Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation
Abstract
:1. Introduction
2. Research Methodology
3. Mechanism of Action
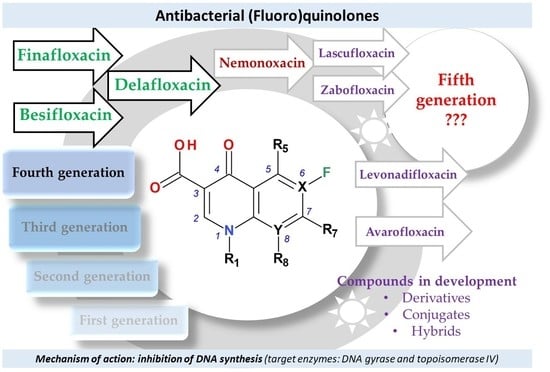
4. Classification into Generations of FQNs Used in Therapy
5. Compounds in Therapy Since 2000
5.1. Besifloxacin
| Properties | Besifloxacin | Besifloxacin Hydrochloride | Ref. |
|---|---|---|---|
| Chemical name (IUPAC) | {7-[(3R)-3-aminohexahydro-1H-azepin-1-yl]-8- chloro-1-clyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid} | (+)-7-[(3R)-3-aminohexahydro-1H-azepin-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride | [46] [97] |
| Chemical formula | C19H21ClFN3O3 | C19H21ClFN3O3·HCl | [97] |
| Molecular weight | 393.8 g/mol | 430.40 g/mol | [116] [117] |
| Aspect | Not available | White to pale yellowish-white powder; white to light brown | [97,117,118] |
| Solubility | Insoluble in water 0.143 mg/mL (predicted values) | 2 mg/mL (DMSO 1) | [46,106,116,118] |
| LogP | 0.7; 0.54 | Not available | [106] |
| pKa |
| Not available | [46,61,106,116] |
| Melting point | Not available | Over 210 °C; 270.04 °C | [117,119] |
| Storage | Not available | At refrigerator; −20 °C | [117,118] |
5.2. Delafloxacin
| Delafloxacin | Properties | Ref. |
|---|---|---|
| Chemical name (IUPAC) | 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid | [38,130] |
| Chemical formula | C18H12ClF3N4O4 | [38,130] |
| Molecular weight | 440.8 | [38] |
| Aspect | Powder, white to beige | [136] |
| Solubility | 0.0699 mg/mL (in water); 20 mg/mL (in DMSO 1) | [136,137] |
| LogP | 1.67 (predicted value) | [136,137] |
| pKa |
| [61,131,137] |
| Melting point | 249.32 °C | [135,138] |
| Storage | Refrigerator: 2–8 °C | [136] |
5.3. Finafloxacin
| Properties | Finafloxacin | References |
|---|---|---|
| Chemical name (IUPAC) | (-)-8-Cyano-1-cyclopropyl-6-fluoro-7-((4aS,7aS)-hexahydropyrrolo(3,4-b)-1,4-oxazin-6(2H)-yl) -4-oxo-1,4-dihydroquinoline-3-carboxylic acid | [49,99,151] |
| Chemical formula | C20H19FN4O4 | [49] |
| Molecular weight | 398.4 | [49] |
| Aspect | Powder, white to beige; white to yellowish (hydrochloride salt); | [152,153] |
| Solubility | 0.208 mg/mL (in water) 2 mg/mL (in DMSO 1) 5.5 mg/mL (in water, hydrochloride salt) | [151,152,153] |
| LogP | −0.5; −1.1 (predicted) | [151] |
| pKa | 5.6 (carboxylate) and 7.8 (nitrogen at C7) | [143,153] |
| Melting point | Not available | |
| Storage | −20 °C | [152] |
5.4. Lascufloxacin
| Lascufloxacin | Properties | References |
|---|---|---|
| Chemical name (IUPAC) | 7-[(3S,4S)-3-[(cyclopropylamino)methyl]-4-fluoropyrrolidin-1-yl]-6-fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxoquinoline-3-carboxylic acid | [159] |
| Chemical formula | C21H24F3N3O4 | [159] |
| Molecular weight | 439.4 g/mol | [159] |
| Aspect | White to off-white solid powder | [160] |
| Solubility | Slightly soluble in chloroform, very slightly soluble in DMSO 1, insoluble in water | [160,161] |
| LogP | 1.79 (nonionic species) | [61] |
| pKa | 5.64 (carboxyl), 9.75 (secondary amino group)—calculated | [61] |
| Melting point | Not available | |
| Storage | 0–4 °C (short term, days to weeks); −20 °C (long term, months), dry and dark conditions | [162] |
5.5. Nadifloxacin and Levonadifloxacin
| Nadifloxacin | Properties | References |
|---|---|---|
| Chemical name (IUPAC) | 7-fluoro-8-(4-hydroxypiperidin-1-yl)-12-methyl-4-oxo-1-azatricyclo[7.3.1.0⁵,¹³]trideca-2,5,7,9(13)-tetraene-3-carboxylic acid | [184] |
| Chemical formula | C19H21FN2O4 | [184] |
| Molecular weight | 360.4 g/mol | [184] |
| Aspect | White; light yellow powder | [185,186] |
| Solubility | 25 mg/mL (in DMF 1), 20 mg/mL (in DMSO 2), 0.25 mg/mL (in ethanol), insoluble in water | [185,186,187,188] |
| LogP | 2.47; 1.77 (calculated) | [61,187] |
| pKa | 5.94 (carboxyl), 0.44 (piperidinic nitrogen atom), 15.18 (hydroxyl) | [61] |
| Melting point | 245–247 °C (decomposition) | [87,189] |
| Storage | −20 °C | [188,189] |
5.6. Nemonoxacin
| Nemonoxacin | Properties | References |
|---|---|---|
| Chemical name (IUPAC) | 7-[(3S,5S)-3-Amino-5-methylpiperidin-1-yl]-1-cyclopropyl-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid | [210,211] |
| Chemical formula | C20H25N3O4 | [210,211] |
| Molecular weight | 371.4 g/mol | [210,211] |
| Aspect | Not available | |
| Solubility | Insoluble in water 0.453 mg/mL (predicted values) | [212] |
| LogP | 0.32; −0.44 | [212] |
| pKa |
| [212] [61] |
| Melting point | Not available | |
| Storage | Not available |
5.7. Zabofloxacin
| Zabofloxacin | Properties | References |
|---|---|---|
| Chemical name (IUPAC) | 1-cyclopropyl-6-fluoro-7-[(8E)-8-methoxyimino-2,6-diazaspiro[3.4]octan-6-yl]-4-oxo-1,8-naphthyridine-3-carboxylic acid | [234] |
| Chemical formula | C19H20FN5O4 | [234] |
| Molecular weight | 401.4 g/mol | [234] |
| Aspect | Not available | |
| Solubility | 0.196 mg/mL in water (calculated) | [235] |
| LogP | −0.89 (calculated); 2.43 for non ionic species | [61,235] |
| pKa |
| [61,235] |
| Melting point | 155 °C | [236] |
| Storage | Not available |
6. Is the Fifth Generation of Antibacterial FQNs Outlined?
- the majority of the new representatives have a broad spectrum of activity, including activity against anaerobic bacteria (except nemonoxacin);
- the new representatives are active against many resistant bacteria (including resistant to FQNs); this is the main advantage of the newly approved compounds;
- some representatives are very active in the environment with acidic pH (delafloxacin, finafloxacin), this being an advantage over previous generations’ representatives;
- some representatives were approved only for a specific type of administration (topic); these are very effective in the treatment of targeted infections (besifloxacin, finafloxacin); for these compounds, there are numerous ongoing clinical trials for oral or parenteral administration;
- lascufloxacin has superior tissue penetration due to its high binding capacity to phosphatidylserine.
7. Antimicrobial Resistance to the Newer FQNs
8. Compounds in Development Based on Antibacterial QNs Structures
8.1. Avarofloxacin (Acorafloxacin)
8.2. Other Derivatives of Antibacterial QNs
8.3. Hybrids
9. Concerning Side Effects
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sheehan, G.; Chew, N.S.Y. The history of quinolones. In Fluoroquinolone Antibiotics; Milestones in Drug Therapy; Ronald, A.R., Low, D.E., Eds.; Birkhäuser: Basel, Switzerland, 2003; pp. 1–10. ISBN 978-3-0348-8103-6. [Google Scholar]
- Oliphant, C.M.; Green, G.M. Quinolones: A Comprehensive Review. Am. Fam. Physician 2002, 65, 455–464. [Google Scholar]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone Antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Lesher, G.Y.; Froelich, E.J.; Gruett, M.D.; Bailey, J.H.; Brundage, R.P. 1,8-Naphthyridine derivatives. A new class of chemotherapeutic agents. J. Med. Pharm. Chem. 1962, 91, 1063–1065. [Google Scholar] [CrossRef]
- Ball, P. Chapter 1-The Quinolones: History and Overview. In The Quinolones, 3rd ed.; Andriole, V.T., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 1–31. ISBN 978-0-12-059517-4. [Google Scholar]
- Emami, S.; Shafiee, A.; Foroumadi, A. Quinolones: Recent Structural and Clinical Developments. Iran. J. Pharm. Res. 2005, 3, 123–136. [Google Scholar] [CrossRef]
- Bisacchi, G.S. Origins of the Quinolone Class of Antibacterials: An Expanded “Discovery Story”. J. Med. Chem. 2015, 58, 4874–4882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, P. Quinolone Generations: Natural History or Natural Selection? J. Antimicrob. Chemother. 2000, 46, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beale, J.M., Jr.; Block, J.H. (Eds.) Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th ed.; Wolters Kluwer Health: Baltimore, MD, USA, 2010; ISBN 978-0-7817-7929-6. [Google Scholar]
- Scoper, S.V. Review of Third-and Fourth-Generation Fluoroquinolones in Ophthalmology: In-Vitro and in-Vivo Efficacy. Adv. Ther. 2008, 25, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Bolon, M.K. The Newer Fluoroquinolones. Infect. Dis. Clin. N. Am. 2009, 23, 1027–1051. [Google Scholar] [CrossRef] [PubMed]
- WHO Operational Handbook on Tuberculosis, Module 4: Treatment—Drug-Resistant Tuberculosis Treatment. Available online: https://www.who.int/publications-detail-redirect/9789240006997 (accessed on 30 June 2021).
- Pranger, A.D.; van der Werf, T.S.; Kosterink, J.G.W.; Alffenaar, J.W.C. The Role of Fluoroquinolones in the Treatment of Tuberculosis in 2019. Drugs 2019, 79, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Sukul, P.; