Investigating the Potential of Transdermal Delivery of Avanafil Using Vitamin E-TPGS Based Mixed Micelles Loaded Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
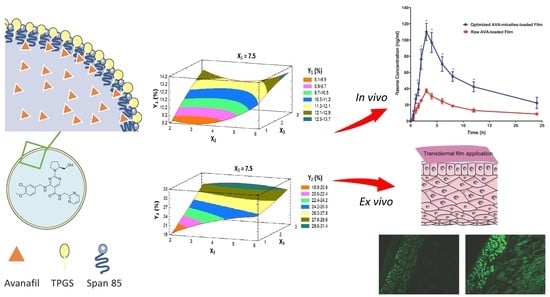
2.2. Formulation and Optimization of AVA-MM-Loaded Transdermal Film
2.3. Ex Vivo Permeation of AVA-Loaded Transdermal Film
2.4. Prediction of the Optimized Formulation
2.5. Study of Optimized Transdermal Film Using a Fluorescence Laser Microscope
2.6. In Vivo Pharmacokinetic Studies
2.6.1. Study Design
2.6.2. Animal Handling
2.6.3. Blood Sampling
2.6.4. Pharmacokinetics Parameters Evaluation
2.6.5. Chromatographic Conditions
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of AVA-MM-Loaded Transdermal Films
3.1.1. Effect of Variables on the AVA Permeation (Y1 and Y2) Behavior
3.1.2. Prediction of the Optimized AVA-MM-Loaded Transdermal Film
3.2. Ex Vivo Fluorescence Microscope Investigation of the Optimized Film
3.3. In Vivo Pharmacokinetic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mulhall, J.P.; Luo, X.; Zou, K.H.; Stecher, V.; Galaznik, A. Relationship between age and erectile dysfunction diagnosis or treatment using real-world observational data in the USA. Int. J. Clin. Pract. 2016, 70, 1012–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvin, E.; Burnett, A.L.; Platz, E.A. Prevalence and Risk Factors for Erectile Dysfunction in the US. Am. J. Med. 2007, 120, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Aboalfotouh, M.A.; Al-elali, N.S. Effect of erectile dysfunction on quality of life. East. Mediterr. Heal. J. 2001, 7, 510–518. [Google Scholar]
- Montague, D.; Jarow, J.; Broderick, G.; Dmochowski, R.; Heaton, J.; Lue, T.; Milbank, A.; Nehra, A.; Sharlip, I. Erectile Dysfunction Guideline Update Panel the management of erectile dysfunction: An aua update. J. Urol. 2005, 174, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Wespes, E.; Amar, E.; Hatzichristou, D.; Hatzimouratidis, K.; Montorsi, F.; Pryor, J.; Vardi, Y. EAU Guidelines on Erectile Dysfunction: An Update. Eur. Urol. 2006, 49, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.A.; Lie, J.D. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T 2013, 38, 407–419. [Google Scholar]
- FDA STENDRATM (Avanafil) Tablets, for Oral Use: HIGHLIGHTS OF PRESCRIBING INFORMATION. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202276s000lbl.pdf (accessed on 20 April 2021).
- European Medicines Agency. Spedra. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/spedra (accessed on 20 April 2021).
