A Dry Powder Platform for Nose-to-Brain Delivery of Dexamethasone: Formulation Development and Nasal Deposition Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Statistical Design of Experiments
2.3. Preparation of Microspheres
2.4. Determination of Entrapment Efficiency and DSP Loading
2.5. Particle Size Distribution Measurement
2.6. Moisture Content Determination
2.7. Swelling Study
2.8. Zeta-Potential Analysis
2.9. In Vitro Release Study
2.10. Preparation of Powder Blends
2.11. Scanning Electron Microscopy
2.12. Powder Flow Properties
2.13. Spray Cone Angle Determination
2.14. In Vitro Mucoadhesion Test
2.15. Cell Culture Conditions
2.16. In Vitro Biocompatibility Studies
2.17. In Vitro Permeability through Epithelial Model Barrier
2.18. Development of Nasal Cavity Model
2.19. Assessment of the Deposition Profile within the Nasal Cavity In Vitro
2.20. Quantitative Determination of DSP and DB
2.21. Statistical Analysis
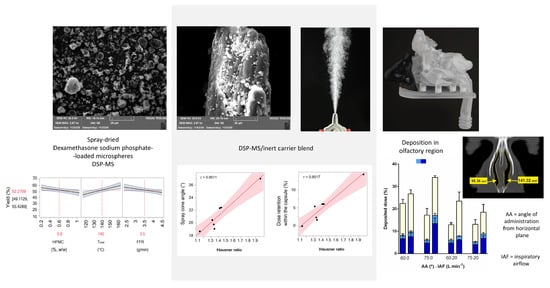
3. Results and Discussion
3.1. Experimental Design: Analysis of the Results
3.1.1. Process Yield
3.1.2. Entrapment Efficiency and Drug Loading
3.1.3. Particle Size
3.1.4. Residual Moisture Content
3.1.5. Swelling of the Microspheres
3.1.6. Selection of the Leading DSP-Loaded Microspheres
3.1.7. In vitro Release of DSP from Microspheres
3.2. Properties of DSP-MS/Inert Carrier Blends
3.3. In Vitro Mucoadhesion Studies
3.4. Biocompatibility of DSP-MS/Inert Carrier Blends
3.5. In Vitro DSP Permeability Studies
3.6. Nasal Deposition Profile of DSP Powder Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higgins, T.S.; Wu, A.W.; Illing, E.A.; Sokoloski, K.J.; Weaver, B.A.; Anthony, B.P.; Hughes, N.; Ting, J. Intranasal antiviral drug delivery and Coronavirus disease 2019 (COVID-19): A State of the Art Review. Otolaryngol. Head Neck Surg. 2020, 163, 682–694. [Google Scholar] [CrossRef]
- Basu, S.; Holbrook, L.T.; Kudlaty, K.; Fasanmade, O.; Wu, J.; Burke, A.; Langworthy, B.W.; Farzal, Z.; Mamdani, M.; Bennett, W.D.; et al. Numerical evaluation of spray position for improved nasal drug delivery. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N.A. Proposed intranasal route for drug administration in the management of central nervous system manifestations of COVID-19. ACS Chem. Neurosci. 2020, 11, 1523–1524. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cárdenas, G.; Torres-García, D.; Cervantes-Torres, J.; Rosales-Mendoza, S.; Fleury, A.; Fragoso, G.; Laclette, J.P.; Sciutto, E. Role of Systemic and Nasal Glucocorticoid Treatment in the Regulation of the Inflammatory Response in Patients with SARS-Cov-2 Infection. Arch. Med. Res. 2020, 52, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Meneses, G.; Gevorkian, G.; Florentino, A.; Bautista Marcel, A.; Espinosa, A.; Acero, G.; Díaz, G.; Fleury, A.; Pérez Osorio, I.N.; Del Rey, A.; et al. Intranasal delivery of dexamethasone efficiently controls LPS-induced murine neuroinflammation. Clin. Exp. Immunol. 2017, 190, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vroegop, A.V.; Eeckels, A.-S.; Van Rompaey, V.; Vanden Abeele, D.; Schiappoli, M.S.; Alobid, I.; Hummel, T.; Dorlodot, C.D.; Levie, P.; Huart, C.; et al. COVID-19 and olfactory dysfunction—An ENT perspective to the current COVID-19 pandemic. B-ENT 2020, 16, 81–85. [Google Scholar] [CrossRef]
- Martins, P.P.; Smyth, H.D.C.; Cui, Z. Strategies to facilitate or block nose-to-brain drug delivery. Int. J. Pharm. 2019, 570, 118635. [Google Scholar] [CrossRef] [PubMed]
- Fasiolo, L.T.; Manniello, M.D.; Tratta, E.; Buttini, F.; Rossi, A.; Sonvico, F.; Bortolotti, F.; Russo, P.; Colombo, G. Opportunity and challenges of nasal powders: Drug formulation and delivery. Eur. J. Pharm. Sci. 2018, 113, 2–17. [Google Scholar] [CrossRef]
- Tanaka, A.; Furubayashi, T.; Tomisaki, M.; Kawakami, M.; Kimura, S.; Inoue, D.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Nasal drug absorption from powder formulations: The effect of three types of hydroxypropyl cellulose (HPC). Eur. J. Pharm. Sci. 2017, 96, 284–289. [Google Scholar] [CrossRef]
