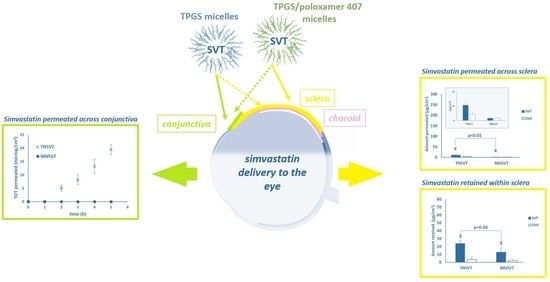
Preliminary Investigation on Simvastatin-Loaded Polymeric Micelles in View of the Treatment of the Back of the Eye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Micelle Preparations
2.2.1. TPGS Micelles (TN)
2.2.2. TPGS/P407 Mixed Micelles (MN)
2.3. SVT Solubility in Micelles
2.4. Viscosity
2.5. Dynamic Light Scattering
2.6. Stability
2.7. Ocular Tissues Preparation
2.8. Validation of SVT and SVA Extraction Method from Sclera and Choroid
2.9. Ex Vivo Experiments
2.9.1. Trans-Conjunctival Permeation
2.9.2. Trans-Scleral Permeation and Retention Experiments
2.10. HPLC/UV-Vis Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Micelles Characterization and Stability
3.2. Trans-Conjunctival Studies
3.3. Trans-Scleral Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
References
- World Health Organization. World Report on Vision; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Apte, R.S. Targeting Tissue Lipids in Age-related Macular Degeneration. EBioMedicine 2016, 5, 26–27. [Google Scholar] [CrossRef] [Green Version]
- Mullins, R.F.; Russell, S.R.; Anderson, D.H.; Hageman, G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000, 14, 835–846. [Google Scholar] [CrossRef]
- Curcio, C.A.; Presley, J.B.; Millican, C.L.; Medeiros, N.E. Basal deposits and drusen in eyes with age-related maculopathy: Evidence for solid lipid particles. Exp. Eye Res. 2005, 80, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roizenblatt, M.; Naranjit, N.; Maia, M.; Gehlbach, P.L. The Question of a Role for Statins in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2018, 19, 3688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooi, K.G.-J.; Khoo, P.; Vaclavik, V.; Watson, S.L. Statins in ophthalmology. Surv. Ophthalmol. 2019, 64, 401–432. [Google Scholar] [CrossRef]
- Vavvas, D.G.; Daniels, A.B.; Kapsala, Z.G.; Goldfarb, J.W.; Ganotakis, E.; Loewenstein, J.I.; Young, L.H.; Gragoudas, E.S.; Eliott, D.; Kim, I.; et al. Regression of Some High-risk Features of Age-related Macular Degeneration (AMD) in Patients Receiving Intensive Statin Treatment. EBioMedicine 2016, 5, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Guymer, R.H.; Baird, P.N.; Varsamidis, M.; Busija, L.; Dimitrov, P.N.; Aung, K.Z.; Makeyeva, G.A.; Richardson, A.J.; Lim, L.; Robman, L.D. Proof of Concept, Randomized, Placebo-Controlled Study of the Effect of Simvastatin on the Course of Age-Related Macular Degeneration. PLoS ONE 2013, 8, e83759. [Google Scholar] [CrossRef]
- Fong, D.S.; Contreras, R. Recent Statin Use and 1-Year Incidence of Exudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2010, 149, 955–958. [Google Scholar] [CrossRef]
- Rajeshuni, N.; Ludwig, C.A.; Moshfeghi, D.M. The effect of statin exposure on choroidal neovascularization in nonexudative age-related macular degeneration patients. Eye 2018, 33, 163–165. [Google Scholar] [CrossRef]
- Mast, N.; Bederman, I.R.; Pikuleva, I.A. Retinal Cholesterol Content Is Reduced in Simvastatin-Treated Mice Due to Inhibited Local Biosynthesis Albeit Increased Uptake of Serum Cholesterol. Drug Metab. Dispos. 2018, 46, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gillies, M.; Wang, Y.; Shen, W.; Bahrami, B.; Zeng, S.; Zhu, M.; Yao, W.; Zhou, F.; Murray, M.; et al. Simvastatin protects photoreceptors from oxidative stress induced by all- trans -retinal, through the up-regulation of interphotoreceptor retinoid binding protein. Br. J. Pharmacol. 2019, 176, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Pikuleva, I.A.; Curcio, C.A. Cholesterol in the retina: The best is yet to come. Prog. Retin. Eye Res. 2014, 41, 64–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef] [Green Version]
- Al-Janabi, A.; Lightman, S.; Tomkins-Netzer, O. ‘Statins in retinal disease’. Eye 2018, 32, 981–991. [Google Scholar] [CrossRef]