- Limin, M.; Johnsen, N.; Hellstrom, W.J. Avanafil, a new rapid-onset phosphodiesterase 5 inhibitor for the treatment of erectile dysfunction. Expert Opin. Investig. Drugs 2010, 19, 1427–1437. [Google Scholar] [CrossRef]
- Alwaal, A.; Al-Mannie, R.; Carrier, S. Future prospects in the treatment of erectile dysfunction: Focus on avanafil. Drug Des. Devel. Ther. 2011, 5, 435–443. [Google Scholar]
- Burke, R.M.; Evans, J.D. Avanafil for treatment of erectile dysfunction: Review of its potential. Vasc. Health Risk Manag. 2012, 8, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Hosny, K.M.; Aldawsari, H.M. Avanafil liposomes as transdermal drug delivery for erectile dysfunction treatment: Preparation, characterization, and in vitro, ex vivo and in vivo studies. Trop. J. Pharm. Res. 2015, 14, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Park, K. Nanotechnology: What it can do for drug delivery. J. Control. Release 2007, 120, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Khemtong, C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Ahmed, O.A.A.; Fahmy, U.A.; Alkhalidi, H.M. Nanovesicular systems loaded with a recently approved second generation type-5 phospodiesterase inhibitor (avanafil): I. Plackett-Burman screening and characterization. J. Drug Deliv. Sci. Technol. 2018, 43, 154–159. [Google Scholar] [CrossRef]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent advances in the application of vitamin E TPGS for drug delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin e TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.A.; El-Say, K.M.; Aljaeid, B.M.; Badr-Eldin, S.M.; Ahmed, T.A. Optimized vinpocetine-loaded vitamin E D-α-tocopherol polyethylene glycol 1000 succinate-alpha lipoic acid micelles as a potential transdermal drug delivery system: In vitro and ex vivo studies. Int. J. Nanomed. 2018, 14, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Bibi, N.; Ahmed, N.; Khan, G.M. Nanostructures in transdermal drug delivery systems. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 639–668. [Google Scholar]
- Cheng, Y.C.; Li, T.S.; Su, H.L.; Lee, P.C.; Wang, H.M.D. Transdermal Delivery Systems of Natural Products Applied to Skin Therapy and Care. Molecules 2020, 25, 5051. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil—An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C 2021, 119, 111591. [Google Scholar] [CrossRef]
- Lalotra, A.S.; Singh, V.; Khurana, B.; Agrawal, S.; Shrestha, S.; Arora, D. A Comprehensive Review on Nanotechnology-Based Innovations in Topical Drug Delivery for the Treatment of Skin Cancer. Curr. Pharm. Des. 2020, 26, 5720–5731. [Google Scholar] [CrossRef]
- Lopes, J.; Ferreira-Gonçalves, T.; Figueiredo, I.V.; Rodrigues, C.M.P.; Ferreira, H.; Ferreira, D.; Viana, A.S.; Faísca, P.; Gaspar, M.M.; Coelho, J.M.P.; et al. Proof-of-concept study of multifunctional hybrid nanoparticle system combined with nir laser irradiation for the treatment of melanoma. Biomolecules 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Mahato, R. Microneedles in Drug Delivery. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 331–353. ISBN 9780323429979. [Google Scholar]
- Wang, M.; Marepally, S.K.; Vemula, P.K.; Xu, C. Inorganic Nanoparticles for Transdermal Drug Delivery and Topical Application. In Nanoscience in Dermatology; Academic Press: Cambridge, MA, USA, 2016; pp. 57–72. ISBN 9780128029268. [Google Scholar]