- Giuliani, A.; Balducci, A.G.; Zironi, E.; Colombo, G.; Bortolotti, F.; Lorenzini, L.; Galligioni, V.; Pagliuca, G.; Scagliarini, A.; Calzà, L.; et al. In vivo nose-to-brain delivery of the hydrophilic antiviral ribavirin by microparticle agglomerates. Drug Deliv. 2018, 25, 376–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release 2015, 201, 68–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgamwar, V.S.; Patel, H.S.; Joshi, A.S.; Agrawal, A.; Surana, S.J.; Tekade, A.R. Design and development of nasal mucoadhesive microspheres containing tramadol HCl for CNS targeting. Drug Deliv. 2011, 18, 353–360. [Google Scholar] [CrossRef]
- Rassu, G.; Ferraro, L.; Pavan, B.; Giunchedi, P.; Gavini, E.; Dalpiaz, A. The role of combined penetration enhancers in nasal microspheres on in vivo drug bioavailability. Pharmaceutics 2018, 10, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavini, E.; Rassu, G.; Ferraro, L.; Beggiato, S.; Alhalaweh, A.; Velaga, S.; Marchetti, N.; Bandiera, P.; Giunchedi, P.; Dalpiaz, A. Influence of polymeric microcarriers on the in vivo intranasal uptake of an anti-migraine drug for brain targeting. Eur. J. Pharm. Biopharm. 2013, 83, 174–183. [Google Scholar] [CrossRef]
- Picher, M.; Burch, L.H.; Hirsh, A.J.; Spychala, J.; Boucher, R.C. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase: Two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J. Biol. Chem. 2003, 278, 13468–13479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes Viegas, D.; Rodrigues, M.; Francisco, J.; Falcão, A.; Alves, G.; Santos, A.O. Development and application of an ex vivo fosphenytoin nasal bioconversion/permeability evaluation method. Eur. J. Pharm. Sci. 2016, 89, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Bourne, G.H. Alkaline Phosphatase in Taste Buds and Nasal Mucosa. Nature 1948, 161, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Santos, L.T.; Rodrigues, M.; Alves, G.; Santos, A.O. Intranasal fosphenytoin: The promise of phosphate esters in nose-to-brain delivery of poorly soluble drugs. Int. J. Pharm. 2021, 592, 120040. [Google Scholar] [CrossRef] [PubMed]
- Fransén, N.; Björk, E.; Nyström, C. Development and characterisation of interactive mixtures with a fine-particulate mucoadhesive carrier for nasal drug delivery. Eur. J. Pharm. Biopharm. 2007, 67, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Trows, S.; Scherließ, R. Carrier-based dry powder formulation for nasal delivery of vaccines utilizing BSA as model drug. Powder Technol. 2016, 292, 223–231. [Google Scholar] [CrossRef]
- Nižić, L.; Potaś, J.; Winnicka, K.; Szekalska, M.; Erak, I.; Gretić, M.; Jug, M.; Hafner, A. Development, characterisation and nasal deposition of melatonin-loaded pectin/hypromellose microspheres. Eur. J. Pharm. Sci. 2020, 141, 105–115. [Google Scholar] [CrossRef]
- Popov, T.A.; Åberg, N.; Emberlin, J.; Josling, P.; Ilyina, N.I.; Nikitin, N.P.; Church, M. Methyl-cellulose powder for prevention and management of nasal symptoms. Expert Rev. Respir. Med. 2017, 11, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Åberg, N.; Ospanova, S.T.; Nikitin, N.P.; Emberlin, J.; Dahl, Å. A nasally applied cellulose powder in seasonal allergic rhinitis in adults with grass pollen allergy: A double-blind, randomized, placebo-controlled, parallel-group study. Int. Arch. Allergy Immunol. 2014, 163, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guan, W.-J.; Sun, S.-X.; Zheng, P.-Y.; Sun, L.-H.; Chen, D.-H.; Wang, D.-D.; Chen, C.; Sun, B.-Q.; Zhang, X.-H.D. Effects of Intranasal Cellulose Powder on Asthma Control in Children with Mild-to-Moderate Perennial Allergic Rhinitis: A Randomized, Placebo-Controlled Trial. Am. J. Rhinol. Allergy 2019, 33, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Martinac, A.; Filipović-Grčić, J.; Perissutti, B.; Voinovich, D.; Pavelić, Ž. Spray-dried chitosan/ethylcellulose microspheres for nasal drug delivery: Swelling study and evaluation of in vitro drug release properties. J. Microencapsul. 2005, 22, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Kaialy, W.; Alhalaweh, A.; Velaga, S.P.; Nokhodchi, A. Influence of lactose carrier particle size on the aerosol performance of budesonide from a dry powder inhaler. Powder Technol. 2012, 227, 74–85. [Google Scholar] [CrossRef]
- Kaialy, W.; Martin, G.P.; Ticehurst, M.D.; Momin, M.N.; Nokhodchi, A. The enhanced aerosol performance of salbutamol from dry powders containing engineered mannitol as excipient. Int. J. Pharm. 2010, 392, 178–188. [Google Scholar] [CrossRef]
- Nižić, L.; Ugrina, I.; Špoljarić, D.; Saršon, V.; Kučuk, M.S.; Pepić, I.; Hafner, A. Innovative sprayable in situ gelling fluticasone suspension: Development and optimization of nasal deposition. Int. J. Pharm. 2019, 563, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Fachel, F.N.S.; Medeiros-Neves, B.; Prá, M.D.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Box-Behnken design optimization of mucoadhesive chitosan-coated nanoemulsions for rosmarinic acid nasal delivery—In vitro studies. Carbohydr. Polym. 2018, 199, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Dukovski, B.J.; Mrak, L.; Winnicka, K.; Szekalska, M.; Juretić, M.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J.; Hafner, A. Spray-dried nanoparticle-loaded pectin microspheres for dexamethasone nasal delivery. Dry Technol. 2019, 39, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Matilainen, L.; Toropainen, T.; Vihola, H.; Hirvonen, J.; Järvinen, T. In vitro toxicity and permeation of cyclodextrins in Calu-3 cells. J. Control. Release 2008, 126, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Lovrić, J.; Voinovich, D.; Filipović-Grčić, J. Melatonin-loaded lecithin/chitosan nanoparticles: Physicochemical characterisation and permeability through Caco-2 cell monolayers. Int. J. Pharm. 2009, 381, 205–213. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Validation of Analytical Procedures: Text and Methodology Q2(R1); European Medicines Agency: London, UK, 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29 Guideline.pdf (accessed on 5 May 2021).
- Food and Drug Administration. Inactive Ingredients in Approved Drug Products; Food and Drug Administration: Silver Spring, MD, USA, 2021.
- Scherließ, R.; Mönckedieck, M.; Young, K.; Trows, S.; Buske, S.; Hook, S. First in vivo evaluation of particulate nasal dry powder vaccine formulations containing ovalbumin in mice. Int. J. Pharm. 2015, 479, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Balducci, A.G.; Ferraro, L.; Bortolotti, F.; Nastruzzi, C.; Colombo, P.; Sonvico, F.; Russo, P.; Colombo, G. Antidiuretic effect of desmopressin chimera agglomerates by nasal administration in rats. Int. J. Pharm. 2013, 440, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bálint, Z.; Krizbai, I.A.; Wilhelm, I.; Farkas, A.E.; Párducz, Á.; Szegletes, Z.; Váró, G. Changes induced by hyperosmotic mannitol in cerebral endothelial cells: An atomic force microscopic study. Eur. Biophys. J. 2007, 36, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, A.J. Altering airway surface liquid volume: Inhalation therapy with amiloride and hyperosmotic agents. Adv. Drug Deliv. Rev. 2002, 54, 1445–1462. [Google Scholar] [CrossRef]
- Dreijer-vander Glas, S.; Hafner, A. Nose. In Practical Pharmaceutics; Bouwman-Boer, Y., Fenton-May, V., Le Brun, P., Eds.; Springer: London, UK, 2015; pp. 139–152. [Google Scholar]
- Goos, P.; Jones, B. Optimal Design of Experiments, 1st ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Focaroli, S.; Mah, P.; Hastedt, J.; Gitlin, I.; Oscarson, S.; Fahy, J.; Healy, A. A Design of Experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery. Int. J. Pharm. 2019, 562, 228–240. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Magri, G.; Franzé, S.; Musazzi, U.M.; Selmin, F.; Cilurzo, F. Data on spray-drying processing to optimize the yield of materials sensitive to heat and moisture content. Data Brief 2019, 23, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.A.B.; Bassani, V.L.; Castellanos, L.; Rodríguez, F.A.R.; Baena, Y. Development of an oral control release system from Physalis peruviana L. fruits extract based on the co-spray-drying method. Powder Technol. 2019, 354, 676–688. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Shahin, H.I.; Vinjamuri, B.P.; Mahmoud, A.A.; Shamma, R.N.; Mansour, S.M.; Ammar, H.O.; Ghorab, M.M.; Chougule, M.B.; Chablani, L. Design and evaluation of novel inhalable sildenafil citrate spray-dried microparticles for pulmonary arterial hypertension. J. Control. Release 2019, 302, 126–139. [Google Scholar] [CrossRef]
- Trenkel, M.; Scherließ, R. Nasal Powder Formulations: In-Vitro characterisation of the impact of powders on nasal residence time and sensory effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Drugbank. Available online: https://www.drugbank.com (accessed on 15 January 2021).
