Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity
Abstract
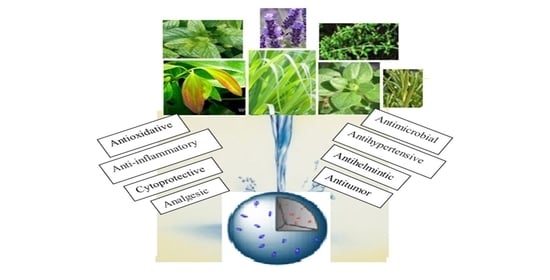
:1. Introduction

2. Polymeric Carriers for EOs Encapsulation
2.1. Natural Polymers
2.1.1. Alginate
2.1.2. Cellulose Derivatives
2.1.3. Chitosan
2.1.4. Starch and Maltodextrin Based Systems
2.1.5. Whey Protein
2.1.6. Silk Fibroin
2.1.7. Gelatin
2.2. Synthetic Macromolecular Structures for EOs Encapsulation
3. Machine Learning Analysis in Support of the EOs Use
4. Opportunities, Challenges, and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Term | Abbreviation |
| 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) | ABTS |
| 2,2-diphenyl-1-picrylhydrazyl | DPPH |
| Amphibious Air Traffic Control Center | AATCC100 |
| Artificial neural network | ANN |
| Chitosan | CS |
| Convolutional Neural Networks | CNN |
| Encapsulation efficiency | EE |
| Essential oil | EO |
| Essential Oil Reduction and Optimization Tool | EOROT |
| Field Emission Scanning Electron Microscopy | FESEM |
| Fumonisin B1 | FB1 |
| Gas chromatography–mass spectrometry | GC-MS |
| Genetic algorithm and multiple linear regression | GA-MLR |
| Genetic algorithm and partial least square | GA-PLS |
| Genetic algorithm and kernel PLS | GA-KPLS |
| High-pressure homogenization | HPH |
| Levenberg Marquardt artificial neural network | L-M ANN |
| Loading capacity | LC |
| Machine learning | ML |
| Maltodextrin | MD |
| Minimum inhibitory concentrations | MIC |
| Multiclass Neural Network | MNN |
| Nanocapsules | NCs |
| Nanoparticles | NP |
| National Committee for Clinical Laboratory Standards | NCCLS |
| Pentasodium tripolyphosphate | TPP |
| Phase inversion temperature | PIT |
| Poly(acrylonitrile) | PAN |
| Poly(methyl methacrylate) | PMMA |
| Poly(vinylidene fluoride) | PVDF |
| Polycaprolactone | PCL |
| Polyethylene oxide | PEO |
| Polylactic acid | PLA |
| Polyvinyl alcohol | PVA |
| Polyvinyl pyrrolidone | PVP |
| Quantitative structure activity relationship | QSAR |
| Quorum sense inhibitor | QSI |
| Retention index | RI |
| Scanning Electron Microscopy | SEM |
| Segment average mass spectra | SAMS |
| Self-nanoemulsifying drug delivery system | SNEDDS |
| Sodium alginate | SA |
| Sodium hexametaphosphate | HMP |
| Soy protein isolate | SPI |
| Total chromatogram average mass spectra | TCAMS |
| US Food and Drug Administration | FDA |
| Whey protein | WP |
| β-cyclodextrin | CD |
References
- Global Essential Oils Market Outlook. Available online: https://www.expertmarketresearch.com/reports/essential-oils-market (accessed on 5 March 2020).
- Essential Oils Market Size, Share & Trends Analysis Report by Application. Available online: https://www.grandviewresearch.com/industry-analysis/essential-oils-market (accessed on 5 March 2020).
