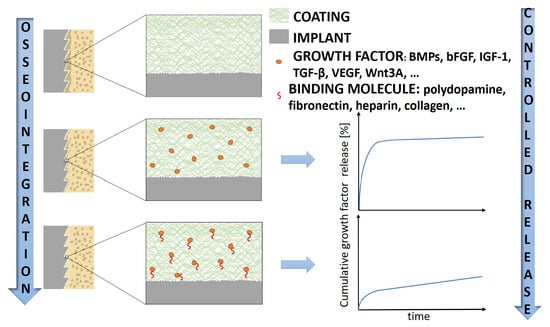
The Role of Growth Factors in Bioactive Coatings
Abstract
:1. Introduction
2. Bioactive Coatings and GFs
2.1. Bone Morphogenic Protein-2 (BMP-2)
2.2. Bone Morphogenic Protein-7 (BMP-7)
2.3. Basic Fibroblast Growth Factor (bFGF)
2.4. The Wingless-Type MMTV Integration Site Family Member 3A (Wnt3A)
2.5. Insulin-Like Growth Factor-1 (IGF-1)
2.6. Vascular Endothelial Growth Factor (VEGF)
2.7. Platelet-derived growth factor BB (PDGF-BB)
2.8. The Influence of Coating Materials and the Indirect Involvement of GFs
2.8.1. Synthetic Coatings
2.8.2. Coatings Based on Naturally Occurring Compounds
3. Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| bFGF | basic fibroblast growth factor |
| BHK-21 | baby hamster kidney cell |
| BMP | bone morphogenic protein |
| BMP-2 | bone morphogenic protein-2 |
| BMP-4 | bone morphogenic protein-4 |
| BMP-6 | bone morphogenic protein-6 |
| BMP-7 | bone morphogenic protein-7 |
| BMSC | bone mesenchymal stem cell |
| COPROG | copolymer-protected gene vector |
| D-RADA16 | biocompatible peptide, comprising arginine (R), alanine (A), and aspartate (D) |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| FDA | Food and Drug Administration |
| FN | fibronectin |
| GF | growth factor |
| HA | hydroxyapatite |
| hAD-MSC | human adipose tissue-derived mesenchymal stem cell |
| IAPP | ion-assisted plasma polymer |
| IGF | insulin-like growth factor |
| IGF-1 | insulin-like growth factor-1 |
| miRNA | micro ribonucleic acid |
| mRNA | messenger ribonucleic acid |
| MSC | mesenchymal stem cell |
| NP | nanoparticle |
| PBMC | peripheral blood mononuclear cell |
| PCL | poly(ε-caprolactone) |
| PDLLA | poly(D,L-lactide) |
| pDNA | plasmid-deoxyribonucleic acid |
| PEEK | polyetheretherketone |
| PEG | poly(ethylene glycol) |
| PLA | polylactic acid |
| PLGA | poly (lactic-co-glycolic acid) |
| PRP | platelet-rich plasma |
| RGD | Arg-Gly-Asp |
| rhPDGF-BB | recombinant human platelet-derived growth factor BB |
| Runx2 | runt-related transcription factor 2 |
| TGF-β | transforming growth factor-β |
| TGF-β1 | transforming growth factor-β1 |
| TGF-β2 | transforming growth factor-β2 |
| Ti-HA | titanium implant infiltrated with hydroxyapatite |
| VEGF | vascular endothelial growth factor |
| VEGFA | vascular endothelial growth factor A |
| Wnt3A | wingless-type MMTV integration site family member 3A |
References
- American Academy of Orthopaedic Surgeons. Total Joint Replacement. Available online: https://orthoinfo.aaos.org/en/treatment/total-joint-replacement/ (accessed on 3 December 2020).
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef]
- Marino, A.; Pontikaki, I.; Truzzi, M.; Menon, A.; Artusi, C.; Di Marco, M.; Randelli, P.S.; Cimaz, R.; Viganò, R. Early joint replacement in juvenile idiopathic arthritis (JIA): Trend over time and factors influencing implant survival. Arthritis Care Res. 2020. [Google Scholar] [CrossRef]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: A population-based cohort study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.T.; Evans, J.P.; Walker, R.W.; Blom, A.W.; Whitehouse, M.R.; Sayers, A. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019, 393, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.-T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Bohm, E.R.; Dunbar, M.J.; Frood, J.J.; Johnson, T.M.; Morris, K.A. Rehospitalizations, Early Revisions, Infections, and Hospital Resource Use in the First Year After Hip and Knee Arthroplasties. J. Arthroplast. 2012, 27, 232–237.e231. [Google Scholar] [CrossRef]
- Dalury, D.F.; Pomeroy, D.L.; Gorab, R.S.; Adams, M.J. Why are Total Knee Arthroplasties Being Revised? J. Arthroplast. 2013, 28, 120–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Q.; Liu, W.; Tang, Y.; Liu, J.; Zhang, H.; Liu, X.; Liu, J.; Yang, J.; Zhang, L.-C.; et al. Multi-scale hybrid modified coatings on titanium implants for non-cytotoxicity and antibacterial properties. Nanoscale 2021, 13, 10587–10599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, L.; Liu, S.; Cao, P.; Yang, J.; Wang, L. Nanostructured Titanium Alloys Surface Modification Technology for Antibacterial and Osteogenic Properties. Curr. Nanosci. 2021, 17, 175–193. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.-W.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef]
- Qiu, J.; Geng, H.; Wang, D.; Qian, S.; Zhu, H.; Qiao, Y.; Qian, W.; Liu, X. Layer-Number Dependent Antibacterial and Osteogenic Behaviors of Graphene Oxide Electrophoretic Deposited on Titanium. ACS Appl. Mater. Interfaces 2017, 9, 12253–12263. [Google Scholar] [CrossRef] [PubMed]
- Rahnamaee, S.Y.; Bagheri, R.; Vossoughi, M.; Ahmadi Seyedkhani, S.; Samadikuchaksaraei, A. Bioinspired multifunctional TiO2 hierarchical micro/nanostructures with tunable improved bone cell growth and inhibited bacteria adhesion. Ceram. Int. 2020, 46, 9669–9679. [Google Scholar] [CrossRef]
- Gu, H.; Ding, Z.; Yang, Z.; Yu, W.; Zhang, W.; Lu, W.; Zhang, L.-C.; Wang, K.; Wang, L.; Fu, Y.-F. Microstructure evolution and electrochemical properties of TiO2/Ti-35Nb-2Ta-3Zr micro/nano-composites fabricated by friction stir processing. Mater. Des. 2019, 169, 107680. [Google Scholar] [CrossRef]
- Azari, R.; Rezaie, H.R.; Khavandi, A. Investigation of functionally graded HA-TiO2 coating on Ti–6Al–4V substrate fabricated by sol-gel method. Ceram. Int. 2019, 45, 17545–17555. [Google Scholar] [CrossRef]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef]
- Yang, S.; Liang, L.; Liu, L.; Yin, Y.; Liu, Y.; Lei, G.; Zhou, K.; Huang, Q.; Wu, H. Using MgO nanoparticles as a potential platform to precisely load and steadily release Ag ions for enhanced osteogenesis and bacterial killing. Mater. Sci. Eng. C 2021, 119, 111399. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Schmidmaier, G.; Wildemann, B.; Bail, H.; Lucke, M.; Stemberger, A.; Flyvbjerg, A.; Raschke, M. Die lokale Freisetzung von IGF-I und TGF-b1 aus einer biodegradierbaren Poly(d,l-Lactid) Beschichtung von Implantaten beschleunigt die Frakturheilung. Der Chir. 2000, 71, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Pankey, S.C.; Bouchard, L.S.; Ranchalis, J.; Puolakkainen, P. Controlled release of TGF-beta 1 from a biodegradable matrix for bone regeneration. J. Biomater. Sci. Polym. Ed. 1993, 5, 49–63. [Google Scholar] [CrossRef]
- Welsh, W.R.; Kim, H.D.; Jong, Y.S.; Valentini, R.F. Controlled release of platelet-derived growth factor using ethylene vinyl acetate copolymer (EVAc) coated on stainless-steel wires. Biomaterials 1995, 16, 1319–1325. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Schwabe, P.; Raschke, M.; Haas, N.P.; Wildemann, B. Collective review: Bioactive implants coated with poly(d,l-lactide) and growth factors IGF-I, TGF-beta1, or BMP-2 for stimulation of fracture healing. J. Long Term Eff. Med. Implant. 2006, 16, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wildemann, B.; Kandziora, F.; Krummrey, G.; Palasdies, N.; Haas, N.P.; Raschke, M.; Schmidmaier, G. Local and controlled release of growth factors (combination of IGF-I and TGF-beta I, and BMP-2 alone) from a polylactide coating of titanium implants does not lead to ectopic bone formation in sheep muscle. J. Control. Release 2004, 95, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Hwang, M.P.; Du, P.; Ko, J.; Ha, C.W.; Do, S.H.; Park, K. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials 2015, 50, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Motamedian, S.R.; Hosseinpour, S.; Ahsaie, M.G.; Khojasteh, A. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J. Stem Cells 2015, 7, 657–668. [Google Scholar] [CrossRef]
- Donos, N.; Dereka, X.; Calciolari, E. The use of bioactive factors to enhance bone regeneration: A narrative review. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 124–161. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [Green Version]
- Linkhart, T.A.; Mohan, S.; Baylink, D.J. Growth factors for bone growth and repair: IGF, TGFβ and BMP. Bone 1996, 19, S1–S12. [Google Scholar] [CrossRef]
- Mundy, G.R.; Bonewald, L.F. Role of TGF beta in bone remodeling. Ann. N. Y. Acad. Sci. 1990, 593, 91–97. [Google Scholar] [CrossRef]
- Seghezzi, G.; Patel, S.; Ren, C.J.; Gualandris, A.; Pintucci, G.; Robbins, E.S.; Shapiro, R.L.; Galloway, A.C.; Rifkin, D.B.; Mignatti, P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: An autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998, 141, 1659–1673. [Google Scholar] [CrossRef]
- Ten Dijke, P.; Iwata, K.K. Growth Factors For Wound Healing. Bio/Technology 1989, 7, 793–798. [Google Scholar] [CrossRef]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: Signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Nakashima, A.; Tamura, M. Regulation of matrix metalloproteinase-13 and tissue inhibitor of matrix metalloproteinase-1 gene expression by WNT3A and bone morphogenetic protein-2 in osteoblastic differentiation. Front. Biosci. 2006, 11, 1667–1678. [Google Scholar] [CrossRef] [Green Version]
- Westendorf, J.J.; Kahler, R.A.; Schroeder, T.M. Wnt signaling in osteoblasts and bone diseases. Gene 2004, 341, 19–39. [Google Scholar] [CrossRef]
- Johnson, M.L.; Harnish, K.; Nusse, R.; Van Hul, W. LRP5 and Wnt signaling: A union made for bone. J. Bone Miner. Res. 2004, 19, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Ravidà, A.; Barootchi, S.; Chambrone, L.; Giannobile, W.V. Recombinant Human Platelet–Derived Growth Factor: A Systematic Review of Clinical Findings in Oral Regenerative Procedures. JDR Clin. Transl. Res. 2020, 6, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Lobb, D.C.; DeGeorge, B.R.; Chhabra, A.B. Bone Graft Substitutes: Current Concepts and Future Expectations. J. Hand Surg. 2019, 44, 497–505.e492. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Saul, D.; Böker, K.O.; Ernst, J.; Lehman, W.; Schilling, A.F. Current Methods for Skeletal Muscle Tissue Repair and Regeneration. BioMed Res. Int. 2018, 2018, 1984879. [Google Scholar] [CrossRef]
- Hutchings, G.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Sibiak, R.; Bryja, A.; Jankowski, M.; Mozdziak, P.; Bukowska, D.; Antosik, P.; et al. Bone Regeneration, Reconstruction and Use of Osteogenic Cells; from Basic Knowledge, Animal Models to Clinical Trials. J. Clin. Med. 2020, 9, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial Bone Grafts Claimed as an Alternative to Autografts: Current Trends for Clinical Applications in Orthopaedics. Materials 2021, 14, 3290. [Google Scholar] [CrossRef]
- Nyberg, E.; Holmes, C.; Witham, T.; Grayson, W.L. Growth factor-eluting technologies for bone tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 184–194. [Google Scholar] [CrossRef]
- Graziani, G.; Govoni, M.; Vivarelli, L.; Boi, M.; De Carolis, M.; Bianchi, M.; Sassoni, E.; Bignozzi, M.C.; Carnevale, G.; Marmi, F.; et al. A Comprehensive Microstructural and Compositional Characterization of Allogenic and Xenogenic Bone: Application to Bone Grafts and Nanostructured Biomimetic Coatings. Coatings 2020, 10, 522. [Google Scholar] [CrossRef]
- Akter, F. Chapter 2—Principles of Tissue Engineering. In Tissue Engineering Made Easy; Akter, F., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 3–16. [Google Scholar] [CrossRef]
- The Editors of Encyclopaedia Britannica. Growth Factor. Available online: https://www.britannica.com/science/growth-factor (accessed on 6 December 2020).
- Ding, Z.Z.; Fan, Z.H.; Huang, X.W.; Bai, S.M.; Song, D.W.; Lu, Q.; Kaplan, D.L. Bioactive Natural Protein-Hydroxyapatite Nanocarriers for Optimizing Osteogenic Differentiation of Mesenchymal Stem Cells. J. Mater. Chem. B 2016, 4, 3555–3561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhang, Y.; Li, B.; Chen, L. Controlled dual delivery of low doses of BMP-2 and VEGF in a silk fibroin-nanohydroxyapatite scaffold for vascularized bone regeneration. J. Mater. Chem. B 2017, 5, 6963–6972. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Zhu, J.; Yuan, Y.; Liu, H.; Qian, J.; Li, Y.; Liu, C. A dual-delivery system of pH-responsive chitosan-functionalized mesoporous silica nanoparticles bearing BMP-2 and dexamethasone for enhanced bone regeneration. J. Mater. Chem. B 2015, 3, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Pearson, H.B.; Mason, D.E.; Kegelman, C.D.; Zhao, L.; Dawahare, J.H.; Kacena, M.A.; Boerckel, J.D. Effects of Bone Morphogenetic Protein-2 on Neovascularization During Large Bone Defect Regeneration. Tissue Eng. Part A 2019, 25, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Fu, H.; Rahaman, M.N.; Liu, Y.; Bal, B.S. Hollow hydroxyapatite microspheres: A novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration. Acta Biomater. 2013, 9, 8374–8383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zhang, Z.; Feng, J.; Guo, Y.; Yu, Y.; Cui, J.; Li, H.; Shang, L. Influence of Mussel-Derived Bioactive BMP-2-Decorated PLA on MSC Behavior in Vitro and Verification with Osteogenicity at Ectopic Sites in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 11961–11971. [Google Scholar] [CrossRef]
- Croes, M.; Akhavan, B.; Sharifahmadian, O.; Fan, H.; Mertens, R.; Tan, R.P.; Chunara, A.; Fadzil, A.A.; Wise, S.G.; Kruyt, M.C.; et al. A multifaceted biomimetic interface to improve the longevity of orthopedic implants. Acta Biomater. 2020, 110, 266–279. [Google Scholar] [CrossRef]