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, C.Y.; Adams, M. DEM analysis of particle adhesion during powder mixing for dry powder inhaler formulation development. Granul. Matter 2013, 15, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Yang, Y.; Xie, X.; Yu, F.; Yang, Y.; Yang, Z.; Zhang, T.; Mei, X. Preparation, characterization and pulmonary pharmacokinetics of a new inhalable zanamivir dry powder. Drug Deliv. 2016, 23, 1962–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvam, P.; Marek, S.; Truman, C.R.; McNair, D.; Smyth, H.D.C. Micronized drug adhesion and detachment from surfaces: Effect of loading conditions. Aerosol Sci. Technol. 2011, 45, 81–87. [Google Scholar] [CrossRef]
- Jug, M.; Hafner, A.; Lovrić, J.; Kregar, M.L.; Pepić, I.; Vanić, Ž.; Cetina-Čižmek, B.; Filipović-Grčić, J. An overview of in vitro dissolution/release methods for novel mucosal drug delivery systems. J. Pharm. Biomed. Anal. 2018, 147, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Soane, R.J.; Hinchcliffe, M.; Davis, S.S.; Illum, L. Clearance characteristics of chitosan based formulations in the sheep nasal cavity. Int. J. Pharm. 2001, 217, 183–191. [Google Scholar] [CrossRef]
- Yarragudi, S.B.; Richter, R.; Lee, H.; Walker, G.F.; Clarkson, A.N.; Kumar, H.; Rizwan, S.B. Formulation of olfactory-targeted microparticles with tamarind seed polysaccharide to improve nose-to-brain transport of drugs. Carbohydr. Polym. 2017, 163, 216–226. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, H.-G.; Kwon, Y.-B.; Kim, J.-Y.; Rhee, Y.-S.; Chon, J.; Park, E.-S.; Kim, D.-W.; Park, C.-W. The role of lactose carrier on the powder behavior and aerodynamic performance of bosentan microparticles for dry powder inhalation. Eur. J. Pharm. Sci. 2018, 117, 279–289. [Google Scholar] [CrossRef]
- Rudén, J.; Frenning, G.; Bramer, T.; Thalberg, K.; An, J.; Alderborn, G. Linking carrier morphology to the powder mechanics of adhesive mixtures for dry powder inhalers via a blend-state model. Int. J. Pharm. 2019, 561, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Jüptner, A.; Scherließ, R. Spray dried formulations for inhalation—Meaningful characterisation of powder properties. Pharmaceutics 2020, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Gänger, S.; Schindowski, K. Tailoring formulations for intranasal nose-to-brain delivery: A review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Dai, L.; Yu, Q.; Yang, Q. Histological and anatomical structure of the nasal cavity of Bama minipigs. PLoS ONE 2017, 12, e0173902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladel, S.; Schlossbauer, P.; Flamm, J.; Luksch, H.; Mizaikoff, B.; Schindowski, K. Improved in vitro model for intranasal mucosal drug delivery: Primary olfactory and respiratory epithelial cells compared with the permanent Nasal cell line RPMI 2650. Pharmaceutics 2019, 11, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joergensen, L.; Klösgen, B.; Simonsen, A.C.; Borch, J.; Hagesaether, E. New insights into the mucoadhesion of pectins by AFM roughness parameters in combination with SPR. Int. J. Pharm. 2011, 411, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose—A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Wang, X.; Tang, L. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev. Ind. Pharm. 2010, 36, 323–336. [Google Scholar] [CrossRef]