- Stea, S.; Beraudi, A.; De Pasquale, D. Essential oils for complementary treatment of surgical patients: State of the art. Evid. Based Complement. Alternat. Med. 2014, 2014, 726341. [Google Scholar] [CrossRef] [Green Version]
- El-Hosseiny, L.; El-Shenawy, M.; Haroun, M.; Abdullah, F. Comparative Evaluation of the Inhibitory Effect of Some Essential Oils with Antibiotics against Pseudomonas aeruginosa. Int. J. Antibiot. 2014, 2014, 586252. [Google Scholar] [CrossRef] [Green Version]
- Kunihiro, K.; Myoda, T.; Tajima, N.; Gotoh, K.; Kaneshima, T.; Someya, T.; Toeda, K.; Fujimori, T.; Nishizawa, M. Volatile Components of the Essential Oil of Artemisia montana and Their Sedative Effects. J. Oleo. Sci. 2017, 66, 843–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doran, A.L.; Morden, W.E.; Dunn, K.; Edwards-Jones, V. Vapour-phase activities of essential oils against antibiotic sensitive and resistant bacteria including MRSA. Lett. Appl. Microbiol. 2009, 48, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Jones, V.; Buck, R.; Shawcross, S.G.; Dawson, M.M.; Dunn, K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns 2004, 30, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Padalia, H.; Moteriya, P.; Baravalia, Y.; Chanda, S. Antimicrobial and synergistic effects of some essential oils to fight against microbial pathogens: A review. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs, 1st ed.; Méndez-Vilas, A., Ed.; Formatex Research Center S.L: Malaga, Spain, 2015; pp. 34–45. [Google Scholar]
- Duarte, A.E.; de Menezes, I.R.; Bezerra Morais Braga, M.F.; Leite, N.F.; Barros, L.M.; Waczuk, E.P.; Pessoa da Silva, M.A.; Boligon, A.; Teixeira Rocha, J.B.; Souza, D.O.; et al. Antimicrobial Activity and Modulatory Effect of Essential Oil from the Leaf of Rhaphiodon echinus (Nees & Mart) Schauer on Some Antimicrobial Drugs. Molecules 2016, 21, 743. [Google Scholar] [CrossRef]
- Karpanen, T.J.; Worthington, T.; Hendry, E.R.; Conway, B.R.; Lambert, P.A. Antimicrobial efficacy of chlorhexidine digluconate alone and in combination with eucalyptus oil, tea tree oil and thymol against planktonic and biofilm cultures of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2008, 62, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.J.; Conway, B.R.; Worthington, T.; Hilton, A.C.; Elliott, T.S.; Lambert, P.A. Enhanced chlorhexidine skin penetration with eucalyptus oil. BMC Infect. Dis. 2010, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Rosato, A.; Vitali, C.; De Laurentis, N.; Armenise, D.; Antonietta Milillo, M. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine 2007, 14, 727–732. [Google Scholar] [CrossRef]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.A.; Rodriguez, D.; Beaumont, C.; Allred, K. The Effects of Various Essential Oils on Epilepsy and Acute Seizure: A Systematic Review. Evid. Based Complement. Alternat. Med. 2019, 2019, 6216745. [Google Scholar] [CrossRef] [Green Version]
- Vaillancourt, K.; LeBel, G.; Yi, L.; Grenier, D. In vitro antibacterial activity of plant essential oils against Staphylococcus hyicus and Staphylococcus aureus, the causative agents of exudative epidermitis in pigs. Arch. Microbiol. 2018, 200, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid. Based Complement. Alternat. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dornic, N.; Ficheux, A.S.; Roudot, A.C. Qualitative and quantitative composition of essential oils: A literature-based database on contact allergens used for safety assessment. Regul. Toxicol. Pharmacol. 2016, 80, 226–232. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [Green Version]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid. Based Complement. Alternat. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [Green Version]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef] [Green Version]
- Bernadelli Sousa Silva, N.; de Andrade Marques, L.; von Dolinger de Brito Röder, D. Antibiofilm Activity of Natural Products: Promising Strategies for Combating Microbial Biofilms. Ann. Public Health Rep. 2020, 4, 92–99. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Xia, Y.-X.; He, Z.-D.; Zhang, H.-J. A Review of Natural Products with Anti-biofilm Activity. Curr. Org. Chem. 2018, 22, 789–817. [Google Scholar] [CrossRef]
- Savoia, D. Plant-derived antimicrobial compounds: Alternatives to antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [Green Version]
- Tobouti, P.L.; de Andrade Martins, T.C.; Pereira, T.J.; Mussi, M.C.M. Antimicrobial activity of copaiba oil: A review and a call for further research. Biomed. Pharmacother. 2017, 94, 93–99. [Google Scholar] [CrossRef]