- Sasaki, M.; Gan, W.L.; Kawasaki, R.; Hodgson, L.; Lee, K.Y.; Wong, T.Y.; Lamoureux, E.; Robman, L.; Guymer, R. Effect of simvastatin on retinal vascular caliber: The Age-Related Maculopathy Statin Study. Acta Ophthalmol. 2013, 91, e418–e419. [Google Scholar] [CrossRef]
- Talwar, N.; Musch, D.C.; Stein, J.D. Association of Daily Dosage and Type of Statin Agent with Risk of Open-Angle Glaucoma. JAMA Ophthalmol. 2017, 135, 263–267. [Google Scholar] [CrossRef]
- Borkar, D.S.; Tham, V.M.; Shen, E.; Parker, J.V.; Uchida, A.; Vinoya, A.C.; Acharya, N.R. Association between statin use and uveitis: Results from the Pacific Ocular Inflammation study. Am. J. Ophthalmol. 2015, 159, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Rojas, B.; Ramírez, A.I.; Salazar, J.J.; De Hoz, R.; Redondo, A.; Raposo, R.; Méndez, T.; Tejerina, T.; Triviño, A.; Ramírez, J.M. Low-dosage statins reduce choroidal damage in hypercholesterolemic rabbits. Acta Ophthalmol. 2010, 89, 660–669. [Google Scholar] [CrossRef]
- Loukovaara, S.; Sahanne, S.; Takala, A.; Haukka, J. Statin use and vitreoretinal surgery: Findings from a Finnish population-based cohort study. Acta Ophthalmol. 2018, 96, 442–451. [Google Scholar] [CrossRef] [Green Version]
- VanderBeek, B.L.; Zacks, D.; Talwar, N.; Nan, B.; Stein, J.D. Role of Statins in the Development and Progression of Age-Related Macular Degeneration. Retina 2013, 33, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Gehlbach, P.; Li, T.; Hatef, E. Statins for age-related macular degeneration. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Mizranita, V.; Pratisto, E.H. Statin-associated ocular disorders: The FDA and ADRAC data. Int. J. Clin. Pharm. 2015, 37, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.G.-J.; Wakefield, D.; Billson, F.A.; Watson, S.L. Efficacy and Safety of Topical Atorvastatin for the Treatment of Dry Eye Associated with Blepharitis: A Pilot Study. Ophthalmic Res. 2015, 54, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, I.R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement from the American Heart Association. Arter. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Schiavone, N.; Aranguez, A.I.G.; Giansanti, F.; Papucci, L.; De Lara, M.J.P.; Singh, M.; Kaur, I.P. Atorvastatin-loaded solid lipid nanoparticles as eye drops: Proposed treatment option for age-related macular degeneration (AMD). Drug Deliv. Transl. Res. 2020, 10, 919–944. [Google Scholar] [CrossRef]
- Pereira-Da-Mota, A.; Vivero-Lopez, M.; Topete, A.; Serro, A.; Concheiro, A.; Alvarez-Lorenzo, C. Atorvastatin-Eluting Contact Lenses: Effects of Molecular Imprinting and Sterilization on Drug Loading and Release. Pharmaceutics 2021, 13, 606. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Topical application of polymeric nanomicelles in ophthalmology: A review on research efforts for the noninvasive delivery of ocular therapeutics. Expert Opin. Drug Deliv. 2019, 16, 397–413. [Google Scholar] [CrossRef]
- Campos, P.M.; Petrilli, R.; Lopez, R.F. The prominence of the dosage form design to treat ocular diseases. Int. J. Pharm. 2020, 586, 119577. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Polymeric micelles as drug stabilizers: The camptothecin and simvastatin cases. J. Drug Deliv. Sci. Technol. 2010, 20, 249–257. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Poloxamer 407/TPGS Mixed Micelles as Promising Carriers for Cyclosporine Ocular Delivery. Mol. Pharm. 2018, 15, 571–584. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, F.; Buckett, L.; Davidson, R.; Holdgate, G.; McCormick, A.; Schneck, D.; Smith, G.; Warwick, M. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy- 3-methylglutaryl coenzyme A reductase inhibitor. Am. J. Cardiol. 2001, 87, 28–32. [Google Scholar] [CrossRef]
- SciFinder. SciFinder-n, Version 2021; Chemical Abstract Service: Columbus, OH, USA, 2021; Available online: https://scifinder.cas.org (accessed on 25 April 2021).
- Joshi, H.N.; Fakes, M.G.; Serajuddin, A.T.M. Differentiation of 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase Inhibitors by Their Relative Lipophilicity. Pharm. Pharmacol. Commun. 1999, 5, 269–271. [Google Scholar] [CrossRef]
- McKay, A.; Leung, B.P.; McInnes, I.B.; Thomson, N.C.; Liew, F.Y. A Novel Anti-Inflammatory Role of Simvastatin in a Murine Model of Allergic Asthma. J. Immunol. 2004, 172, 2903–2908. [Google Scholar] [CrossRef] [Green Version]
- Clementino, A.; Sonvico, F. Development and validation of a RP-HPLC method for the simultaneous detection and quantification of simvastatin’s isoforms and coenzyme Q10 in lecithin/chitosan nanoparticles. J. Pharm. Biomed. Anal. 2018, 155, 33–41. [Google Scholar] [CrossRef]
- Pescina, S.; Lucca, L.G.; Govoni, P.; Padula, C.; Del Favero, E.; Cantù, L.; Santi, P.; Nicoli, S. Ex Vivo Conjunctival Retention and Transconjunctival Transport of Poorly Soluble Drugs Using Polymeric Micelles. Pharmaceutics 2019, 11, 476. [Google Scholar] [CrossRef] [Green Version]
- Pescina, S.; Govoni, P.; Antopolsky, M.; Murtomaki, L.; Padula, C.; Santi, P.; Nicoli, S. Permeation of Proteins, Oligonucleotide and Dextrans Across Ocular Tissues: Experimental Studies and a Literature Update. J. Pharm. Sci. 2015, 104, 2190–2202. [Google Scholar] [CrossRef]
- Pescina, S.; Santi, P.; Ferrari, G.; Padula, C.; Cavallini, P.; Govoni, P.; Nicoli, S. Ex vivo models to evaluate the role of ocular melanin in trans-scleral drug delivery. Eur. J. Pharm. Sci. 2012, 46, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Batger, M.; Garrastazu, G.; Pozzoli, M.; Del Favero, E.; Rondelli, V.; Gutfilen, B.; Barboza, T.; Sukkar, M.B.; Souza, S.A.L.; et al. The nasal delivery of nanoencapsulated statins—an approach for brain delivery. Int. J. Nanomed. 2016, 11, 6575–6590. [Google Scholar] [CrossRef] [Green Version]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef] [PubMed]
- FDA. [Email Protected]. Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/ (accessed on 7 February 2012).
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Salehi, R.; Jelvehgari, M. Preparation and evaluation of PCL-PEG-PCL micelles as potential nanocarriers for ocular delivery of dexamethasone. Iran. J. Basic Med. Sci. 2018, 21, 153–164. [Google Scholar]
- Vaishya, R.D.; Gokulgandhi, M.; Patel, S.; Minocha, M.; Mitra, A.K. Novel Dexamethasone-Loaded Nanomicelles for the Intermediate and Posterior Segment Uveitis. AAPS PharmSciTech 2014, 15, 1238–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsaid, N.; Somavarapu, S.; Jackson, T.L. Cholesterol-poly(ethylene) glycol nanocarriers for the transscleral delivery of sirolimus. Exp. Eye Res. 2014, 121, 121–129. [Google Scholar] [CrossRef]
- Suksiriworapong, J.; Rungvimolsin, T.; A-gomol, A.; Junyaprasert, V.B.; Chantasart, D. Development and Characterization of Lyophilized Diazepam-Loaded Polymeric Micelles. AAPS PharmSciTech 2013, 15, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Owen, S.C.; Chan, D.P.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- González-López, J.; Sandez-Macho, I.; Concheiro, A.; Alvarez-Lorenzo, C. Poloxamines and Poloxamers as Polymeric Micellar Carriers for Simvastatin: Interactions at the Air−Water Interface and in Bulk Solution. J. Phys. Chem. C 2010, 114, 1181–1189. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Vickers, S.; Duncan, C.A.; Chen, I.W.; Rosegay, A.; Duggan, E.D. Metabolic disposition studies on simvastatin, a cholesterol-lowering prodrug. Drug Metab. Dispos. 1990, 18, 138–145. [Google Scholar] [PubMed]
- Duvvuri, S.; Majumdar, S.; Mitra, A.K. Role of Metabolism in Ocular Drug Delivery. Curr. Drug Metab. 2004, 5, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, E.M.; del Amo, E.M.; Ranta, V.-P.; Urtti, A.; Vellonen, K.-S.; Ruponen, M. Esterase activity in porcine and albino rabbit ocular tissues. Eur. J. Pharm. Sci. 2018, 123, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Vree, T.B.; Dammers, E.; Ulč, I.; Horkovics-Kovats, S.; Ryska, M.; Merkx, I. Differences between Lovastatin and Simvastatin Hydrolysis in Healthy Male and Female Volunteers: Gut Hydrolysis of Lovastatin is Twice that of Simvastatin. Sci. World J. 2003, 3, 1332–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Tommaso, C.; Bourges, J.-L.; Valamanesh, F.; Trubitsyn, G.; Torriglia, A.; Jeanny, J.-C.; Behar-Cohen, F.; Gurny, R.; Möller, M. Novel micelle carriers for cyclosporin A topical ocular delivery: In vivo cornea penetration, ocular distribution and efficacy studies. Eur. J. Pharm. Biopharm. 2012, 81, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.-I.; Lee, V.H.; Kim, K.-J. Roles of the conjunctiva in ocular drug delivery: A review of conjunctival transport mechanisms and their regulation. Eur. J. Pharm. Biopharm. 2005, 60, 227–240. [Google Scholar] [CrossRef]
- Pepic, I.; Lovrić, J.; Filipovic-Grcic, J. Polymeric micelles in ocular drug delivery: Rationale, strategies and challenges. Chem. Biochem. Eng. Q. 2012, 26, 365–377. [Google Scholar]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathalla, Z.M.A.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Watt, R.P.; Khatri, H.; Dibble, A.R.G. Injectability as a function of viscosity and dosing materials for subcutaneous administration. Int. J. Pharm. 2019, 554, 376–386. [Google Scholar] [CrossRef]
- Leblanc, B.; Jezequel, S.; Davies, T.; Hanton, G.; Taradach, C. Binding of Drugs to Eye Melanin Is Not Predictive of Ocular Toxicity. Regul. Toxicol. Pharmacol. 1998, 28, 124–132. [Google Scholar] [CrossRef]
- Sajjan, S.S.; SantoshKumar, M.; Sanjeevkumar, S.; Karegoudar, T.B. Binding affinity of amlodipine, atorvastatin and telmisartan drugs to purified bacterial melanin pigment: A kinetic study. J. Pharm. Investig. 2013, 43, 267–278. [Google Scholar] [CrossRef]
- Bridelli, M.G.; Ciati, A.; Crippa, P.R. Binding of chemicals to melanins re-examined: Adsorption of some drugs to the surface of melanin particles. Biophys. Chem. 2006, 119, 137–145. [Google Scholar] [CrossRef]



| Time Zero | 48 h | 1 Month | ||||
|---|---|---|---|---|---|---|
| Code a | PdI | Size (nm and % Intensity) | PdI | Size (nm and % Intensity) | PdI | Size (nm and % Intensity) |
| TN | 0.081 | 12.4 ± 3.6 (100) | 0.024 | 11.7 ± 2.8 (100) | 0.046 | 11.6 ± 3.0 (100) |
| TNSVT | 0.142 | 13.2 ± 3.8 (100) | 0.087 | 12.3 ± 3.8 (100) | 0.032 | 11.7 ± 3.0 (100) |
| MN | 0.26 | 5.0 ± 1.5 (49) | 0.436 | 6.2 ± 2.5 (49.7) | 0.201 | 4.9 ± 1.5 (41.6) |
| 27.8 ± 8.8 (42.7) | 34.0 ± 15.1 (40.6) | 31.0 ± 10.8 (51.6) | ||||
| 165.1 ± 52.3 (8.3) | 873.1 ± 480.8 (5.2) | 158.8 ± 41.4 (6.9) | ||||
| MNSVT | 0.348 | 5.0 ± 1.2 (48.9) | 0.367 | 5.3 ± 1.5 (42.1) | 0.509 | 5.7 ± 1.6 (42.3) |
| 25.5 ± 6.7 (40.2) | 25.0 ± 6.5 (40.7) | 32.7 ± 10.8 (39.5) | ||||
| 98.6 ± 17.8 (10.9) | 636.6 ± 124.6 (16.7) | 1443 ± 794.2 (18.2) | ||||
| 4 °C | 25 °C | |||
|---|---|---|---|---|
| Storage Time | TNSVT (SVT%) | MNSVT (SVT%) | TNSVT (SVT%) | MNSVT (SVT%) |
| 24 h | 96.9 ± 1.1 | 94.6 ± 6.8 | 92.7 ± 3.9 | 87.0 ± 13.9 |
| 48 h | 91.8 ± 6.1 | 90.3 ± 12.1 | 86.7 ± 4.2 | 80.3 ± 8.8 |
| 1 month | 79.5 ± 3.1 | 100.7 ± 0.3 | 32.4 ± 1.7 | 80.6 ± 4.1 |
| Permeation | Retention | |||
|---|---|---|---|---|
| Code a | SVT (µg/cm2) | SVA (µg/cm2) | SVT (µg/cm2) | SVA (µg/cm2) |
| TNSVT | 10.61 ± 3.27 b | 4.26 ± 1.06 | 23.81 ± 3.88 c | 3.54 ± 2.69 |
| MNSVT | 1.55 ± 0.36 b | 1.61 ± 0.66 | 12.65 ± 5.39 c | 1.95 ± 1.56 |
| SVA sol | 0 d | 251.93 ± 31.23 | 0 d | 51.42 ± 23.60 |
| Sclera | Choroid | |||
|---|---|---|---|---|
| SVT (µg/cm2) | SVA (µg/cm2) | SVT (µg/cm2) | SVA (µg/cm2) | |
| Pigmented Choroid | 30.07 ± 4.97 | 2.59 ± 0.74 | 1.01 ± 1.29 | 0 a |
| Not pigmented Choroid | 30.15 ± 11.98 | 3.38 ± 1.05 | 1.06 ± 0.66 | 0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pescina, S.; Sonvico, F.; Clementino, A.; Padula, C.; Santi, P.; Nicoli, S. Preliminary Investigation on Simvastatin-Loaded Polymeric Micelles in View of the Treatment of the Back of the Eye. Pharmaceutics 2021, 13, 855. https://doi.org/10.3390/pharmaceutics13060855
Pescina S, Sonvico F, Clementino A, Padula C, Santi P, Nicoli S. Preliminary Investigation on Simvastatin-Loaded Polymeric Micelles in View of the Treatment of the Back of the Eye. Pharmaceutics. 2021; 13(6):855. https://doi.org/10.3390/pharmaceutics13060855
Chicago/Turabian StylePescina, Silvia, Fabio Sonvico, Adryana Clementino, Cristina Padula, Patrizia Santi, and Sara Nicoli. 2021. "Preliminary Investigation on Simvastatin-Loaded Polymeric Micelles in View of the Treatment of the Back of the Eye" Pharmaceutics 13, no. 6: 855. https://doi.org/10.3390/pharmaceutics13060855
APA StylePescina, S., Sonvico, F., Clementino, A., Padula, C., Santi, P., & Nicoli, S. (2021). Preliminary Investigation on Simvastatin-Loaded Polymeric Micelles in View of the Treatment of the Back of the Eye. Pharmaceutics, 13(6), 855. https://doi.org/10.3390/pharmaceutics13060855