- Furubayashi, T.; Inoue, D.; Nishiyama, N.; Tanaka, A.; Yutani, R.; Kimura, S.; Katsumi, H.; Yamamoto, A.; Sakane, T. Comparison of various cell lines and three-dimensional mucociliary tissue model systems to estimate drug permeability using an in vitro transport study to predict nasal drug absorption in rats. Pharmaceutics 2020, 12, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, D.; Furubayashi, T.; Tanaka, A.; Sakane, T.; Sugano, K. Quantitative estimation of drug permeation through nasal mucosa using in vitro membrane permeability across Calu-3 cell layers for predicting in vivo bioavailability after intranasal administration to rats. Eur. J. Pharm. Biopharm. 2020, 149, 145–153. [Google Scholar] [CrossRef]
- Salade, L.; Wauthoz, N.; Deleu, M.; Vermeersch, M.; De Vriese, C.; Amighi, K.; Goole, J. Development of coated liposomes loaded with ghrelin for nose-to-brain delivery for the treatment of cachexia. Int. J. Nanomed. 2017, 12, 8531–8543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salade, L.; Wauthoz, N.; Vermeersch, M.; Amighi, K.; Goole, J. Chitosan-coated liposome dry-powder formulations loaded with ghrelin for nose-to-brain delivery. Eur. J. Pharm. Biopharm. 2018, 129, 257–266. [Google Scholar] [CrossRef]
- Zhang, L.; Du, S.Y.; Lu, Y.; Liu, C.; Tian, Z.H.; Yang, C.; Wu, H.C.; Wang, Z. Puerarin transport across a Calu-3 cell monolayer—An in vitro model of nasal mucosa permeability and the influence of paeoniflorin and menthol. Drug Des. Dev. Ther. 2016, 10, 2227–2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, H.X.; Traini, D.; Young, P.M. Pharmaceutical applications of the Calu-3 lung epithelia cell line. Expert Opin. Drug Deliv. 2013, 10, 1287–1302. [Google Scholar] [CrossRef]
- Sibinovska, N.; Žakelj, S.; Roškar, R.; Kristan, K. Suitability and functional characterization of two Calu-3 cell models for prediction of drug permeability across the airway epithelial barrier. Int. J. Pharm. 2020, 585, 19484. [Google Scholar] [CrossRef] [PubMed]
- Pepić, I.; Lovrić, J.; Cetina-Čižmek, B.; Reichl, S.; Filipović-Grčić, J. Toward the practical implementation of eye-related bioavailability prediction models. Drug Discov. Today 2014, 19, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Sibinovska, N.; Božič, D.; Ribarski, M.B.; Kristan, K. Prediction of pharmacokinetic studies outcome for locally acting nasal sprays by using different in vitro methods. Int. J. Pharm. 2021. [Google Scholar] [CrossRef] [PubMed]
- Noach, A.B.; Sakai, M.; Blom-Roosemalen, M.C.; de Jonge, H.R.; de Boer, A.G.; Breimer, D.D. Effect of anisotonic conditions on the transport of hydrophilic model compounds across monolayers of human colonic cell lines. J. Pharmacol. Exp. Ther. 1994, 270, 1373–1380. [Google Scholar] [PubMed]
- Brophy, T.R.O.R.; Mccafferty, J.; Tyrer, J.H.; Eadie, M.J. Bioavailability of Oral Dexamethasone During High Dose Steroid Therapy in Neurological Patients. Eur. J. Clin. Pharmacol. 1983, 24, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Crixivan Summary of Product Characteristics. Available online: https://www.ema.europa.eu (accessed on 25 February 2021).