- Araújo Nogueira, J.W.; Albuquerque Costa, R.; Turini da Cunha, M.; Arruda Cavalcante, T.T. Antibiofilm activity of natural substances derived from plants. Afr. J. Microbiol. Res. 2017, 11, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Juárez, I.; Maeda, T.; Mandujano-Tinoco, E.A.; Tomás, M.; Pérez-Eretza, B.; García-Contreras, S.J.; Wood, T.K.; García-Contreras, R. Role of quorum sensing in bacterial infections. World J. Clin. Cases 2015, 3, 575–598. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New Perspectives on the Use of Phytochemicals as an Emergent Strategy to Control Bacterial Infections Including Biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Borges, A.; Saavedra, M.J.; Simões, M. Insights on antimicrobial resistance, biofilms and the use of phytochemicals as new antimicrobial agents. Curr. Med. Chem. 2015, 22, 2590–2614. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.H.N.; Casey, E. A Review of Nanomaterials and Technologies for Enhancing the Antibiofilm Activity of Natural Products and Phytochemicals. ACS Appl. Nano Mater. 2020, 3, 8537–8556. [Google Scholar] [CrossRef]
- Franklyne, J.S.; Mukherjee, A.; Chandrasekaran, N. Essential oil micro- and nanoemulsions: Promising roles in antimicrobial therapy targeting human pathogens. Lett. Appl. Microbiol. 2016, 63, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Knapp, H.R.; Melly, M.A. Bactericidal effects of polyunsaturated fatty acids. J. Infect. Dis. 1986, 154, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Karlsson, S.M.; Steingrímsson, O.; Thormar, H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 1998, 42, 2290–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.; Thormar, H. Killing of Gram-positive cocci by fatty acids and monoglycerides. Apmis 2001, 109, 670–678. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef] [Green Version]
- Orchard, A.; Kamatou, G.; Viljoen, A.M.; Patel, N.; Mawela, P.; van Vuuren, S.F. The Influence of Carrier Oils on the Antimicrobial Activity and Cytotoxicity of Essential Oils. Evid. Based Complement. Alternat. Med. 2019, 2019, 6981305. [Google Scholar] [CrossRef] [Green Version]
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Alternat. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef] [Green Version]
- Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Encapsulation of Flavours and Fragrances into Polymeric Capsules and Cyclodextrins Inclusion Complexes: An Update. Molecules 2020, 25, 5878. [Google Scholar] [CrossRef] [PubMed]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of phytochemicals with cyclodextrin derivatives—An insight. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef]
- Basavegowda, N.; Patra, J.K.; Baek, K.H. Essential Oils and Mono/bi/tri-Metallic Nanocomposites as Alternative Sources of Antimicrobial Agents to Combat Multidrug-Resistant Pathogenic Microorganisms: An Overview. Molecules 2020, 25, 1058. [Google Scholar] [CrossRef] [Green Version]
- Luna, E.C.; Luna, I.S.; Scotti, L.; Monteiro, A.F.M.; Scotti, M.T.; de Moura, R.O.; de Araújo, R.S.A.; Monteiro, K.L.C.; de Aquino, T.M.; Ribeiro, F.F.; et al. Active Essential Oils and Their Components in Use against Neglected Diseases and Arboviruses. Oxid. Med. Cell Longev. 2019, 2019, 6587150. [Google Scholar] [CrossRef] [Green Version]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles-A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Georg Jensen, M.; Kristensen, M.; Astrup, A. Effect of alginate supplementation on weight loss in obese subjects completing a 12-wk energy-restricted diet: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Volić, M.; Pajić-Lijaković, I.; Djordjević, V.; Knežević-Jugović, Z.; Pećinar, I.; Stevanović-Dajić, Z.; Veljović, Đ.; Hadnadjev, M.; Bugarski, B. Alginate/soy protein system for essential oil encapsulation with intestinal delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef]
- Fernandes Nassar, S.; Dombre, C.; Gastaldi, E.; Touchaleaume, F.; Chalier, P. Soy protein isolate nanocomposite film enriched with eugenol, an antimicrobial agent: Interactions and properties. J. Appl. Polym. Sci. 2018, 135, 45941. [Google Scholar] [CrossRef]
- Barboza, J.N.; da Silva Maia Bezerra Filho, C.; Silva, R.O.; Medeiros, J.V.R.; de Sousa, D.P. An Overview on the Anti-inflammatory Potential and Antioxidant Profile of Eugenol. Oxid. Med. Cell Longev. 2018, 2018, 3957262. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef]
- Martins, E.; Renard, D.; Davy, J.; Marquis, M.; Poncelet, D. Oil core microcapsules by inverse gelation technique. J. Microencapsul. 2015, 32, 86–95. [Google Scholar] [CrossRef]
- Martins, E.; Renard, D.; Adiwijaya, Z.; Karaoglan, E.; Poncelet, D. Oil encapsulation in core-shell alginate capsules by inverse gelation. I: Dripping methodology. J. Microencapsul. 2017, 34, 82–90. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation in core-shell alginate capsules by inverse gelation II: Comparison between dripping techniques using W/O or O/W emulsions. J. Microencapsul. 2017, 34, 522–534. [Google Scholar] [CrossRef]
- Kokina, M.; Salević, A.; Kalušević, A.; Lević, S.; Pantić, M.; Pljevljakušić, D.; Šavikin, K.; Shamtsyan, M.; Nikšić, M.; Nedović, V. Characterization, Antioxidant and Antibacterial Activity of Essential Oils and Their Encapsulation into Biodegradable Material Followed by Freeze Drying. Food Technol. Biotechnol. 2019, 57, 282–289. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, E.F.; Paula, H.C.; de Paula, R.C. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B Biointerfaces 2014, 113, 146–151. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ansari, M.T.; Sami, F.; Bera, H.; Hasnain, M.S. Chapter 11—Cashew gum in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 263–283. [Google Scholar]
- Pitombeira, N.A.O.; Veras Neto, J.G.; Silva, D.A.; Feitosa, J.P.A.; Paula, H.C.B.; de Paula, R.C.M. Self-assembled nanoparticles of acetylated cashew gum: Characterization and evaluation as potential drug carrier. Carbohydr. Polym. 2015, 117, 610–615. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; de Souza, F.R.L.; Bezerra, J.; Oliveira, C.; Nadvorny, D.; de La Roca Soares, M.F.; Nunes, L.C.C.; Silva-Filho, E.C.; Veiga, F.; Soares Sobrinho, J.L. Gums’ based delivery systems: Review on cashew gum and its derivatives. Carbohydr. Polym. 2016, 147, 188–200. [Google Scholar] [CrossRef]
- Kumar, A.; Moin, A.; Shruthi, R.; Ahmed, A.; Shivakumar, H.G. Cashew gum a versatile hydrophyllic polymer: A review. Curr. Drug Ther. 2012, 7, 2–12. [Google Scholar] [CrossRef]
- Castro Porto, B.; Cristianini, M. Evaluation of cashew tree gum (Anacardium occidentale L.) emulsifying properties. LWT 2014, 59, 1325–1331. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate-lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Đorđević, V.; Karlović, S.; Pavlović, V.; Komes, D.; Ježek, D.; Bugarski, B.; Nedović, V. Protein-reinforced and chitosan-pectin coated alginate microparticles for delivery of flavan-3-ol antioxidants and caffeine from green tea extract. Food Hydrocoll. 2015, 51, 361–374. [Google Scholar] [CrossRef]
- Kayaci, F.; Uyar, T. Encapsulation of vanillin/cyclodextrin inclusion complex in electrospun polyvinyl alcohol (PVA) nanowebs: Prolonged shelf-life and high temperature stability of vanillin. Food Chem. 2012, 133, 641–649. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Thakur, R.A.; Florek, C.A.; Kohn, J.; Michniak, B.B. Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int. J. Pharm. 2008, 364, 87–93. [Google Scholar] [CrossRef]
- El-Aassar, M.R. Functionalized electrospun nanofibers from poly (AN-co-MMA) for enzyme immobilization. J. Mol. Catal. B Enzym. 2013, 85–86, 140–148. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, G.; Hu, H.; Yang, J.; Qian, B.; Ling, Z.; Qiu, J. Ultrasound-assisted preparation of electrospun carbon nanofiber/graphene composite electrode for supercapacitors. J. Power Source 2013, 243, 350–353. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Ataei, S.; Azari, P.; Hassan, A.; Pingguan-Murphy, B.; Yahya, R.; Muhamad, F. Essential Oils-Loaded Electrospun Biopolymers: A Future Perspective for Active Food Packaging. Adv. Polym. Technol. 2020, 2020, 9040535. [Google Scholar] [CrossRef]
- Rafiq, M.; Hussain, T.; Abid, S.; Nazir, A.; Masood, R. Development of sodium alginate/PVA antibacterial nanofibers by the incorporation of essential oils. Mater. Res. Express 2018, 5, 035007. [Google Scholar] [CrossRef]
- Sibanda, W.; Pillay, V.; Danckwerts, M.P.; Viljoen, A.M.; van Vuuren, S.; Khan, R.A. Experimental design for the formulation and optimization of novel cross-linked oilispheres developed for in vitro site-specific release of Mentha piperita oil. AAPS PharmSciTech 2004, 5, E18. [Google Scholar] [CrossRef] [Green Version]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha spp. Essential Oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef] [Green Version]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Abu-Zaitoun, S.Y.; Khasati, A.I.; Kalbouneh, S.R. Biological Properties and Bioactive Components of Mentha spicata L. Essential Oil: Focus on Potential Benefits in the Treatment of Obesity, Alzheimer’s Disease, Dermatophytosis, and Drug-Resistant Infections. Evid. Based Complement. Altern. Med. 2019, 2019, 3834265. [Google Scholar] [CrossRef] [Green Version]
- Liakos, I.L.; D’Autilia, F.; Garzoni, A.; Bonferoni, C.; Scarpellini, A.; Brunetti, V.; Carzino, R.; Bianchini, P.; Pompa, P.P.; Athanassiou, A. All natural cellulose acetate-Lemongrass essential oil antimicrobial nanocapsules. Int. J. Pharm. 2016, 510, 508–515. [Google Scholar] [CrossRef]
- Liakos, I.L.; Iordache, F.; Carzino, R.; Scarpellini, A.; Oneto, M.; Bianchini, P.; Grumezescu, A.M.; Holban, A.M. Cellulose acetate—essential oil nanocapsules with antimicrobial activity for biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 471–479. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Aksentijevic, K.; Stojanovic, D.B.; Ivanovic, J.; Zizovic, I. Application of cellulose acetate for controlled release of thymol. Carbohydr. Polym. 2016, 147, 344–353. [Google Scholar] [CrossRef]
- Wadhwa, G.; Kumar, S.; Mittal, V.; Rao, R. Encapsulation of babchi essential oil into microsponges: Physicochemical properties, cytotoxic evaluation and anti-microbial activity. J. Food Drug Anal. 2019, 27, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mele, E. Electrospinning of Essential Oils. Polymers 2020, 12, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liakos, I.; Rizzello, L.; Hajiali, H.; Brunetti, V.; Carzino, R.; Pompa, P.P.; Athanassiou, A.; Mele, E. Fibrous wound dressings encapsulating essential oils as natural antimicrobial agents. J. Mater. Chem B 2015, 3, 1583–1589. [Google Scholar] [CrossRef]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun Fiber Pads of Cellulose Acetate and Essential Oils with Antimicrobial Activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemzadeh Rahbardar, M.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed. Pharmacother. 2017, 86, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Villalobos, K.L.; Déciga-Campos, M.; Aguilar-Mariscal, H.; González-Trujano, M.E.; Martínez-Salazar, M.F.; Ramírez-Cisneros, M.; Rios, M.Y.; López-Muñoz, F.J. Synergistic antinociceptive interaction of Syzygium aromaticum or Rosmarinus officinalis coadministered with ketorolac in rats. Biomed. Pharmacother. 2017, 94, 858–864. [Google Scholar] [CrossRef]
- Einbond, L.S.; Wu, H.A.; Kashiwazaki, R.; He, K.; Roller, M.; Su, T.; Wang, X.; Goldsberry, S. Carnosic acid inhibits the growth of ER-negative human breast cancer cells and synergizes with curcumin. Fitoterapia 2012, 83, 1160–1168. [Google Scholar] [CrossRef]
- González-Vallinas, M.; Reglero, G.; Ramírez de Molina, A. Rosemary (Rosmarinus officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr. Cancer 2015, 67, 1221–1229. [Google Scholar] [CrossRef]
- Petiwala, S.M.; Johnson, J.J. Diterpenes from rosemary (Rosmarinus officinalis): Defining their potential for anti-cancer activity. Cancer Lett. 2015, 367, 93–102. [Google Scholar] [CrossRef]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharmacother. 2018, 103, 1643–1651. [Google Scholar] [CrossRef]
- Wang, T.; Dou, Y.; Lin, G.; Li, Q.; Nie, J.; Chen, B.; Xie, J.; Su, Z.; Zeng, H.; Chen, J.; et al. The anti-hepatocellular carcinoma effect of Brucea javanica oil in ascitic tumor-bearing mice: The detection of brusatol and its role. Biomed. Pharmacother. 2021, 134, 111122. [Google Scholar] [CrossRef]
- Chen, M.; Chen, R.; Wang, S.; Tan, W.; Hu, Y.; Peng, X.; Wang, Y. Chemical components, pharmacological properties, and nanoparticulate delivery systems of Brucea javanica. Int. J. Nanomed. 2013, 8, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti. Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Echazú, M.I.; Olivetti, C.E.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development and evaluation of thymol-chitosan hydrogels with antimicrobial-antioxidant activity for oral local delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 588–596. [Google Scholar] [CrossRef]
- Mihailovic-Stanojevic, N.; Belscak-Cvitanovic, A.; Grujic-Milanovic, J.; Ivanov, M.; Jovovic, D.; Bugarski, D.; Miloradovic, Z. Antioxidant and antihypertensive activity of extract from Thymus serpyllum L. in experimental hypertension. Plant. Foods Hum. Nutr. 2013, 68, 235–240. [Google Scholar] [CrossRef]
- Trifkovic, K.T.; Milasinovic, N.Z.; Djordjevic, V.B.; Krusic, M.T.; Knezevic-Jugovic, Z.D.; Nedovic, V.A.; Bugarski, B.M. Chitosan microbeads for encapsulation of thyme (Thymus serpyllum L.) polyphenols. Carbohydr. Polym. 2014, 111, 901–907. [Google Scholar] [CrossRef]
- Sotelo-Boyas, M.; Correa-Pacheco, Z.; Bautista-Banos, S.; Gomez, Y.G.Y. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef]
- Fachel, F.N.S.; Medeiros-Neves, B.; Dal Prá, M.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Box-Behnken design optimization of mucoadhesive chitosan-coated nanoemulsions for rosmarinic acid nasal delivery—In vitro studies. Carbohydr. Polym. 2018, 199, 572–582. [Google Scholar] [CrossRef]
- Matshetshe, K.I.; Parani, S.; Manki, S.M.; Oluwafemi, O.S. Preparation, characterization and in vitro release study of beta-cyclodextrin/chitosan nanoparticles loaded Cinnamomum zeylanicum essential oil. Int. J. Biol. Macromol. 2018, 118, 676–682. [Google Scholar] [CrossRef]