- Somagoni, J.; Boakye, C.H.A.; Godugu, C.; Patel, A.R.; Mendonca Faria, H.A.; Zucolotto, V.; Singh, M. Nanomiemgel—A Novel Drug Delivery System for Topical Application—In Vitro and In Vivo Evaluation. PLoS ONE 2014, 9, e115952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, O.A.A.; Badr-Eldin, S.M. Development of an optimized avanafil-loaded invasomal transdermal film: Ex vivo skin permeation and in vivo evaluation. Int. J. Pharm. 2019, 570. [Google Scholar] [CrossRef] [PubMed]
- Basahih, T.S.; Alamoudi, A.A.; El-Say, K.M.; Alhakamy, N.A.; Ahmed, O.A.A. Improved Transmucosal Delivery of Glimepiride via Unidirectional Release Buccal Film Loaded With Vitamin E TPGS-Based Nanocarrier. Dose-Response 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Aldawsari, H.; Fahmy, U.; Abd-Allah, F.; Ahmed, O. Formulation and Optimization of Avanafil Biodegradable Polymeric Nanoparticles: A Single-Dose Clinical Pharmacokinetic Evaluation. Pharmaceutics 2020, 12, 596. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Ahmed, O.A.A.; Badr-Eldin, S.M. In situ misemgel as a multifunctional dual-absorption platform for nasal delivery of raloxifene hydrochloride: Formulation, characterization, and in vivo performance. Int. J. Nanomed. 2018, 13, 6325–6335. [Google Scholar] [CrossRef] [Green Version]
- Dintaman, J.M.; Silverman, J.A. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm. Res. 1999, 16, 1550–1556. [Google Scholar] [CrossRef]
- Collnot, E.M.; Baldes, C.; Wempe, M.F.; Hyatt, J.; Navarro, L.; Edgar, K.J.; Schaefer, U.F.; Lehr, C.M. Influence of vitamin E TPGS poly(ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J. Control. Release 2006, 111, 35–40. [Google Scholar] [CrossRef]
- Muthu, M.S.; Avinash Kulkarni, S.; Liu, Y.; Feng, S.S. Development of docetaxel-loaded vitamin e TPGS micelles: Formulation optimization, effects on brain cancer cells and biodistribution in rats. Nanomedicine 2012, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.B. Penetration Enhancement Techniques. J. Appl. Pharm. 2017, 9, 1–5. [Google Scholar] [CrossRef]
- Ng, K.W. Penetration enhancement of topical formulations. Pharmaceutics 2018, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, K.W.; Lau, W.M. Skin deep: The basics of human skin structure and drug penetration. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Drug Manipulation Strategies and Vehicle Effects; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. ISBN 9783662450130. [Google Scholar]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M.; Ali, A. Role of novel terpenes in transcutaneous permeation of valsartan: Effectiveness and mechanism of action. Drug Dev. Ind. Pharm. 2011, 37, 583–596. [Google Scholar] [CrossRef]
- Dos Anjos, J.L.V.; de Sousa Neto, D.; Alonso, A. Effects of 1,8-cineole on the dynamics of lipids and proteins of stratum corneum. Int. J. Pharm. 2007, 345, 81–87. [Google Scholar] [CrossRef]





| Factor | Low | High | Units |
|---|---|---|---|
| X1: HLB of surfactant | 5.60 | 9.40 | |
| X2: Concentration of MMs in the film | 0.20 | 2.00 | % |
| X3: The percentage of penetration enhancer in the film | 1.00 | 3.00 | % |
| Response | Low | High | Goal |
| Y1: Initial permeation after 1 h (%) | 5.96 | 17.81 | Maximize |
| Y2: Cumulative permeation after 24 h (%) | 18.86 | 43.95 | Maximize |
| Run | HLB of Surfactant | Conc of MM in the Film | Penetration Enhancer | Initial Permeation (%) | Cumulative Permeation (%) | ||
|---|---|---|---|---|---|---|---|
| Observed Value | Fitted Value | Observed Value | Fitted Value | ||||
| 1 | 7.50 | 4.00 | 2.00 | 10.40 | 10.51 | 26.46 | 26.70 |
| 2 | 9.40 | 4.00 | 1.00 | 11.55 | 12.68 | 30.91 | 30.67 |
| 3 | 5.60 | 4.00 | 3.00 | 9.45 | 8.32 | 23.35 | 23.59 |