- Indu, P.; Rameshkumar, M.R.; Arunagirinathan, N.; Al-Dhabi, N.A.; Valan Arasu, M.; Ignacimuthu, S. Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine against main protease and RNA-dependent RNA polymerase of SARS-CoV-2: A molecular docking and drug repurposing approach. J. Infect. Public Health 2020, 13, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnken, Z.N.; Smyth, H.D.C.; Davis, D.A.; Weitman, S.; Kuhn, J.G.; Williams, R.O. Personalized medicine in nasal delivery: The use of patient-specific administration parameters to improve nasal drug targeting using 3D-printed nasal replica casts. Mol. Pharm. 2018, 15, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Mahmoud, R.A. Role of nasal casts for in vitro evaluation of nasal drug delivery and quantitative evaluation of various nasal casts. Ther. Deliv. 2020, 11, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Goldstein, B.J. Olfactory epithelium: Cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig. Otolaryngol. 2018, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Wang, Z.; Nevorski, D.; White, T.; Zhou, Y. Nasal and olfactory deposition with normal and bidirectional intranasal delivery techniques: In vitro tests and numerical simulations. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 118–131. [Google Scholar]
- Xi, J.; Wang, Z.; Si, X.A.; Zhou, Y. Nasal dilation effects on olfactory deposition in unilateral and bi-directional deliveries: In vitro tests and numerical modeling. Eur. J. Pharm. Sci. 2018, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Assessment Report on Dexamethasone in Hospitalized Patients with Covid-19; European Medicines Agency: Amsterdam, The Netherlands, 2020; Volume 5.











| HPMC (%; w/w) | DSP (%; w/w) | Tinlet (°C) | FFR (g min−1) | Yield (%) | DL (%) | EE (%) | Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | D[4,3] (µm) | MC (%) | VSNF (µL mg−1) | Vwater (µL mg−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.2 | 0.20 | 120 | 4.5 | 48.2 | 32.4 ± 0.5 | 97.2 ± 1.6 | 1.6 ± 0.0 | 2.7 ± 0.0 | 5.8 ± 0.1 | 3.3 ± 0.0 | 6.9 ± 0.6 | 13.6 ± 2.9 | 26.2 ± 4.2 |
| 2 | 1.0 | 0.20 | 160 | 4.5 | 52.4 | 14.6 ± 0.0 | 101.9 ± 0.3 | 1.9 ± 0.0 | 5.0 ± 0.0 | 17.7 ± 0.4 | 7.7 ± 0.1 | 4.7 ± 0.2 | 15.5 ± 1.4 | 22.4 ± 1.0 |
| 3 | 0.2 | 0.02 | 120 | 2.5 | 58.5 | 5.1 ± 1.6 | 97.9 ± 10.1 | 1.4 ± 0.0 | 2.2 ± 0.0 | 4.9 ± 0.0 | 2.0 ± 1.2 | 6.2 ± 0.4 | 11.7 ± 1.8 | 37.0 ± 2.0 |
| 4 | 0.6 | 0.11 | 140 | 3.5 | 63.5 | 11.7 ± 0.15 | 97.1 ± 1.2 | 1.6 ± 0.0 | 2.9 ± 0.0 | 8.1 ± 0.0 | 4.0 ± 0.0 | 5.0 ± 0.1 | 16.5 ± 2.6 | 20.7 ± 3.2 |
| 5 | 0.6 | 0.11 | 140 | 3.5 | 65.2 | 11.7 ± 0.15 | 97.1 ± 1.3 | 1.6 ± 0.0 | 3.0 ± 0.0 | 11.4 ± 0.4 | 5.0 ± 0.1 | 5.0 ± 0.1 | 16.1 ± 0.7 | 24.5 ± 0.4 |
| 6 | 0.2 | 0.02 | 120 | 4.5 | 50.7 | 4.3 ± 0.2 | 91.3 ± 3.2 | 1.4 ± 0.0 | 2.2 ± 0.0 | 4.4 ± 0.0 | 2.7 ± 0.0 | 5.7 ± 1.4 | 17.5 ± 2.2 | 39.2 ± 3.4 |
| 7 | 1.0 | 0.02 | 120 | 2.5 | 38.8 | 1.5 ± 0.0 | 91.8 ± 0.4 | 1.8 ± 0.0 | 3.3 ± 0.0 | 12.3 ± 0.1 | 5.4 ± 0.0 | 3.6 ± 0.5 | 16.3 ± 3.7 | 27.9 ± 2.0 |
| 8 | 1.0 | 0.20 | 120 | 4.5 | 25.2 | 13.2 ± 0.0 | 96.6 ± 5.8 | 1.8 ± 0.0 | 7.3 ± 0.9 | 36.6 ± 2.4 | 13.6 ± 0.3 | 3.2 ± 0.8 | 9.7 ± 1.4 | 16.0 ± 1.6 |
| 9 | 0.2 | 0.02 | 160 | 2.5 | 56.9 | 4.7 ± 0.0 | 98.6 ± 0.6 | 1.4 ± 0.0 | 2.2 ± 0.0 | 6.3 ± 0.0 | 3.6 ± 0.0 | 6.9 ± 0.5 | 14.8 ± 1.6 | 40.6 ± 4.0 |