- Antunes, J.C.; Tavares, T.D.; Teixeira, M.A.; Teixeira, M.O.; Homem, N.C.; Amorim, M.T.P.; Felgueiras, H.P. Eugenol-Containing Essential Oils Loaded onto Chitosan/Polyvinyl Alcohol Blended Films and Their Ability to Eradicate Staphylococcus aureus or Pseudomonas aeruginosa from Infected Microenvironments. Pharmaceutics 2021, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H.; Imran, M. Encapsulation of Cardamom Essential Oil in Chitosan Nano-composites: In-vitro Efficacy on Antibiotic-Resistant Bacterial Pathogens and Cytotoxicity Studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef] [PubMed]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Barreto da Silva, L.; Camargo, S.B.; Moraes, R.d.A.; Medeiros, C.F.; Jesus, A.d.M.; Evangelista, A.; Villarreal, C.F.; Quintans-Júnior, L.J.; Silva, D.F. Antihypertensive effect of carvacrol is improved after incorporation in β-cyclodextrin as a drug delivery system. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1798–1807. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef]
- Yogalakshmi, B.; Viswanathan, P.; Anuradha, C.V. Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 2010, 268, 204–212. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.D.; Mendonça, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Turasan, H.; Sahin, S.; Sumnu, G. Encapsulation of rosemary essential oil. LWT 2015, 64, 112–119. [Google Scholar] [CrossRef]
- Sotelo-Bautista, M.; Bello-Perez, L.A.; Gonzalez-Soto, R.A.; Yañez-Fernandez, J.; Alvarez-Ramirez, J. OSA-maltodextrin as wall material for encapsulation of essential avocado oil by spray drying. J. Dispers. Sci. Technol. 2020, 41, 235–242. [Google Scholar] [CrossRef]
- Burhan, A.M.; Abdel-Hamid, S.M.; Soliman, M.E.; Sammour, O.A. Optimisation of the microencapsulation of lavender oil by spray drying. J. Microencapsul. 2019, 36, 250–266. [Google Scholar] [CrossRef]
- Veiga, R.D.S.D.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Partheniadis, I.; Vergkizi, S.; Lazari, D.; Reppas, C.; Nikolakakis, I. Formulation, characterization and antimicrobial activity of tablets of essential oil prepared by compression of spray-dried powder. J. Drug Deliv. Sci. Technol. 2019, 50, 226–236. [Google Scholar] [CrossRef]
- Sarkar, P.; Bhunia, A.K.; Yao, Y. Impact of starch-based emulsions on the antibacterial efficacies of nisin and thymol in cantaloupe juice. Food Chem. 2017, 217, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Jelkmann, M.; Leichner, C.; Menzel, C.; Kreb, V.; Bernkop-Schnürch, A. Cationic starch derivatives as mucoadhesive and soluble excipients in drug delivery. Int. J. Pharm. 2019, 570, 118664. [Google Scholar] [CrossRef]
- Biduski, B.; Kringel, D.H.; Colussi, R.; Hackbart, H.; Lim, L.T.; Dias, A.R.G.; Zavareze, E.D.R. Electrosprayed octenyl succinic anhydride starch capsules for rosemary essential oil encapsulation. Int. J. Biol. Macromol. 2019, 132, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.; Ji, N.; Liu, J.; Xiong, L.; Sun, Q. Morphology and Characteristics of Starch Nanoparticles Self-Assembled via a Rapid Ultrasonication Method for Peppermint Oil Encapsulation. J. Agric. Food Chem. 2017, 65, 8363–8373. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of spray drying microencapsulation of almond oil into taro starch spherical aggregates. LWT 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Varona, S.; Rodríguez-Rojo, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M.M. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J. Supercrit. Fluids 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Fang, Y.; Fu, J.; Liu, P.; Cu, B. Morphology and characteristics of 3D nanonetwork porous starch-based nanomaterial via a simple sacrifice template approach for clove essential oil encapsulation. Ind. Crops Prod. 2020, 143, 111939. [Google Scholar] [CrossRef]
- Vilas Dhumal, C.; Pal, K.; Sarkar, P. Synthesis, characterization, and antimicrobial efficacy of composite films from guar gum/sago starch/whey protein isolate loaded with carvacrol, citral and carvacrol-citral mixture. J. Mater. Sci. Mater. Med. 2019, 30, 117. [Google Scholar] [CrossRef]
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; El-Nekeety, A.A.; Hassan, N.S.; Gibriel, A.A.Y.; Abdel-Wahhab, K.G. Encapsulation of cinnamon essential oil in whey protein enhances the protective effect against single or combined sub-chronic toxicity of fumonisin B(1) and/or aflatoxin B(1) in rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 29144–29161. [Google Scholar] [CrossRef]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.Y.; Chuah, Y.J.; Goh, J.C.H.; Li, J.L.; Xu, H. Design and engineering of silk fibroin scaffolds with biomimetic hierarchical structures. Chem. Comm. 2013, 49, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Correia, I.J. Electrospun Polycaprolactone/Aloe Vera_Chitosan Nanofibrous Asymmetric Membranes Aimed for Wound Healing Applications. Polymers 2017, 9, 183. [Google Scholar] [CrossRef]
- Figueira, D.R.; Miguel, S.P.; de Sá, K.D.; Correia, I.J. Production and characterization of polycaprolactone- hyaluronic acid/chitosan- zein electrospun bilayer nanofibrous membrane for tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1100–1110. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef]