| 4 | 5.60 | 6.00 | 2.00 | 9.31 | 9.64 | 22.24 | 21.81 |
| 5 | 9.40 | 6.00 | 2.00 | 17.81 | 17.34 | 35.97 | 37.46 |
| 6 | 7.50 | 2.00 | 3.00 | 12.3 | 12.97 | 28.29 | 29.54 |
| 7 | 7.50 | 6.00 | 1.00 | 11.89 | 11.22 | 26.99 | 25.74 |
| 8 | 7.50 | 4.00 | 2.00 | 10.44 | 10.51 | 26.09 | 26.70 |
| 9 | 7.50 | 2.00 | 1.00 | 9.32 | 8.52 | 18.86 | 18.67 |
| 10 | 5.60 | 2.00 | 2.00 | 7.64 | 8.11 | 20.99 | 19.51 |
| 11 | 9.40 | 4.00 | 3.00 | 17.61 | 17.28 | 43.95 | 42.28 |
| 12 | 9.40 | 2.00 | 2.00 | 16.08 | 15.75 | 32.26 | 32.69 |
| 13 | 7.50 | 6.00 | 3.00 | 12.59 | 13.39 | 29.35 | 29.54 |
| 14 | 5.60 | 4.00 | 1.00 | 5.96 | 6.30 | 18.86 | 20.53 |
| 15 | 7.50 | 4.00 | 2.00 | 10.69 | 10.51 | 27.55 | 26.70 |
| Factors | Initial Permeation (Y1), % | Cumulative Permeation (Y2), % | ||||
|---|---|---|---|---|---|---|
| Estimate | F-Ratio | P-Value | Estimate | F-Ratio | P-Value | |
| X1 | 7.6725 | 103.9100 | 0.0002 * | 14.4125 | 140.14 | 0.0001 * |
| X2 | 1.5650 | 4.3200 | 0.0921 | 3.5375 | 8.4400 | 0.0336 * |
| X3 | 3.3075 | 19.3100 | 0.0071 * | 7.3300 | 36.2500 | 0.0018 * |
| X1 X1 | 1.8175 | 2.6900 | 0.1618 | 4.5600 | 6.4700 | 0.0516 |
| X1 X2 | 0.0300 | 0.0000 | 0.9786 | 1.2300 | 0.5100 | 0.5069 |
| X1 X3 | 1.2850 | 1.4600 | 0.2813 | 4.2750 | 6.1600 | 0.0556 |
| X2 X2 | 2.5825 | 5.4300 | 0.0671 | −2.2300 | 1.5500 | 0.2685 |
| X2 X3 | −1.1400 | 1.1500 | 0.3331 | −3.5350 | 4.2200 | 0.0953 |
| X3 X3 | −0.5525 | 0.2500 | 0.6392 | 0.5750 | 0.1000 | 0.7613 |
| R2 | 96.51 | 97.61 | ||||
| Adj. R2 | 90.23 | 93.31 | ||||
| SE | 1.06 | 1.72 | ||||
| MAE | 0.52 | 0.81 | ||||
| Factor | Optimum | Response | Observed Value | Fitted Value | Residual |
|---|---|---|---|---|---|
| X1: the HLB of surfactant | 9.40 | Y1: Initial permeation (%) | 16.21 | 17.81 | 1.60 |
| X2: the percentage of MMs in the film | 5.12 | ||||
| X3: the percentage of penetration enhancer in the film | 2.99 | Y2: Cumulative permeation (%) | 43.95 | 42.26 | 1.69 |
| Parameter | Unit | Optimized AVA-TPGS MM-Loaded FIlm | Raw AVA-Loaded Film |
|---|---|---|---|
| Average ± SD | Average ± SD | ||
| Ke | 1/h | 0.06 ± 0.02 | 0.06 ± 0.01 |
| t1/2 | h | 13.04 ± 4.84 | 12.73 ± 3.58 |
| Tmax | h | 3.0 ± 0.0 | 3.0 ± 0.0 |
| Cmax | ng/ml | 110.0 ± 10.54 * | 37.33 ± 2.52 |
| AUC 0–24 | ng/mL*h | 1129.417 ± 155.54 * | 382.58 ± 40.06 |
| AUC 0–inf | ng/mL*h | 1579.78 ± 391.17 * | 545.68 ± 108.95 |
| AUMC 0–inf | ng/mL*h2 | 31,162.81 ± 17,693.69 | 10,740.85 ± 4486.28 |
| MRT 0–inf | h | 18.77 ± 6.50 | 19.15 ± 4.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamoudi, A.A.; Ahmed, O.A.A.; El-Say, K.M. Investigating the Potential of Transdermal Delivery of Avanafil Using Vitamin E-TPGS Based Mixed Micelles Loaded Films. Pharmaceutics 2021, 13, 739. https://doi.org/10.3390/pharmaceutics13050739
Alamoudi AA, Ahmed OAA, El-Say KM. Investigating the Potential of Transdermal Delivery of Avanafil Using Vitamin E-TPGS Based Mixed Micelles Loaded Films. Pharmaceutics. 2021; 13(5):739. https://doi.org/10.3390/pharmaceutics13050739
Chicago/Turabian StyleAlamoudi, Abdullah A., Osama A. A. Ahmed, and Khalid M. El-Say. 2021. "Investigating the Potential of Transdermal Delivery of Avanafil Using Vitamin E-TPGS Based Mixed Micelles Loaded Films" Pharmaceutics 13, no. 5: 739. https://doi.org/10.3390/pharmaceutics13050739
APA StyleAlamoudi, A. A., Ahmed, O. A. A., & El-Say, K. M. (2021). Investigating the Potential of Transdermal Delivery of Avanafil Using Vitamin E-TPGS Based Mixed Micelles Loaded Films. Pharmaceutics, 13(5), 739. https://doi.org/10.3390/pharmaceutics13050739