| 10 | 0.2 | 0.20 | 160 | 4.5 | 68.6 | 31.7 ± 0.3 | 95.1 ± 1.0 | 1.6 ± 0.0 | 2.6 ± 0.0 | 5.1 ± 0.1 | 3.0 ± 0.0 | 6.6 ± 0.1 | 8.8 ± 1.0 | 24.8 ± 0.8 |
| 11 | 1.0 | 0.20 | 120 | 2.5 | 55.0 | 13.8 ± 0.3 | 99.4 ± 4.6 | 1.9 ± 0.0 | 4.1 ± 0.0 | 13.5 ± 0.3 | 6.9 ± 0.2 | 6.6 ± 0.0 | 15.0 ± 1.1 | 15.0 ± 3.7 |
| 12 | 0.2 | 0.02 | 160 | 4.5 | 53.2 | 4.6 ± 0.1 | 97.1 ± 1.6 | 1.4 ± 0.0 | 2.2 ± 0.0 | 4.4 ± 0.0 | 2.6 ± 0.0 | 7.7 ± 0.2 | 14.7 ± 2.9 | 41.6 ± 3.4 |
| 13 | 1.0 | 0.02 | 160 | 4.5 | 59.2 | 1.3 ± 0.0 | 85.2 ± 4.8 | 1.7 ± 0.0 | 3.6 ± 0.0 | 15.3 ± 0.3 | 5.4 ± 0.0 | 5.9 ± 0.0 | 14.3 ± 0.5 | 23.9 ± 1.0 |
| 14 | 0.2 | 0.20 | 160 | 4.5 | 66.6 | 31.7 ± 0.3 | 95.1 ± 1.0 | 1.7 ± 0.0 | 2.9 ± 0.0 | 6.0 ± 0.0 | 3.4 ± 0.0 | 6.6 ± 0.1 | 9.4 ± 1.0 | 21.9 ± 0.7 |
| 15 | 1.0 | 0.02 | 160 | 2.5 | 55.1 | 1.5 ± 0.0 | 97.6 ± 8.7 | 1.6 ± 0.0 | 3.1 ± 0.0 | 12.9 ± 0.0 | 5.4 ± 0.0 | 4.5 ± 1.9 | 23.5 ± 1.9 | 32.7 ± 0.5 |
| 16 | 1.0 | 0.02 | 120 | 2.5 | 65.9 | 1.5 ± 0.0 | 91.8 ± 0.6 | 2.0 ± 0.0 | 4.0 ± 0.0 | 10.8 ± 0.2 | 5.3 ± 0.1 | 3.6 ± 0.5 | 13.8 ± 2.7 | 30.2 ± 1.7 |
| 17 | 1.0 | 0.20 | 160 | 2.5 | 46.6 | 14.3 ± 0.1 | 101.6 ± 1.6 | 2.2 ± 0.1 | 13.7 ± 0.5 | 21.4 ± 0.9 | 13.3 ± 0.6 | 5.1 ± 0.1 | 19.5 ± 2.8 | 24.2 ± 2.2 |
| 18 | 0.2 | 0.20 | 120 | 2.5 | 56.7 | 32.9 ± 0.2 | 92.3 ± 8.9 | 1.5 ± 0.0 | 2.4 ± 0.0 | 4.8 ± 0.0 | 2.8 ± 0.0 | 9.1 ± 0.2 | 21.5 ± 1.4 | 35.7 ± 3.8 |
| 19 | 0.2 | 0.02 | 120 | 4.5 | 32.8 | 4.6 ± 0.4 | 96.7 ± 8.8 | 1.5 ± 0.0 | 2.2 ± 0.0 | 3.7 ± 0.0 | 2.4 ± 0.0 | 5.7 ± 1.4 | 14.7 ± 0.7 | 37.9 ± 3.0 |
| 20 | 0.2 | 0.02 | 160 | 4.5 | 51.7 | 4.6 ± 0.1 | 97.1 ± 1.6 | 1.5 ± 0.0 | 2.7 ± 0.0 | 7.1 ± 0.3 | 3.9 ± 0.2 | 7.7 ± 0.2 | 20.2 ± 2.9 | 32.1 ± 2.7 |
| 21 | 1.0 | 0.02 | 120 | 4.5 | 30.9 | 1.6 ± 0.1 | 100.4 ± 6.4 | 2.3 ± 0.0 | 12.7 ± 0.1 | 42.9 ± 0.6 | 18.0 ± 0.2 | 4.1 ± 0.4 | 9.9 ± 2.1 | 18.3 ± 0.4 |
| 22 | 1.0 | 0.20 | 120 | 4.5 | 13.9 | 13.2 ± 0.0 | 87.4 ± 7.2 | 2.0 ± 0.0 | 5.1 ± 0.2 | 34.0 ± 4.0 | 11.4 ± 0.3 | 3.2 ± 0.8 | 9.7 ± 1.4 | 16.0 ± 1.6 |
| 23 | 0.6 | 0.11 | 140 | 3.5 | 62.8 | 11.7 ± 0.2 | 97.1 ± 1.3 | 1.6 ± 0.0 | 2.8 ± 0.0 | 9.2 ± 0.4 | 4.4 ± 0.2 | 6.0 ± 0.2 | 6.1 ± 1.6 | 17.0 ± 3.3 |
| 24 | 0.2 | 0.20 | 160 | 2.5 | 57.0 | 33.5 ± 0.4 | 100.6 ± 1.3 | 1.5 ± 0.0 | 2.9 ± 0.0 | 8.2 ± 0.2 | 4.3 ± 0.1 | 8.7 ± 0.3 | 7.4 ± 2.1 | 23.6 ± 1.1 |
| 25 | 0.6 | 0.11 | 140 | 3.5 | 60.4 | 11.7 ± 0.2 | 97.1 ± 1.3 | 1.6 ± 0.0 | 2.7 ± 0.0 | 9.8 ± 0.3 | 4.7 ± 0.2 | 6.0 ± 0.2 | 9.4 ± 1.9 | 20.6 ± 0.9 |
| 26 | 0.2 | 0.20 | 120 | 4.5 | 42.8 | 32.4 ± 0.5 | 97.2 ± 1.6 | 1.7 ± 0.0 | 2.8 ± 0.0 | 5.3 ± 0.0 | 3.2 ± 0.0 | 6.9 ± 0.6 | 16.0 ± 0.6 | 23.8 ± 1.5 |
| 27 | 1.0 | 0.20 | 160 | 4.5 | 59.4 | 13.8 ± 0.2 | 96.6 ± 1.2 | 2.5 ± 0.0 | 6.5 ± 0.1 | 14.8 ± 0.6 | 7.7 ± 0.2 | 4.7 ± 0.2 | 15.5 ± 1.4 | 22.4 ± 1.0 |
| 28 | 1.0 | 0.02 | 120 | 4.5 | 25.9 | 1.6 ± 0.1 | 100.4 ± 6.4 | 1.7 ± 0.0 | 3.5 ± 0.0 | 36.0 ± 1.1 | 12.2 ± 0.4 | 4.1 ± 0.4 | 13.5 ± 0.7 | 22.5 ± 0.9 |
| 29 | 1.0 | 0.02 | 160 | 4.5 | 67.3 | 1.5 ± 0.1 | 93.9 ± 9.1 | 1.9 ± 0.0 | 3.9 ± 0.0 | 12.7 ± 0.1 | 5.8 ± 0.0 | 5.9 ± 0.0 | 14.3 ± 0.5 | 23.9 ± 1.0 |
| 30 | 0.2 | 0.20 | 120 | 2.5 | 57.5 | 32.9 ± 0.2 | 96.5 ± 3.0 | 1.5 ± 0.0 | 2.6 ± 0.0 | 6.3 ± 0.1 | 3.8 ± 0.1 | 9.1 ± 0.2 | 21.5 ± 1.4 | 35.7 ± 3.8 |