- Silva, L.S.; Mar, J.M.; Azevedo, S.G.; Rabelo, M.S.; Bezerra, J.A.; Campelo, P.H.; Machado, M.B.; Trovati, G.; Dos Santos, A.L.; da Fonseca Filho, H.D.; et al. Encapsulation of Piper aduncum and Piper hispidinervum essential oils in gelatin nanoparticles: A possible sustainable control tool of Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. J. Sci. Food Agric. 2019, 99, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Fabre, T.; Schappacher, M.; Bareille, R.; Dupuy, B.; Soum, A.; Bertrand-Barat, J.; Baquey, C. Study of a (trimethylenecarbonate-co-epsilon-caprolactone) polymer—Part 2: In vitro cytocompatibility analysis and in vivo ED1 cell response of a new nerve guide. Biomaterials 2001, 22, 2951–2958. [Google Scholar] [CrossRef]
- Jin, W.-J.; Lee, H.K.; Jeong, E.H.; Park, W.H.; Youk, J.H. Preparation of Polymer Nanofibers Containing Silver Nanoparticles by Using Poly(N-vinylpyrrolidone). Macromol. Rapid Commun. 2005, 26, 1903–1907. [Google Scholar] [CrossRef]
- Karami, Z.; Rezaeian, I.; Zahedi, P.; Abdollahi, M. Preparation and performance evaluations of electrospun poly(ε-caprolactone), poly(lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J. Appl. Polym. Sci. 2013, 129, 756–766. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef]
- Suganya, S.; Senthil Ram, T.; Lakshmi, B.S.; Giridev, V.R. Herbal drug incorporated antibacterial nanofibrous mat fabricated by electrospinning: An excellent matrix for wound dressings. J. Appl. Polym. Sci. 2011, 121, 2893–2899. [Google Scholar] [CrossRef]
- Koushki, P.; Bahrami, S.H.; Ranjbar-Mohammadi, M. Coaxial nanofibers from poly(caprolactone)/poly(vinyl alcohol)/Thyme and their antibacterial properties. J. Ind. Text. 2018, 47, 834–852. [Google Scholar] [CrossRef]
- Ivanovic, J.; Knauer, S.; Fanovich, A.; Milovanovic, S.; Stamenic, M.; Jaeger, P.; Zizovic, I.; Eggers, R. Supercritical CO2 sorption kinetics and thymol impregnation of PCL and PCL-HA. J. Supercrit. Fluids 2016, 107, 486–498. [Google Scholar] [CrossRef]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun Nanofibres Containing Antimicrobial Plant Extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Souza, M.A.; Oliveira, J.E.; Medeiros, E.S.; Glenn, G.M.; Mattoso, L.H. Controlled Release of Linalool Using Nanofibrous Membranes of Poly(lactic acid) Obtained by Electrospinning and Solution Blow Spinning: A Comparative Study. J. Nanosci. Nanotechnol. 2015, 15, 5628–5636. [Google Scholar] [CrossRef] [PubMed]
- Mori, C.L.S.d.O.; dos Passos, N.A.; Oliveira, J.E.; Altoé, T.F.; Mori, F.A.; Mattoso, L.H.C.; Scolforo, J.R.; Tonoli, G.H.D. Nanostructured Polylactic Acid/Candeia Essential Oil Mats Obtained by Electrospinning. J. Nanomater. 2015, 2015, 439253. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React. Funct. Polym. 2017, 117, 106–111. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Marino, A.; Nostro, A. Antimicrobial additives for poly(lactic acid) materials and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 7739–7756. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Nostro, A. Poly(lactic acid)/carvacrol-based materials: Preparation, physicochemical properties, and antimicrobial activity. Appl. Microbiol. Biotechnol. 2020, 104, 1823–1835. [Google Scholar] [CrossRef]
- Dusankova, M.; Pummerova, M.; Sedlarik, V. Microspheres of essential oil in polylactic acid and poly(methyl methacrylate) matrices and their blends. J. Microencapsul. 2019, 36, 305–316. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Kodam, K.M. Encapsulation of therapeutic lavender oil in an electrolyte assisted polyacrylonitrile nanofibres for antibacterial applications. RSC Adv. 2014, 4, 54892–54901. [Google Scholar] [CrossRef]
- Kesici Güler, H.; Cengiz Çallıoğlu, F.; Sesli Çetin, E. Antibacterial PVP/cinnamon essential oil nanofibers by emulsion electrospinning. J. Text. Inst. 2019, 110, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, F.; Shazly, G.A.; Raish, M.; Ahmad, A.; Kalam, M.A.; Ali, N.; Ansari, M.A.; Elosaily, G.M. Biological investigation of a supersaturated self-nanoemulsifying drug delivery system of Piper cubeba essential oil. RSC Adv. 2015, 5, 105206–105217. [Google Scholar] [CrossRef]
- Shakeel, F.; Alam, P.; Anwer, M.K.; Alanazi, S.A.; Alsarra, I.A.; Alqarni, M.H. Wound healing evaluation of self-nanoemulsifying drug delivery system containing Piper cubeba essential oil. 3 Biotech 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Balzamo, G.; Willcock, H.; Ali, J.; Ratcliffe, E.; Mele, E. Bioinspired Poly(vinylidene fluoride) Membranes with Directional Release of Therapeutic Essential Oils. Langmuir 2018, 34, 8652–8660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aouf, A.; Ali, H.; Al-Khalifa, A.R.; Mahmoud, K.F.; Farouk, A. Influence of Nanoencapsulation Using High-Pressure Homogenization on the Volatile Constituents and Anticancer and Antioxidant Activities of Algerian Saccocalyx satureioides Coss. et Durieu. Molecules 2020, 25, 756. [Google Scholar] [CrossRef]