- Alba-Perez, A.; Jayawarna, V.; Childs, P.G.; Dalby, M.J.; Salmeron-Sanchez, M. Plasma polymerised nanoscale coatings of controlled thickness for efficient solid-phase presentation of growth factors. Mater. Sci. Eng. C 2020, 113, 110966. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.-J.; Song, E.-H.; Park, C.; Lee, H.; Kang, I.-G.; Kim, H.-E.; Jeong, S.-H. Porous calcium phosphate–collagen composite microspheres for effective growth factor delivery and bone tissue regeneration. Mater. Sci. Eng. C 2020, 109, 110480. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lin, J.; Li, J.; Wang, X.; Zhuang, J.; Wang, H.; Cheng, K.; Weng, W. Spatially-controlled distribution of HACC in mineralized collagen coatings for improving rhBMP-2 loading and release behavior. Colloids Surf. B Biointerfaces 2016, 145, 114–121. [Google Scholar] [CrossRef]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Poh, C.K.; Wang, W. Surface Functionalization of Titanium with Carboxymethyl Chitosan and Immobilized Bone Morphogenetic Protein-2 for Enhanced Osseointegration. Biomacromolecules 2009, 10, 1603–1611. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Xing, W.; Cao, L.; Liu, C. Surface-modified pliable PDLLA/PCL/β-TCP scaffolds as a promising delivery system for bone regeneration. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Hyzy, S.L.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. BMP2 induces osteoblast apoptosis in a maturation state and noggin-dependent manner. J. Cell. Biochem. 2012, 113, 3236–3245. [Google Scholar] [CrossRef] [Green Version]
- Riley, E.H.; Lane, J.M.; Urist, M.R.; Lyons, K.M.; Lieberman, J.R. Bone Morphogenetic Protein-2: Biology and Applications. Clin. Orthop. Relat. Res. 1996, 324, 39–46. [Google Scholar] [CrossRef]
- Chen, S.-H.; Zheng, L.-Z.; Xie, X.-H.; Wang, X.-L.; Lai, Y.-X.; Chen, S.-K.; Zhang, M.; Wang, Y.-X.; Griffith, J.F.; Qin, L. Comparative study of poly (lactic-co-glycolic acid)/tricalcium phosphate scaffolds incorporated or coated with osteogenic growth factors for enhancement of bone regeneration. J. Orthop. Transl. 2014, 2, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Behr, B.; Sorkin, M.; Lehnhardt, M.; Renda, A.; Longaker, M.T.; Quarto, N. A Comparative Analysis of the Osteogenic Effects of BMP-2, FGF-2, and VEGFA in a Calvarial Defect Model. Tissue Eng. Part A 2011, 18, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Heim, S.E.; Gornet, M.F.; Zdeblick, T.A. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J. Spinal Disord. Tech. 2003, 16, 113–122. [Google Scholar] [CrossRef]
- Baskin, D.S.; Ryan, P.; Sonntag, V.; Westmark, R.; Widmayer, M.A. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine 2003, 28, 1219–1224. [Google Scholar] [CrossRef]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G.; et al. Recombinant Human Bone Morphogenetic Protein-2 for Treatment of Open Tibial Fractures: A Prospective, Controlled, Randomized Study of Four Hundred and Fifty Patients. JBJS 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Devine, J.G.; Dettori, J.R.; France, J.C.; Brodt, E.; McGuire, R.A. The use of rhBMP in spine surgery: Is there a cancer risk? Evid. Based Spine Care J. 2012, 3, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Pountos, I.; Georgouli, T.; Henshaw, K.; Bird, H.; Jones, E.; Giannoudis, P.V. The Effect of Bone Morphogenetic Protein-2, Bone Morphogenetic Protein-7, Parathyroid Hormone, and Platelet-Derived Growth Factor on the Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells Derived From Osteoporotic Bone. J. Orthop. Trauma 2010, 24. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Sefcik, R.S.; Bishop, T.J.; Montelone, S.M.; Crouser, N.; Welter, J.F.; Caplan, A.I.; Dean, D. Growth Factor Dose Tuning for Bone Progenitor Cell Proliferation and Differentiation on Resorbable Poly(propylene fumarate) Scaffolds. Tissue Eng. Part C Methods 2016, 22, 904–913. [Google Scholar] [CrossRef] [Green Version]
- Gu, K.; Zhang, L.; Jin, T.; Rutherford, R.B. Identification of Potential Modifiers of Runx2/Cbfa1 Activity in C2C12 Cells in Response to Bone Morphogenetic Protein-7. Cells Tissues Organs 2004, 176, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Wei, A.; Whittaker, S.; Williams, L.A.; Tao, H.; Ma, D.D.; Diwan, A.D. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J. Cell. Biochem. 2010, 109, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Polini, A.; Wang, J.; Bai, H.; Zhu, Y.; Tomsia, A.P.; Mao, C. Stable biofunctionalization of hydroxyapatite (HA) surfaces by HA-binding/osteogenic modular peptides for inducing osteogenic differentiation of mesenchymal stem cells. Biomater. Sci. 2014, 2, 1779–1786. [Google Scholar] [CrossRef]
- Brigaud, I.; Agniel, R.; Leroy-Dudal, J.; Kellouche, S.; Ponche, A.; Bouceba, T.; Mihailescu, N.; Sopronyi, M.; Viguier, E.; Ristoscu, C.; et al. Synergistic effects of BMP-2, BMP-6 or BMP-7 with human plasma fibronectin onto hydroxyapatite coatings: A comparative study. Acta Biomater. 2017, 55, 481–492. [Google Scholar] [CrossRef]
- Tan, H.C.; Poh, C.K.; Cai, Y.; Wang, W. Anti-fibrosis effect of BMP-7 peptide functionalization on cobalt chromium alloy. J. Orthop. Res. 2013, 31, 983–990. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, L.-F.; Lin, H.-S.; Yin, M.-N.; Tong, Y.-Q.; Shi, G.-S. The optimal dose of recombinant human osteogenic protein-1 enhances differentiation of mouse osteoblast-like cells: An in vitro study. Arch. Oral. Biol. 2012, 57, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, M.; Helder, M.N.; Zandieh Doulabi, B.; Wuisman, P.I.J.M.; Klein-Nulend, J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem. Biophys. Res. Commun. 2006, 342, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Al-Jarsha, M.; Moulisová, V.; Leal-Egaña, A.; Connell, A.; Naudi, K.B.; Ayoub, A.F.; Dalby, M.J.; Salmerón-Sánchez, M. Engineered Coatings for Titanium Implants To Present Ultralow Doses of BMP-7. ACS Biomater. Sci. Eng. 2018, 4, 1812–1819. [Google Scholar] [CrossRef]
- Diwan, A.D.; Leong, A.; Appleyard, R.; Bhargav, D.; Fang, Z.M.; Wei, A. Bone morphogenetic protein-7 accelerates fracture healing in osteoporotic rats. Indian J. Orthop. 2013, 47, 540–546. [Google Scholar] [CrossRef]
- Bosemark, P.; Perdikouri, C.; Pelkonen, M.; Isaksson, H.; Tägil, M. The masquelet induced membrane technique with BMP and a synthetic scaffold can heal a rat femoral critical size defect. J. Orthop. Res. 2015, 33, 488–495. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Gan, H.; Wang, H.; Li, Q.; Wang, Z. Application of combined porous tantalum scaffolds loaded with bone morphogenetic protein 7 to repair of osteochondral defect in rabbits. Int. Orthop. 2018, 42, 1437–1448. [Google Scholar] [CrossRef]
- Westhauser, F.; Höllig, M.; Reible, B.; Xiao, K.; Schmidmaier, G.; Moghaddam, A. Bone formation of human mesenchymal stem cells harvested from reaming debris is stimulated by low-dose bone morphogenetic protein-7 application in vivo. J. Orthop. 2016, 13, 404–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, A.P.; Vaccaro, A.R.; Hall, J.A.; Whang, P.G.; Friel, B.C.; McKee, M.D. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int. Orthop. 2007, 31, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Negut, I.; Dumitrescu, M.F.; Stan, M.S.; Nica, I.C.; Holban, A.M.; Socol, G.; Andronescu, E. Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics 2021, 10, 160. [Google Scholar] [CrossRef]
