, K.; Diwan, P. Factorial design based curcumin ethosomal nanocarriers for the skin cancer delivery: In vitro evaluation. J. Liposome Res. 2019, 29, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1471–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruela, A.L.M.; Perissinato, A.G.; de Sousa Lino, M.E.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef] [Green Version]
- Chantasart, D.; Kevin, S.K. Structure enhancement relationship of chemical penetration enhancers in drug transport across the stratum corneum. Pharmaceutics 2012, 4, 71–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, R.; Chowdhury, R.; Wakabayashi, R.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic liquid-in-oil microemulsions prepared with biocompatible choline carboxylic acids for improving the transdermal delivery of a sparingly soluble drug. Pharmaceutics 2020, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscariol, R.; Caetano, É.A.; Silva, E.C.; Oliveira, T.J.; Rosa-Castro, R.M.; Vila, M.M.D.C.; Balcão, V.M. Performance of Choline Geranate Deep Eutectic Solvent as Transdermal Permeation Enhancer: An In Vitro Skin Histological Study. Pharmaceutics 2021, 13, 540. https://doi.org/10.3390/pharmaceutics13040540
Boscariol R, Caetano ÉA, Silva EC, Oliveira TJ, Rosa-Castro RM, Vila MMDC, Balcão VM. Performance of Choline Geranate Deep Eutectic Solvent as Transdermal Permeation Enhancer: An In Vitro Skin Histological Study. Pharmaceutics. 2021; 13(4):540. https://doi.org/10.3390/pharmaceutics13040540
Chicago/Turabian StyleBoscariol, Rodrigo, Érika A. Caetano, Erica C. Silva, Thais J. Oliveira, Raquel M. Rosa-Castro, Marta M. D. C. Vila, and Victor M. Balcão. 2021. "Performance of Choline Geranate Deep Eutectic Solvent as Transdermal Permeation Enhancer: An In Vitro Skin Histological Study" Pharmaceutics 13, no. 4: 540. https://doi.org/10.3390/pharmaceutics13040540
APA StyleBoscariol, R., Caetano, É. A., Silva, E. C., Oliveira, T. J., Rosa-Castro, R. M., Vila, M. M. D. C., & Balcão, V. M. (2021). Performance of Choline Geranate Deep Eutectic Solvent as Transdermal Permeation Enhancer: An In Vitro Skin Histological Study. Pharmaceutics, 13(4), 540. https://doi.org/10.3390/pharmaceutics13040540