- Fernandes, C.P.; Mascarenhas, M.P.; Zibetti, F.M.; Lima, B.G.; Oliveira, R.P.R.F.; Rocha, L.; Falcão, D.Q. HLB value, an important parameter for the development of essential oil phytopharmaceuticals. Rev. Bras. Farmacogn. 2013, 23, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Borges, R.S.; Lima, E.S.; Keita, H.; Ferreira, I.M.; Fernandes, C.P.; Cruz, R.A.S.; Duarte, J.L.; Velázquez-Moyado, J.; Ortiz, B.L.S.; Castro, A.N.; et al. Anti-inflammatory and antialgic actions of a nanoemulsion of Rosmarinus officinalis L. essential oil and a molecular docking study of its major chemical constituents. Inflammopharmacology 2018, 26, 183–195. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Macsim, A.-M.; Tudorachi, N.; Rosca, I.; Stoica, I.; Tampu, D.; Aflori, M.; Doroftei, F. Synthesis of Poly(Ethylene Brassylate-Co-squaric Acid) as Potential Essential Oil Carrier. Pharmaceutics 2021, 13, 477. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Montenegro, L.; Campisi, A.; Sarpietro, M.G.; Carbone, C.; Acquaviva, R.; Raciti, G.; Puglisi, G. In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. Drug Dev. Ind. Pharm. 2011, 37, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, C.; Martins-Gomes, C.; Caddeo, C.; Silva, A.M.; Musumeci, T.; Pignatello, R.; Puglisi, G.; Souto, E.B. Mediterranean essential oils as precious matrix components and active ingredients of lipid nanoparticles. Int. J. Pharm. 2018, 548, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.; Oprea, T.I.; Baskin, I.I.; Varnek, A.; Roitberg, A.; et al. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K.; Wilson, P.B. Structure-activity modelling of essential oils, their components, and key molecular parameters and descriptors. Mol. Cell Probes. 2018, 38, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Noorizadeh, H.; Farmany, A.; Noorizadeh, M. Quantitative structure-retention relationships analysis of retention index of essential oils. Quim. Nova 2011, 34, 242–249. [Google Scholar] [CrossRef]
- Raymond, L. The introduction of artificial intelligence to help in the analysis of the chromatographic big data. J. Chromatogr. Sep. Tech. 2019, 10. [Google Scholar] [CrossRef]
- Lebanov, L.; Tedone, L.; Ghiasvand, A.; Paull, B. Random Forests machine learning applied to gas chromatography—Mass spectrometry derived average mass spectrum data sets for classification and characterisation of essential oils. Talanta 2020, 208, 120471. [Google Scholar] [CrossRef]
- Artini, M.; Patsilinakos, A.; Papa, R.; Božović, M.; Sabatino, M.; Garzoli, S.; Vrenna, G.; Tilotta, M.; Pepi, F.; Ragno, R.; et al. Antimicrobial and Antibiofilm Activity and Machine Learning Classification Analysis of Essential Oils from Different Mediterranean Plants against Pseudomonas aeruginosa. Molecules 2018, 23, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daynac, M.; Cortes-Cabrera, A.; Prieto, J.M. Application of Artificial Intelligence to the Prediction of the Antimicrobial Activity of Essential Oils. Evid. Based Complement. Alternat. Med. 2015, 2015, 561024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragno, R. Machine learning analyses on data including essential oil chemical composition and in vitro experimental antibiofilm activities from different bacterial belonging to either grampositive or gram-negative species. J. Dermatol. Res. Skin. Care 2019, 3, 42. [Google Scholar]
- El-Attar, N.E.; Awad, W.A. Computational tool for optimizing the essential oils utilization in inhibiting the bacterial growth. Adv. Appl. Bioinform. Chem. 2017, 10, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Attar, N.E.; Hassan, M.K.; Alghamdi, O.A.; Awad, W.A. Deep learning model for classification and bioactivity prediction of essential oil-producing plants from Egypt. Sci. Rep. 2020, 10, 21349. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity. Pharmaceutics 2021, 13, 631. https://doi.org/10.3390/pharmaceutics13050631
Chiriac AP, Rusu AG, Nita LE, Chiriac VM, Neamtu I, Sandu A. Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity. Pharmaceutics. 2021; 13(5):631. https://doi.org/10.3390/pharmaceutics13050631
Chicago/Turabian StyleChiriac, Aurica P., Alina G. Rusu, Loredana E. Nita, Vlad M. Chiriac, Iordana Neamtu, and Alina Sandu. 2021. "Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity" Pharmaceutics 13, no. 5: 631. https://doi.org/10.3390/pharmaceutics13050631
APA StyleChiriac, A. P., Rusu, A. G., Nita, L. E., Chiriac, V. M., Neamtu, I., & Sandu, A. (2021). Polymeric Carriers Designed for Encapsulation of Essential Oils with Biological Activity. Pharmaceutics, 13(5), 631. https://doi.org/10.3390/pharmaceutics13050631