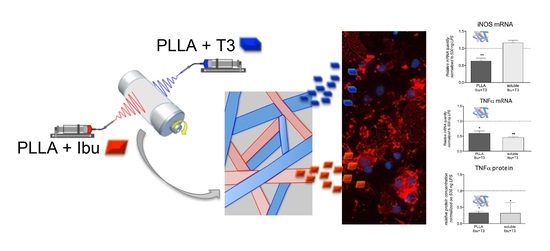
Design and In Vitro Study of a Dual Drug-Loaded Delivery System Produced by Electrospinning for the Treatment of Acute Injuries of the Central Nervous System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Scaffold Fabrication
2.2.2. Morphology, Diameter Distribution, Encapsulation Efficiency, and Thermal Characterization of the Fibers
2.2.3. HPLC-UV Method for Ibu Determination
2.2.4. UPLC–MS/MS Method for T3 Determination
2.3. In Vitro Drug Release Studies
2.4. In Vitro Efficacy and In Vitro Toxicity Tests
2.4.1. Cell Line Cultures (RAW 264.7 and DITNC1)
2.4.2. Cell Cultures of Primary Neural Stem Cell-Derived OPCs
2.4.3. Immunocytochemistry
2.4.4. Preparation of Conditioned Medium for Efficacy Studies on RAW 264.7
2.4.5. GLP Toxicity Assay for Ibuprofen and T3 Combination
2.4.6. GLP Toxicity Assay for Ibuprofen and T3 Released from PLLA Electrospun Scaffolds
2.5. RNA Isolation, Reverse Transcription RT-PCR, and Real-Time PCR
2.6. Cytokine Assay
3. Results and Discussion
3.1. Design of the Dual Drug-Loaded Delivery System
3.2. Development and Characterization of the Ibu-Loaded Electrospun Fibers
3.3. Preparation and Characterization of the Dual Drug-Loaded Delivery System
3.4. In Vitro Efficacy and In Vitro Toxicity Tests of the Dual Drug-Loaded Delivery System
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Donkor, E.S. Stroke in the21stCentury: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [Green Version]
- Mckee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.-R. Traumatic Brain Injury. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinstein, A.A. Treatment of Acute Ischemic Stroke. Contin. Lifelong Learn. Neurol. 2017, 23, 62–81. [Google Scholar] [CrossRef]
- Manners, J.; Steinberg, A.; Shutter, L. Early management of acute cerebrovascular accident. Curr. Opin. Crit. Care 2017, 23, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Faden, A.I. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010, 31, 596–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vink, R.; Nimmo, A.J. Multifunctional drugs for head injury. Neurotherapeutics 2009, 6, 28–42. [Google Scholar] [CrossRef] [Green Version]
- Bolognesi, M.L. Harnessing Polypharmacology with Medicinal Chemistry. ACS Med. Chem. Lett. 2019, 10, 273–275. [Google Scholar] [CrossRef] [Green Version]
- Barabási, A.-L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2010, 12, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Albertini, C.; Salerno, A.; Pinheiro, P.D.S.M.; Bolognesi, M.L. From combinations to multitarget-directed ligands: A continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2020. [Google Scholar] [CrossRef]
- Aryal, S.; Hu, C.-M.J.; Zhang, L. Combinatorial Drug Conjugation Enables Nanoparticle Dual-Drug Delivery. Small 2010, 6, 1442–1448. [Google Scholar] [CrossRef]
- Bighinati, A.; Focarete, M.L.; Gualandi, C.; Pannella, M.; Giuliani, A.; Beggiato, S.; Ferraro, L.; Lorenzini, L.; Giardino, L.; Calzà, L. Improved Functional Recovery in Rat Spinal Cord Injury Induced by a Drug Combination Administered with an Implantable Polymeric Delivery System. J. Neurotrauma 2020, 37, 1708–1719. [Google Scholar] [CrossRef]
- Fernández-Carballido, A.; Herrero-Vanrell, R.; Molina-Martínez, I.; Pastoriza, P. Biodegradable ibuprofen-loaded PLGA microspheres for intraarticular administration. Int. J. Pharm. 2004, 279, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Csányi, E.; Sütő, B.; Berkó, S.; Kozma, G.; Kukovecz, Á.; Budai-Szűcs, M.; Erős, G.; Kemény, L.; Sztojkov-Ivanov, A.; Gaspar, R. Development of ibuprofen-loaded nanostructured lipid carrier-based gels: Characterization and investigation of in vitro and in vivo penetration through the skin. Int. J. Nanomed. 2016, 11, 1201–1212. [Google Scholar] [CrossRef] [Green Version]
- Kuhlmann, T.; Miron, V.; Cuo, Q.; Wegner, C.; Antel, J.; Bruck, W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 2008, 131, 1749–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guideline on Bioanalytical Method Validation. EMEA/CHMP/EWP/192217/2009. 2009. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 21 July 2011).