- Levy, I.; Sher, I.; Corem-Salkmon, E.; Ziv-Polat, O.; Meir, A.; Treves, A.J.; Nagler, A.; Kalter-Leibovici, O.; Margel, S.; Rotenstreich, Y. Bioactive magnetic near Infra-Red fluorescent core-shell iron oxide/human serum albumin nanoparticles for controlled release of growth factors for augmentation of human mesenchymal stem cell growth and differentiation. J. Nanobiotechnol. 2015, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, Y.; Zhou, A.; Chen, X.; Li, K.; Chen, S.; Qiao, B.; Jiang, D. Controlled release of basic fibroblast growth factor from a peptide biomaterial for bone regeneration. R. Soc. Open Sci. 2020, 7, 191830. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Choi, D.; Han, U.; Hong, J. Inkjet-based multilayered growth factor-releasing nanofilms for enhancing proliferation of mesenchymal stem cells in vitro. J. Ind. Eng. Chem. 2017, 50, 36–40. [Google Scholar] [CrossRef]
- Zomer Volpato, F.; Almodóvar, J.; Erickson, K.; Popat, K.C.; Migliaresi, C.; Kipper, M.J. Preservation of FGF-2 bioactivity using heparin-based nanoparticles, and their delivery from electrospun chitosan fibers. Acta Biomater. 2012, 8, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Shim, I.K.; Chung, H.J.; Jung, M.R.; Nam, S.Y.; Lee, S.Y.; Lee, H.; Heo, S.J.; Lee, S.J. Biofunctional porous anodized titanium implants for enhanced bone regeneration. J. Biomed. Mater. Res. A 2014, 102, 3639–3648. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Kubo, T.; Doi, K.; Morita, K.; Takeshita, R.; Katoh, S.; Shiba, T.; Gong, P.; Akagawa, Y. Effect of combined application of bFGF and inorganic polyphosphate on bioactivities of osteoblasts and initial bone regeneration. Acta Biomater. 2009, 5, 1716–1724. [Google Scholar] [CrossRef]
- Fakhry, A.; Ratisoontorn, C.; Vedhachalam, C.; Salhab, I.; Koyama, E.; Leboy, P.; Pacifici, M.; Kirschner, R.E.; Nah, H.D. Effects of FGF-2/-9 in calvarial bone cell cultures: Differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone 2005, 36, 254–266. [Google Scholar] [CrossRef]
- Tsurushima, H.; Marushima, A.; Suzuki, K.; Oyane, A.; Sogo, Y.; Nakamura, K.; Matsumura, A.; Ito, A. Enhanced bone formation using hydroxyapatite ceramic coated with fibroblast growth factor-2. Acta Biomater. 2010, 6, 2751–2759. [Google Scholar] [CrossRef] [Green Version]
- Shiba, T.; Nishimura, D.; Kawazoe, Y.; Onodera, Y.; Tsutsumi, K.; Nakamura, R.; Ohshiro, M. Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J. Biol. Chem. 2003, 278, 26788–26792. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.; Yan, J.; Sun, H.; Wang, H. Multifunctional Nanocomposite Films for Synergistic Delivery of bFGF and BMP-2. ACS Omega 2017, 2, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.Y.; Kim, H.J.; Lee, M.H.; Kwon, T.G.; Nah, H.D.; Furuichi, T.; Komori, T.; Nam, S.H.; Kim, Y.J.; Kim, H.J.; et al. Runx2 regulates FGF2-induced Bmp2 expression during cranial bone development. Dev. Dyn. 2005, 233, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast Growth Factor 2-A Review of Stabilisation Approaches for Clinical Applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef]
- Lind, M. Growth factor stimulation of bone healing. Acta Orthop. Scand. 1998, 69, i-37. [Google Scholar] [CrossRef]
- Li, Y.; Lee, I.S.; Cui, F.Z.; Choi, S.H. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium. Biomaterials 2008, 29, 2025–2032. [Google Scholar] [CrossRef]
- Boteanu, R.M.; Suica, V.I.; Ivan, L.; Safciuc, F.; Uyy, E.; Dragan, E.; Croitoru, S.M.; Grumezescu, V.; Chiritoiu, M.; Sima, L.E.; et al. Proteomics of regenerated tissue in response to a titanium implant with a bioactive surface in a rat tibial defect model. Sci. Rep. 2020, 10, 18493. [Google Scholar] [CrossRef]
- Nakajima, F.; Ogasawara, A.; Goto, K.; Moriya, H.; Ninomiya, Y.; Einhorn, T.A.; Yamazaki, M. Spatial and temporal gene expression in chondrogenesis during fracture healing and the effects of basic fibroblast growth factor. J. Orthop. Res. 2001, 19, 935–944. [Google Scholar] [CrossRef]
- Ma, X.Y.; Feng, Y.F.; Ma, Z.S.; Li, X.; Wang, J.; Wang, L.; Lei, W. The promotion of osteointegration under diabetic conditions using chitosan/hydroxyapatite composite coating on porous titanium surfaces. Biomaterials 2014, 35, 7259–7270. [Google Scholar] [CrossRef]
- Moschouris, P.; Retzepi, M.; Petrie, A.; Donos, N. Effect of Wnt3a delivery on early healing events during guided bone regeneration. Clin. Oral Implant. Res. 2017, 28, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.M.; Reinkemeier, F.; Dadras, M.; Wallner, C.; Huber, J.; Sogorski, A.; Sacher, M.; Schmidt, S.; Drysch, M.; Dittfeld, S.; et al. Local Wnt3a treatment restores bone regeneration in large osseous defects after surgical debridement of osteomyelitis. J. Mol. Med. 2020, 98, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Jia, G.; Jiang, Y.; Liu, Q.; Yang, X.; Pan, S. Improving osteogenesis of PLGA/HA porous scaffolds based on dual delivery of BMP-2 and IGF-1 via a polydopamine coating. RSC Adv. 2017, 7, 56732–56742. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Deng, Y.; Yang, L.; Shi, X.; Dong, T.; Tai, Y.; Yang, W.; Chen, Z.G. Graphene-Oxide-Decorated Microporous Polyetheretherketone with Superior Antibacterial Capability and In Vitro Osteogenesis for Orthopedic Implant. Macromol. Biosci. 2018, 18, e1800036. [Google Scholar] [CrossRef]
- Wan, T.; Li, L.; Guo, M.; Jiao, Z.; Wang, Z.; Ito, Y.; Wan, Y.; Zhang, P.; Liu, Q. Immobilization via polydopamine of dual growth factors on polyetheretherketone: Improvement of cell adhesion, proliferation, and osteo-differentiation. J. Mater. Sci. 2019, 54, 11179–11196. [Google Scholar] [CrossRef]
- Chen, F.-M.; Chen, R.; Wang, X.-J.; Sun, H.-H.; Wu, Z.-F. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials 2009, 30, 5215–5224. [Google Scholar] [CrossRef]
- Lamberg, A.; Bechtold, J.E.; Baas, J.; Søballe, K.; Elmengaard, B. Effect of local TGF-β1 and IGF-1 release on implant fixation: Comparison with hydroxyapatite coating. Acta Orthop. 2009, 80, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschke, M.; Wildemann, B.; Inden, P.; Bail, H.; Flyvbjerg, A.; Hoffmann, J.; Haas, N.P.; Schmidmaier, G. Insulin-like growth factor-1 and transforming growth factor-β1 accelerates osteotomy healing using polylactide-coated implants as a delivery system: A biomechanical and histological study in minipigs. Bone 2002, 30, 144–151. [Google Scholar] [CrossRef]