| 31 | 1.0 | 0.20 | 120 | 2.5 | 43.3 | 13.8 ± 0.3 | 96.4 ± 1.9 | 2.3 ± 0.0 | 14.9 ± 3.4 | 36.7 ± 7.8 | 18.5 ± 3.8 | 6.6 ± 0.0 | 13.4 ± 0.9 | 17.3 ± 0.9 |
| 32 | 1.0 | 0.20 | 160 | 2.5 | 55.6 | 14.3 ± 0.1 | 99.2 ± 1.9 | 2.6 ± 0.3 | 14.8 ± 2.0 | 26.7 ± 0.1 | 13.8 ± 0.0 | 5.1 ± 0.1 | 14.1 ± 0.5 | 19.4 ± 1.6 |
| Powder Sample | Homogeneity | Flowability | Spray Properties | ||
|---|---|---|---|---|---|
| D (%) | RSD (%) | Hausner Ratio | Powder Retention within Capsule (%) | CA (°) | |
| DSP-MS | / | / | 1.96 ± 0.18 | 14.8 ± 2.8 | 26.5 ± 0.3 |
| DSP-MS/Lactose 1:9 | 100.2 | 4.6 | 1.43 ± 0.00 | 6.3 ± 1.7 | 22.0 ± 0.5 |
| DSP-MS/Lactose 1:19 | 102.1 | 1.6 | 1.43 ± 0.10 | 6.0 ± 4.0 | 21.3 ± 0.4 |
| DSP-MS/Mannitol 1:9 | 96.9 | 4.1 | 1.33 ± 0.00 | 1.7 ± 0.0 | 20.6 ± 0.2 |
| DSP-MS/Mannitol 1:19 | 100.3 | 3.2 | 1.32 ± 0.07 | 1.7 ± 1.7 | 19.6 ± 1.0 |
| Lactose | / | / | 1.31 ± 0.10 | 5.5 ± 0.4 | 20.5 ± 0.6 |
| Mannitol | / | / | 1.14 ± 0.00 | 0.0 ± 0.0 | 18.7 ± 0.5 |
| – | Medium | Osmolality (mOsm kg−1) | TEER % of Initial Value in the Period 30–120 min of Experiment | Papp (10−6 cm s−1) |
|---|---|---|---|---|
| DSP-MS/Mannitol 1:19 | HBSS-Ca2+/water | 613 ± 4 | 63 ± 2–94 ± 5 | 0.65 ± 0.12 ‡ |
| DSP-MS | HBSS-Ca2+/water | 160 ± 3 | 16 ± 2–22 ± 3 | 3.03 ± 0.01 ‡ |
| DSP/Mannitol | HBSS-Ca2+/water | 470 ± 1 | 53 ± 5–93 ± 5 | 0.39 ± 0.01 |
| DSP | HBSS-Ca2+/water | 162 ± 1 | 17 ± 1–20 ± 2 | 3.43 ± 0.62 ‡ |
| DSP * | HBSS-Ca2+ | 304 ± 1 | 97 ± 4–103 ± 8 | 0.38 ± 0.06 |
| DB/Mannitol | HBSS-Ca2+/water | 460 ± 2 | 48 ± 6–73 ± 9 | 17.94 ± 1.61 |
| DB | HBSS-Ca2+/water | 161 ± 10 | 9 ± 1–13 ± 2 | 26.46 ± 2.94 † |
| DB * | HBSS-Ca2+ | 298 ± 1 | 102 ± 4–104 ± 12 | 18.65 ± 1.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nižić Nodilo, L.; Ugrina, I.; Špoljarić, D.; Amidžić Klarić, D.; Jakobušić Brala, C.; Perkušić, M.; Pepić, I.; Lovrić, J.; Saršon, V.; Safundžić Kučuk, M.; et al. A Dry Powder Platform for Nose-to-Brain Delivery of Dexamethasone: Formulation Development and Nasal Deposition Studies. Pharmaceutics 2021, 13, 795. https://doi.org/10.3390/pharmaceutics13060795
Nižić Nodilo L, Ugrina I, Špoljarić D, Amidžić Klarić D, Jakobušić Brala C, Perkušić M, Pepić I, Lovrić J, Saršon V, Safundžić Kučuk M, et al. A Dry Powder Platform for Nose-to-Brain Delivery of Dexamethasone: Formulation Development and Nasal Deposition Studies. Pharmaceutics. 2021; 13(6):795. https://doi.org/10.3390/pharmaceutics13060795
Chicago/Turabian StyleNižić Nodilo, Laura, Ivo Ugrina, Drago Špoljarić, Daniela Amidžić Klarić, Cvijeta Jakobušić Brala, Mirna Perkušić, Ivan Pepić, Jasmina Lovrić, Vesna Saršon, Maša Safundžić Kučuk, and et al. 2021. "A Dry Powder Platform for Nose-to-Brain Delivery of Dexamethasone: Formulation Development and Nasal Deposition Studies" Pharmaceutics 13, no. 6: 795. https://doi.org/10.3390/pharmaceutics13060795
APA StyleNižić Nodilo, L., Ugrina, I., Špoljarić, D., Amidžić Klarić, D., Jakobušić Brala, C., Perkušić, M., Pepić, I., Lovrić, J., Saršon, V., Safundžić Kučuk, M., Zadravec, D., Kalogjera, L., & Hafner, A. (2021). A Dry Powder Platform for Nose-to-Brain Delivery of Dexamethasone: Formulation Development and Nasal Deposition Studies. Pharmaceutics, 13(6), 795. https://doi.org/10.3390/pharmaceutics13060795