- Baldassarro, V.A.; Krezel, W.; Fernández, M.; Schuhbaur, B.; Giardino, L.; Calzà, L. The role of nuclear receptors in the differentiation of oligodendrocyte precursor cells derived from fetal and adult neural stem cells. Stem Cell Res. 2019, 37, 101443. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef]
- Fernández, M.; Baldassarro, V.A.; Sivilia, S.; Giardino, L.; Calzà, L. Inflammation severely alters thyroid hormone signaling in the central nervous system during experimental allergic encephalomyelitis in rat: Direct impact on OPCs differentiation failure. Glia 2016, 64, 1573–1589. [Google Scholar] [CrossRef] [PubMed]
- Bergold, P.J. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp. Neurol. 2016, 275, 367–380. [Google Scholar] [CrossRef]
- Nishio, Y.; Koda, M.; Kitajo, K.; Seto, M.; Hata, K.; Taniguchi, J.; Moriya, H.; Fujitani, M.; Kubo, T.; Yamashita, T. Delayed treatment with Rho-kinase inhibitor does not enhance axonal regeneration or functional recovery after spinal cord injury in rats. Exp. Neurol. 2006, 200, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Z.; Qin, H.; Yao, Z.-X. Thyroid Hormone Potentially Benefits Multiple Sclerosis via Facilitating Remyelination. Mol. Neurobiol. 2015, 53, 4406–4416. [Google Scholar] [CrossRef] [PubMed]
- Calzà, L.; Baldassarro, V.A.; Fernandez, M.; Giuliani, A.; Lorenzini, L.; Giardino, L. Chapter Eleven—Thyroid Hormone and the White Matter of the Central Nervous System: From Development to Repair. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 106, pp. 253–281. ISBN 0083-6729. [Google Scholar]
- Breton, J.; Long, K.; Barraza, M.; Perloff, O.; Kaufer, D. Hormonal Regulation of Oligodendrogenesis II: Implications for Myelin Repair. Biomolecules 2021, 11, 290. [Google Scholar] [CrossRef]
- Wooliscroft, L.; Altowaijri, G.; Hildebrand, A.; Samuels, M.; Oken, B.; Bourdette, D.; Cameron, M. Phase I randomized trial of liothyronine for remyelination in multiple sclerosis: A dose-ranging study with assessment of reliability of visual outcomes. Mult. Scler. Relat. Disord. 2020, 41, 102015. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, X.; He, Y.; Ma, J.; Ni, G.; Zhou, S. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications. Prog. Polym. Sci. 2018, 81, 80–113. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Bravo, J.M.C.; Medina, A.S.; González, G.L.P.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.J.; Kaplan, D.L. Poly(lactic-co-glycolic) Acid-Controlled-Release Systems: Experimental and Modeling Insights. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 257–276. [Google Scholar] [CrossRef]
- Lambrechts, M.J.; Cook, J.L. Nonsteroidal Anti-Inflammatory Drugs and Their Neuroprotective Role after an Acute Spinal Cord Injury: A Systematic Review of Animal Models. Glob. Spine J. 2021, 11, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, P.; Marracci, G.; Calkins, E.; Pocius, E.; Bensen, A.; Scanlan, T.; Emery, B.; Bourdette, D. Thyroid hormone and thyromimetics inhibit myelin and axonal degeneration and oligodendrocyte loss in EAE. J. Neuroimmunol. 2021, 352, 577468. [Google Scholar] [CrossRef]
- Fernández, M.; Baldassarro, V.A.; Capirossi, R.; Montevecchi, R.; Bonavita, J.; Cescatti, M.; Giovannini, T.; Giovannini, G.; Uneddu, M.; Giovanni, G.; et al. Possible Strategies to Optimize a Biomarker Discovery Approach to Correlate with Neurological Outcome in Patients with Spinal Cord Injury: A Pilot Study. J. Neurotrauma 2020, 37, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Guideline on the Non-Clinical Development of Fixed Combinations of Medicinal Products. EMEA/CHMP/SWP/258498/2005. 2008. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-non-clinical-development-fixed-combinations-medicinal-products_en.pdf (accessed on 24 January 2008).