- Lamberg, A.; Schmidmaier, G.; Søballe, K.; Elmengaard, B. Locally delivered TGF-β1 and IGF-1 enhance the fixation of titanium implants: A study in dogs. Acta Orthop. 2006, 77, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Overgaard, S.; Nguyen, T.; Ongpipattanakul, B.; Bünger, C.; Søballe, K. Transforming growth factor-beta stimulates bone ongrowth. Hydroxyapatite-coated implants studied in dogs. Acta Orthop. Scand. 1996, 67, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.G.; Striker, L.J.; Elliot, S.J.; Andreani, D.; Striker, G.E. Synthesis and release of insulinlike growth factor I by mesangial cells in culture. Am. J. Physiol. 1988, 255, F1214–F1219. [Google Scholar] [CrossRef]
- Howell, T.H.; Fiorellini, J.P.; Paquette, D.W.; Offenbacher, S.; Giannobile, W.V.; Lynch, S.E. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J. Periodontol. 1997, 68, 1186–1193. [Google Scholar] [CrossRef]
- Feito, M.J.; Serrano, M.C.; Oñaderra, M.; Matesanz, M.C.; Sánchez-Salcedo, S.; Arcos, D.; Vallet-Regí, M.; Portolés, M.T. Effects of immobilized VEGF on endothelial progenitor cells cultured on silicon substituted and nanocrystalline hydroxyapatites. RSC Adv. 2016, 6, 92586–92595. [Google Scholar] [CrossRef] [Green Version]
- Guang, M.; Huang, B.; Yao, Y.; Zhang, L.; Yang, B.; Gong, P. Effects of vascular endothelial growth factor on osteoblasts around dental implants in vitro and in vivo. J. Oral Sci. 2017, 59, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Leedy, M.R.; Jennings, J.A.; Haggard, W.O.; Bumgardner, J.D. Effects of VEGF-loaded chitosan coatings. J. Biomed. Mater. Res. Part A 2014, 102, 752–759. [Google Scholar] [CrossRef]
- Chen, S.; Shi, Y.; Zhang, X.; Ma, J. Evaluation of BMP-2 and VEGF loaded 3D printed hydroxyapatite composite scaffolds with enhanced osteogenic capacity in vitro and in vivo. Mater. Sci. Eng. C 2020, 112, 110893. [Google Scholar] [CrossRef]
- Yanoso-Scholl, L.; Jacobson, J.A.; Bradica, G.; Lerner, A.L.; O’Keefe, R.J.; Schwarz, E.M.; Zuscik, M.J.; Awad, H.A. Evaluation of dense polylactic acid/beta-tricalcium phosphate scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2010, 95A, 717–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roland, L.; Backhaus, S.; Grau, M.; Matena, J.; Teske, M.; Beyerbach, M.; Murua Escobar, H.; Haferkamp, H.; Gellrich, N.-C.; Nolte, I. Evaluation of Functionalized Porous Titanium Implants for Enhancing Angiogenesis in Vitro. Materials 2016, 9, 304. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Neoh, K.-G.; Shi, Z.; Kang, E.-T.; Poh, C.; Wang, W. An in vitro assessment of titanium functionalized with polysaccharides conjugated with vascular endothelial growth factor for enhanced osseointegration and inhibition of bacterial adhesion. Biomaterials 2010, 31, 8854–8863. [Google Scholar] [CrossRef] [PubMed]
- Wolf-Brandstetter, C.; Lode, A.; Hanke, T.; Scharnweber, D.; Worch, H. Influence of modified extracellular matrices on TI6AL4V implants on binding and release of VEGF. J. Biomed. Mater. Res. Part A 2006, 79A, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.; Grau, M.; Matena, J.; Teske, M.; Gieseke, M.; Kampmann, A.; Beyerbach, M.; Murua Escobar, H.; Haferkamp, H.; Gellrich, N.-C.; et al. Poly-ε-caprolactone Coated and Functionalized Porous Titanium and Magnesium Implants for Enhancing Angiogenesis in Critically Sized Bone Defects. Int. J. Mol. Sci. 2016, 17, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Wu, Z.; Lan, J.; Li, Y.; Xie, L.; Huang, X.; Zhang, A.; Qiao, H.; Chang, X.; Lin, H.; et al. Surface modification of titanium implants by silk fibroin/Ag co-functionalized strontium titanate nanotubes for inhibition of bacterial-associated infection and enhancement of in vivo osseointegration. Surf. Coat. Technol. 2021, 405, 126700. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Santos-Ruiz, L.; Becerra, J.; Feito, M.J.; Fernández-Villa, D.; Serrano, M.C.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; et al. Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomater. 2019, 83, 456–466. [Google Scholar] [CrossRef]
- Wagner, Q.; Offner, D.; Idoux-Gillet, Y.; Saleem, I.; Somavarapu, S.; Schwinté, P.; Benkirane-Jessel, N.; Keller, L. Advanced nanostructured medical device combining mesenchymal cells and VEGF nanoparticles for enhanced engineered tissue vascularization. Nanomedicine 2016, 11, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Ramazanoglu, M.; Lutz, R.; Ergun, C.; von Wilmowsky, C.; Nkenke, E.; Schlegel, K.A. The effect of combined delivery of recombinant human bone morphogenetic protein-2 and recombinant human vascular endothelial growth factor 165 from biomimetic calcium-phosphate-coated implants on osseointegration. Clin. Oral Implant. Res. 2011, 22, 1433–1439. [Google Scholar] [CrossRef]
- Du, B.; Gao, Y.; Deng, Y.; Zhao, Y.; Lai, C.; Guo, Z.; Rong, M.; Zhou, L. Local delivery of rhVEGF165 through biocoated nHA/coral block grafts in critical-sized dog mandible defects: A histological study at the early stages of bone healing. Int. J. Clin. Exp. Med. 2015, 8, 4940–4953. [Google Scholar]
- Ai, C.; Sheng, D.; Chen, J.; Cai, J.; Wang, S.; Jiang, J.; Chen, S. Surface modification of vascular endothelial growth factor-loaded silk fibroin to improve biological performance of ultra-high-molecular-weight polyethylene via promoting angiogenesis. Int. J. Nanomed. 2017, 12, 7737–7750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, H.-P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef]
- Wernike, E.; Montjovent, M.O.; Liu, Y.; Wismeijer, D.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W.; Klenke, F.M. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur. Cell Mater. 2010, 19, 30–40. [Google Scholar] [CrossRef]
- Kleinheinz, J.; Stratmann, U.; Joos, U.; Wiesmann, H.P. VEGF-activated angiogenesis during bone regeneration. J. Oral Maxillofac. Surg. 2005, 63, 1310–1316. [Google Scholar] [CrossRef]
- Kovacevic, D.; Gulotta, L.V.; Ying, L.; Ehteshami, J.R.; Deng, X.H.; Rodeo, S.A. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin. Orthop. Relat. Res. 2015, 473, 1644–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canalis, E.; McCarthy, T.L.; Centrella, M. Effects of platelet-derived growth factor on bone formation in vitro. J. Cell. Physiol. 1989, 140, 530–537. [Google Scholar] [CrossRef]
- Hee, C.K.; Dines, J.S.; Dines, D.M.; Roden, C.M.; Wisner-Lynch, L.A.; Turner, A.S.; McGilvray, K.C.; Lyons, A.S.; Puttlitz, C.M.; Santoni, B.G. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. Am. J. Sports Med. 2011, 39, 1630–1639. [Google Scholar] [CrossRef]
- Lynch, S.E.; Trippel, S.B.; Finkelman, R.D.; Hernandez, R.A.; Kiritsy, C.P.; Antoniades, H.N. The combination of platelet-derived growth factor-BB and insulin-like growth factor-I stimulates bone repair in adult Yucatan miniature pigs. Wound Repair Regen. 1994, 2, 182–190. [Google Scholar] [CrossRef]
- Uggen, C.; Dines, J.; McGarry, M.; Grande, D.; Lee, T.; Limpisvasti, O. The effect of recombinant human platelet-derived growth factor BB-coated sutures on rotator cuff healing in a sheep model. Arthroscopy 2010, 26, 1456–1462. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Nam, S.-H.; Im, S.-Y.; Park, Y.-J.; Lee, Y.-M.; Seol, Y.-J.; Chung, C.-P.; Lee, S.-J. Enhanced bone formation by controlled growth factor delivery from chitosan-based biomaterials. J. Control. Release 2002, 78, 187–197. [Google Scholar] [CrossRef]
- Al-Hezaimi, K.; Nevins, M.; Kim, S.-W.; Fateh, A.; Kim, D.M. Efficacy of Growth Factor in Promoting Early Osseointegration. J. Oral Implantol. 2014, 40, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Cãlin, C.; Pãtraşcu, I. Growth factors and beta-tricalcium phosphate in the treatment of periodontal intraosseous defects: A systematic review and meta-analysis of randomised controlled trials. Arch. Oral Biol. 2016, 66, 44–54. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Oechsner, M.; Naumann, A.; Gronwald, R.G.; Minne, H.W.; Ziegler, R. Stimulation of bone matrix apposition in vitro by local growth factors: A comparison between insulin-like growth factor I, platelet-derived growth factor, and transforming growth factor beta. Endocrinology 1990, 127, 69–75. [Google Scholar] [CrossRef]
- Lee, J.Y.; Na, H.J.; Kim, H.M.; Lee, S.C.; Lee, J.Y.; Chung, C.P.; Seol, Y.J.; Park, Y.J. Comparative Study of rhPDGF-BB Plus Equine-Derived Bone Matrix Versus rhPDGF-BB Plus β-TCP in the Treatment of Periodontal Defects. Int. J. Periodont. Restor. Dent. 2017, 37, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, R.P. Molecular regulation of prostaglandin synthesis: Implications for endocrine systems. Trends Endocrinol. Metab. 1995, 6, 293–297. [Google Scholar] [CrossRef]
- Anouz, R.; Repanas, A.; Schwarz, E.; Groth, T. Novel Surface Coatings Using Oxidized Glycosaminoglycans as Delivery Systems of Bone Morphogenetic Protein 2 (BMP-2) for Bone Regeneration. Macromol. Biosci. 2018, 18, e1800283. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y. A mussel-inspired osteogenesis microenvironment with bioactive peptides for the dual-functionalization of biomedical substrates. New J. Chem. 2020, 44, 14256–14265. [Google Scholar] [CrossRef]
- Haidari, S.; Boskov, M.; Schillinger, U.; Bissinger, O.; Wolff, K.D.; Plank, C.; Kolk, A. Functional analysis of bioactivated and antiinfective PDLLA—Coated surfaces. J. Biomed. Mater. Res. A 2017, 105, 1672–1683. [Google Scholar] [CrossRef]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials 2001, 22, 471–480. [Google Scholar] [CrossRef]
- Kurosaki, T.; Kitahara, T.; Fumoto, S.; Nishida, K.; Nakamura, J.; Niidome, T.; Kodama, Y.; Nakagawa, H.; To, H.; Sasaki, H. Ternary complexes of pDNA, polyethylenimine, and γ-polyglutamic acid for gene delivery systems. Biomaterials 2009, 30, 2846–2853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolk, A.; Haczek, C.; Koch, C.; Vogt, S.; Kullmer, M.; Pautke, C.; Deppe, H.; Plank, C. A strategy to establish a gene-activated matrix on titanium using gene vectors protected in a polylactide coating. Biomaterials 2011, 32, 6850–6859. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Alvarez-Urena, P.; Ducheyne, P.; Srinivasan, A.; Baskin, J.; Waters, H.; Gruber, R. 6.2 Bone Tissue Engineering: Growth Factors and Cytokines. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 20–53. [Google Scholar] [CrossRef]
- Jo, J.Y.; Jeong, S.I.; Shin, Y.M.; Kang, S.S.; Kim, S.E.; Jeong, C.M.; Huh, J.B. Sequential delivery of BMP-2 and BMP-7 for bone regeneration using a heparinized collagen membrane. Int. J. Oral Maxillofac. Surg. 2015, 44, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Webster, T.J. Increased osteoblast functions in the presence of BMP-7 short peptides for nanostructured biomaterial applications. J. Biomed. Mater. Res. A 2009, 91, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Gospodarowicz, D. Fibroblast Growth Factor: Chemical Structure and Biologic Function. Clin. Orthop. Relat. Res. 1990, 257, 231–248. [Google Scholar] [CrossRef]
- Park, O.J.; Kim, H.J.; Woo, K.M.; Baek, J.H.; Ryoo, H.M. FGF2-activated ERK mitogen-activated protein kinase enhances Runx2 acetylation and stabilization. J. Biol. Chem. 2010, 285, 3568–3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Chilkoti, A. Protein-polymer conjugation-moving beyond PEGylation. Curr. Opin. Chem. Biol. 2015, 28, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damon, D.H.; Lobb, R.R.; D’Amore, P.A.; Wagner, J.A. Heparin potentiates the action of acidic fibroblast growth factor by prolonging its biological half-life. J. Cell. Physiol. 1989, 138, 221–226. [Google Scholar] [CrossRef]
- Kim, M.S.; Shin, Y.M.; Lee, J.H.; Kim, S.I.; Nam, Y.S.; Shin, C.S.; Shin, H. Release kinetics and in vitro bioactivity of basic fibroblast growth factor: Effect of the thickness of fibrous matrices. Macromol. Biosci. 2011, 11, 122–130. [Google Scholar] [CrossRef]
- Schlessinger, J.; Plotnikov, A.N.; Ibrahimi, O.A.; Eliseenkova, A.V.; Yeh, B.K.; Yayon, A.; Linhardt, R.J.; Mohammadi, M. Crystal Structure of a Ternary FGF-FGFR-Heparin Complex Reveals a Dual Role for Heparin in FGFR Binding and Dimerization. Mol. Cell 2000, 6, 743–750. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef] [Green Version]
- Walker, M.; Will, I.; Pratt, A.; Chechik, V.; Genever, P.; Ungar, D. Magnetically Triggered Release of Entrapped Bioactive Proteins from Thermally Responsive Polymer-Coated Iron Oxide Nanoparticles for Stem-Cell Proliferation. ACS Appl. Nano Mater. 2020, 3, 5008–5013. [Google Scholar] [CrossRef] [PubMed]
- Willert, K.; Nusse, R. Wnt proteins. Cold Spring Harb. Perspect. Biol. 2012, 4, a007864. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun Fibrous Mats with High Porosity as Potential Scaffolds for Skin Tissue Engineering. Biomacromolecules 2008, 9, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Cai, J.-Y. Enhanced cell affinity of poly(l-lactic acid) modified by base hydrolysis: Wettability and surface roughness at nanometer scale. Curr. Appl. Phys. 2007, 7, e108–e111. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Bail, H.; Lucke, M.; Fuchs, T.; Stemberger, A.; Flyvbjerg, A.; Haas, N.P.; Raschke, M. Local application of growth factors (insulin-like growth factor-1 and transforming growth factor-β1) from a biodegradable poly(d,l-lactide) coating of osteosynthetic implants accelerates fracture healing in rats. Bone 2001, 28, 341–350. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Lübberstedt, M.; Haas, N.P.; Raschke, M. IGF-I and TGF-Beta 1 incorporated in a poly(d,l-lactide) implant coating stimulates osteoblast differentiation and collagen-1 production but reduces osteoblast proliferation in cell culture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 65B, 157–162. [Google Scholar] [CrossRef]
- Xing, H.; Wang, X.; Xiao, S.; Zhang, G.; Li, M.; Wang, P.; Shi, Q.; Qiao, P.; E, L.; Liu, H. Osseointegration of layer-by-layer polyelectrolyte multilayers loaded with IGF1 and coated on titanium implant under osteoporotic condition. Int. J. Nanomed. 2017, 12, 7709–7720. [Google Scholar] [CrossRef] [Green Version]
- Villars, F.; Bordenave, L.; Bareille, R.; Amédée, J. Effect of human endothelial cells on Human Bone Marrow Stromal Cell phenotype: Role of VEGF? J. Cell. Biochem. 2000, 79, 672–685. [Google Scholar] [CrossRef]
- Murphy, W.L.; Peters, M.C.; Kohn, D.H.; Mooney, D.J. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2000, 21, 2521–2527. [Google Scholar] [CrossRef]
- Vordemvenne, T.; Paletta, J.R.; Hartensuer, R.; Pap, T.; Raschke, M.J.; Ochman, S. Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC Musculoskelet. Disord. 2011, 12, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, C.; Marino, V.; Fazzalari, N.L.; Bartold, P.M. Soft Tissue Attachment to Titanium Implants Coated with Growth Factors. Clin. Implant Dent. Relat. Res. 2013, 15, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, R.C.; Marino, V.; Bartold, P.M. The effect of Emdogain and platelet-derived growth factor on the osteoinductive potential of hydroxyapatite tricalcium phosphate. Clin. Oral Investig. 2012, 16, 1217–1227. [Google Scholar] [CrossRef]
- Park, Y.J.; Ku, Y.; Chung, C.P.; Lee, S.J. Controlled release of platelet-derived growth factor from porous poly(l-lactide) membranes for guided tissue regeneration. J. Control. Release 1998, 51, 201–211. [Google Scholar] [CrossRef]
- Phipps, M.C.; Xu, Y.; Bellis, S.L. Delivery of platelet-derived growth factor as a chemotactic factor for mesenchymal stem cells by bone-mimetic electrospun scaffolds. PLoS ONE 2012, 7, e40831. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.E.; Yun, Y.P.; Lee, J.Y.; Shim, J.S.; Park, K.; Huh, J.B. Co-delivery of platelet-derived growth factor (PDGF-BB) and bone morphogenic protein (BMP-2) coated onto heparinized titanium for improving osteoblast function and osteointegration. J. Tissue Eng. Regen. Med. 2015, 9, E219–E228. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, R.; Yang, Y.; Liang, C.; Yu, X.; Liu, Y.; Wang, T.; Yu, Y.; Deng, F. Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J. Nanobiotechnol. 2021, 19, 78. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Kramer, S.; Cameron, N.R.; Krajnc, P. Porous Polymers from High Internal Phase Emulsions as Scaffolds for Biological Applications. Polymers 2021, 13, 1786. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Saito, N. Biodegradable Polymers as Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Icriverzi, M.; Bonciu, A.; Rusen, L.; Sima, L.E.; Brajnicov, S.; Cimpean, A.; Evans, R.W.; Dinca, V.; Roseanu, A. Human Mesenchymal Stem Cell Response to Lactoferrin-based Composite Coatings. Materials 2019, 12, 3414. [Google Scholar] [CrossRef] [Green Version]
- Serafim, A.; Cecoltan, S.; Olăreț, E.; Dragusin, D.-M.; Vasile, E.; Popescu, V.; Manolescu Mastalier, B.S.; Iovu, H.; Stancu, I.-C. Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair. Polymers 2020, 12, 1677. [Google Scholar] [CrossRef]
- Lao, J.; Dieudonné, X.; Benbakkar, M.; Jallot, É. Bioactive glass coating on gelatin scaffolds at ambient temperature: Easy route to make polymer scaffolds become bioactive. J. Mater. Sci. 2017, 52, 9129–9139. [Google Scholar] [CrossRef]
- Björkenheim, R.; Strömberg, G.; Pajarinen, J.; Ainola, M.; Uppstu, P.; Hupa, L.; Böhling, T.O.; Lindfors, N.C. Polymer-coated bioactive glass S53P4 increases VEGF and TNF expression in an induced membrane model in vivo. J. Mater. Sci. 2017, 52, 9055–9065. [Google Scholar] [CrossRef] [Green Version]
- De Lima, J.M.; Pinheiro Ferreira, E.; Bonan, R.F.; Silva-Teixeira, D.N.; Goulart, L.R.; de Souza, J.R.; de Medeiros, E.S.; Bonan, P.R.F.; Castellano, L.R.C. Cytokine Regulation from Human Peripheral Blood Leukocytes Cultured In Vitro with Silver Doped Bioactive Glasses Microparticles. Biomed. Res. Int. 2019, 2019, 3210530. [Google Scholar] [CrossRef] [Green Version]
- Deliormanlı, A.M.; Türk, M.; Atmaca, H. Response of mouse bone marrow mesenchymal stem cells to graphene-containing grid-like bioactive glass scaffolds produced by robocasting. J. Biomater. Appl. 2018, 33, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.C.; Widdows, K.L.; Erol, M.M.; Nandakumar, A.; Roqan, I.S.; Ansari, T.; Boccaccini, A.R. Neocellularization and neovascularization of nanosized bioactive glass-coated decellularized trabecular bone scaffolds. J. Biomed. Mater. Res. A 2013, 101, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chang, J. Well-ordered mesoporous bioactive glasses (MBG): A promising bioactive drug delivery system. J. Control. Release 2006, 110, 522–530. [Google Scholar] [CrossRef]
- Sengottuvelan, A.; Mederer, M.; Boccaccini, A.R. Preparation and characterization of mesoporous calcium-doped silica-coated TiO2scaffolds and their drug releasing behavior. Int. J. Appl. Ceram. Technol. 2018, 15, 892–902. [Google Scholar] [CrossRef]
- Qi, X.; Wang, H.; Zhang, Y.; Pang, L.; Xiao, W.; Jia, W.; Zhao, S.; Wang, D.; Huang, W.; Wang, Q. Mesoporous bioactive glass-coated 3D printed borosilicate bioactive glass scaffolds for improving repair of bone defects. Int. J. Biol. Sci. 2018, 14, 471–484. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Roohani-Esfahani, S.I.; Dunstan, C.R.; Quach, T.; Steck, R.; Saifzadeh, S.; Pivonka, P.; Zreiqat, H. Efficacy of novel synthetic bone substitutes in the reconstruction of large segmental bone defects in sheep tibiae. Biomed. Mater. 2016, 11, 015016. [Google Scholar] [CrossRef]
- Kalinichenko, S.G.; Matveeva, N.Y.; Kostiv, R.Y.; Edranov, S.S. The topography and proliferative activity of cells immunoreactive to various growth factors in rat femoral bone tissues after experimental fracture and implantation of titanium implants with bioactive biodegradable coatings. Biomed. Mater. Eng. 2019, 30, 85–95. [Google Scholar] [CrossRef]
- Lucaciu, O.; Soriţău, O.; Gheban, D.; Ciuca, D.R.; Virtic, O.; Vulpoi, A.; Dirzu, N.; Câmpian, R.; Băciuţ, G.; Popa, C.; et al. Dental follicle stem cells in bone regeneration on titanium implants. BMC Biotechnol. 2015, 15, 114. [Google Scholar] [CrossRef] [Green Version]
- Brie, I.C.; Soritau, O.; Dirzu, N.; Berce, C.; Vulpoi, A.; Popa, C.; Todea, M.; Simon, S.; Perde-Schrepler, M.; Virag, P.; et al. Comparative in vitro study regarding the biocompatibility of titanium-base composites infiltrated with hydroxyapatite or silicatitanate. J. Biol. Eng. 2014, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Xu, L.; Li, K.; Xie, N.; Xi, Y.; Wang, Y.; Zheng, X.; Chen, X.; Wang, M.; Ye, X. Zinc-modified Calcium Silicate Coatings Promote Osteogenic Differentiation through TGF-β/Smad Pathway and Osseointegration in Osteopenic Rabbits. Sci. Rep. 2017, 7, 3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rammal, H.; Dubus, M.; Aubert, L.; Reffuveille, F.; Laurent-Maquin, D.; Terryn, C.; Schaaf, P.; Alem, H.; Francius, G.; Quilès, F.; et al. Bioinspired Nanofeatured Substrates: Suitable Environment for Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 12791–12801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalinichenko, S.G.; Matveeva, N.Y.; Kostiv, R.E.; Puz, A.V. Role of Vascular Endothelial Growth Factor and Transforming Growth Factor-β2 in Rat Bone Tissue after Bone Fracture and Placement of Titanium Implants with Bioactive Bioresorbable Coatings. Bull. Exp. Biol. Med. 2017, 162, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Cecchinato, F.; Xue, Y.; Karlsson, J.; He, W.; Wennerberg, A.; Mustafa, K.; Andersson, M.; Jimbo, R. In vitro evaluation of human fetal osteoblast response to magnesium loaded mesoporous TiO2 coating. J. Biomed. Mater. Res. A 2014, 102, 3862–3871. [Google Scholar] [CrossRef] [PubMed]
- Dubus, M.; Rammal, H.; Alem, H.; Bercu, N.B.; Royaud, I.; Quilès, F.; Boulmedais, F.; Gangloff, S.C.; Mauprivez, C.; Kerdjoudj, H. Boosting mesenchymal stem cells regenerative activities on biopolymers-calcium phosphate functionalized collagen membrane. Colloids Surf. B Biointerfaces 2019, 181, 671–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Li, X.; Li, Z.; Liu, C.; Zhao, J.; Wang, J.; Liu, Y.; Yuan, X.; Cui, Z.; Yang, X. Surface Functionalization of Titanium Alloy with miR-29b Nanocapsules To Enhance Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 5783–5793. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, Z.; Jiang, B.; Li, L.; Yu, X. Surface modification of strontium-doped porous bioactive ceramic scaffolds via poly(DOPA) coating and immobilizing silk fibroin for excellent angiogenic and osteogenic properties. Biomater. Sci. 2016, 4, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Pullisaar, H.; Reseland, J.E.; Haugen, H.J.; Brinchmann, J.E.; Østrup, E. Simvastatin coating of TiO2 scaffold induces osteogenic differentiation of human adipose tissue-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014, 447, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, A.J.; Detamore, M.S. Bioactive Microsphere-Based Scaffolds Containing Decellularized Cartilage. Macromol. Biosci. 2015, 15, 979–989. [Google Scholar] [CrossRef]
- Sanghani-Kerai, A.; Coathup, M.; Brown, R.; Lodge, G.; Osagie-Clouard, L.; Graney, I.; Skinner, J.; Gikas, P.; Blunn, G. The development of a novel autologous blood glue aiming to improve osseointegration in the bone-implant interface. Bone Jt. Res. 2020, 9, 402–411. [Google Scholar] [CrossRef]
- Himmlova, L.; Kubies, D.; Hulejova, H.; Bartova, J.; Riedel, T.; Stikarova, J.; Suttnar, J.; Pesakova, V. Effect of Blood Component Coatings of Enosseal Implants on Proliferation and Synthetic Activity of Human Osteoblasts and Cytokine Production of Peripheral Blood Mononuclear Cells. Mediat. Inflamm. 2016, 2016, 8769347. [Google Scholar] [CrossRef] [Green Version]








| GF | Study | Advantages | Disadvantages |
|---|---|---|---|
| BMP-2 | In vitro | Enhances proliferation and osteogenesis [48,49,50,51,52,53,54,55,56,57,58,59] | Short half-life [19]; toxic at 200 ng/ml [60] |
| In vivo | Faster healing and more newly formed bone tissue [48,49,50,52,54,56,59,61,62]; increased angiogenic potential and bone regeneration capacity [51] (compared to bFGF [63]) | Short half-life [19,54] | |
| Clinical trials | Eliminates the pain, scarring, and morbidity of bone harvesting [64,65]; reduces the risk of implant failure; faster healing, fewer infections [66] | Dose-dependent risk of cancer [67] | |
| BMP-7 | In vitro | Enhances osteogenic differentiation [68,69,70,71,72]; higher mineralization than in BMP-4 and BMP-4 [69]; lower doses required compared to BMP-2 and BMP-6 [73]; can act as a fibroblast inhibitor [74] | Higher concentration required for osteogenic differentiation, ALP activity, collagen deposition [71,75,76]; cell differentiation rather than proliferation [77] |
| In vivo | Improves the healing and the quality of bone tissue [68,78,79]; induces bone formation and tissue calcification [80,81] | Cell differentiation rather than proliferation [73,77] | |
| Clinical trials | Enhances healing; induces bridging of the bone with an autograft [82] | Dose-dependent risk of cancer [67] | |
| bFGF | In vitro | Induces cell proliferation [69,83,84,85,86,87]; induces osteogenic marker gene expression [88,89,90,91,92,93,94] | Low cytotoxic effect possible [93]; unstable, short half-life [95,96] |
| In vivo | Upregulates the expression of osteoblast-related genes [89,92]; promotes bone tissue maturation [85,97,98,99]; upregulates BMP-2 expression [91,94]; enhances osseointegration [88] | Unstable, short half-life [95,96] | |
| Clinical trials | * | * | |
| Wnt3A | In vitro | Improves cell adhesion and cell density on scaffolds [100]; improves healing [101]; can inhibit osteoclast activity [102] | * |
| In vivo | Promotes woven bone formation in critical-size defects [101] | * | |
| Clinical trials | * | * | |
| IGF-1 | In vitro | Improves cell adhesion [103]; induces osteo-differentiation [104,105] | Greater cell adhesion in combination with BMP-2 [103,106] |
| In vivo | Improves fracture healing [107,108,109,110]; maintains bone density [111] | Higher healing rate and osteoconductivity in combination with other GFs [103,106,107,108,109,110] | |
| Clinical trials | Improves wound healing [112] | * | |
| VEGF | In vitro | Enhances cell proliferation [49,113,114,115,116,117,118,119,120,121,122,123]; enhances the effect of BMP-2 [116]; enhances angiogenesis [118,121,124] | * |
| In vivo | Improves angiogenic potential and bone regeneration capacity [49,114,116,117,123,124,125,126,127,128,129,130] | Combination with other GFs required for greater effect [98,117,125] | |
| Clinical trials | * | * | |
| PDGF-BB | In vitro | Induces cell proliferation and enhances osteogenesis [68,69,131] | Increases collagenase activity [132] |
| In vivo | Improves healing [133,134,135,136]; induces bone tissue formation [137,138] | Higher bone matrix deposition in combination with other GFs [139] | |
| Clinical trials | Improves healing of periodontal lesions [112]; maintains crestal bone height [140] | Can have a resorption effect on bone tissue [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjelić, D.; Finšgar, M. The Role of Growth Factors in Bioactive Coatings. Pharmaceutics 2021, 13, 1083. https://doi.org/10.3390/pharmaceutics13071083
Bjelić D, Finšgar M. The Role of Growth Factors in Bioactive Coatings. Pharmaceutics. 2021; 13(7):1083. https://doi.org/10.3390/pharmaceutics13071083
Chicago/Turabian StyleBjelić, Dragana, and Matjaž Finšgar. 2021. "The Role of Growth Factors in Bioactive Coatings" Pharmaceutics 13, no. 7: 1083. https://doi.org/10.3390/pharmaceutics13071083
APA StyleBjelić, D., & Finšgar, M. (2021). The Role of Growth Factors in Bioactive Coatings. Pharmaceutics, 13(7), 1083. https://doi.org/10.3390/pharmaceutics13071083