- Karimi-Abdolrezaee, S.; Billakanti, R. Reactive Astrogliosis after Spinal Cord Injury—Beneficial and Detrimental Effects. Mol. Neurobiol. 2012, 46, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2015, 7, a020420. [Google Scholar] [CrossRef] [Green Version]
- Dezonne, R.S.; Lima, F.R.S.; Trentin, A.G.; Gomes, F.C.A. Thyroid Hormone and Astroglia: Endocrine Control of the Neural Environment. J. Neuroendocr. 2015, 27, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, C.; Govoni, M.; Foroni, L.; Valente, S.; Bianchi, M.; Giordano, E.; Pasquinelli, G.; Biscarini, F.; Focarete, M.L. Ethanol disinfection affects physical properties and cell response of electrospun poly(l-lactic acid) scaffolds. Eur. Polym. J. 2012, 48, 2008–2018. [Google Scholar] [CrossRef]
- Mukherjee, S.; Gualandi, C.; Focarete, M.L.; Ravichandran, R.; Venugopal, J.R.; Raghunath, M.; Ramakrishna, S. Elastomeric electrospun scaffolds of poly(l-lactide-co-trimethylene carbonate) for myocardial tissue engineering. J. Mater. Sci. Mater. Electron. 2011, 22, 1689–1699. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Mannila, A.; Rautio, J.; Lehtonen, M.; Järvinen, T.; Savolainen, J. Inefficient central nervous system delivery limits the use of ibuprofen in neurodegenerative diseases. Eur. J. Pharm. Sci. 2005, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bannwarth, B.; Lapicque, F.; Pehourcq, F.; Gillet, P.; Schaeverbeke, T.; Laborde, C.; Dehais, J.; Gaucher, A.; Netter, P. Stereoselective disposition of ibuprofen enantiomers in human cerebrospinal fluid. Br. J. Clin. Pharmacol. 1995, 40, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Calzà, L.; Fernández, M.; Giardino, L. Role of the Thyroid System in Myelination and Neural Connectivity. In Comprehensive Physiology; American Cancer Society: Atlanta, GA, USA, 2015; Volume 5, pp. 1405–1421. [Google Scholar]








| Sample | Flow Rate (mL/h) | Needle-to-Collector Distance (cm) | Voltage (kV) | Ibu EE (% w/w) |
|---|---|---|---|---|
| PLGA 50:50 | 0.8 | 20 | 20 | 108.2 ± 0.8 |
| PLGA 75:25 | 1.5 | 20 | 18 | 109.0 ± 3.2 |
| PLGA 85:15 | 0.8 | 20 | 18 | 102.5 ± 2.1 |
| PLLA-micro | 2.4 | 20 | 18 | 101.0 ± 8.3 |
| PLLA-nano | 1.2 | 20 | 18 | 95.0 ± 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolci, L.S.; Perone, R.C.; Di Gesù, R.; Kurakula, M.; Gualandi, C.; Zironi, E.; Gazzotti, T.; Tondo, M.T.; Pagliuca, G.; Gostynska, N.; et al. Design and In Vitro Study of a Dual Drug-Loaded Delivery System Produced by Electrospinning for the Treatment of Acute Injuries of the Central Nervous System. Pharmaceutics 2021, 13, 848. https://doi.org/10.3390/pharmaceutics13060848
Dolci LS, Perone RC, Di Gesù R, Kurakula M, Gualandi C, Zironi E, Gazzotti T, Tondo MT, Pagliuca G, Gostynska N, et al. Design and In Vitro Study of a Dual Drug-Loaded Delivery System Produced by Electrospinning for the Treatment of Acute Injuries of the Central Nervous System. Pharmaceutics. 2021; 13(6):848. https://doi.org/10.3390/pharmaceutics13060848
Chicago/Turabian StyleDolci, Luisa Stella, Rosaria Carmela Perone, Roberto Di Gesù, Mallesh Kurakula, Chiara Gualandi, Elisa Zironi, Teresa Gazzotti, Maria Teresa Tondo, Giampiero Pagliuca, Natalia Gostynska, and et al. 2021. "Design and In Vitro Study of a Dual Drug-Loaded Delivery System Produced by Electrospinning for the Treatment of Acute Injuries of the Central Nervous System" Pharmaceutics 13, no. 6: 848. https://doi.org/10.3390/pharmaceutics13060848
APA StyleDolci, L. S., Perone, R. C., Di Gesù, R., Kurakula, M., Gualandi, C., Zironi, E., Gazzotti, T., Tondo, M. T., Pagliuca, G., Gostynska, N., Baldassarro, V. A., Cescatti, M., Giardino, L., Focarete, M. L., Calzà, L., Passerini, N., & Bolognesi, M. L. (2021). Design and In Vitro Study of a Dual Drug-Loaded Delivery System Produced by Electrospinning for the Treatment of Acute Injuries of the Central Nervous System. Pharmaceutics, 13(6), 848. https://doi.org/10.3390/pharmaceutics13060848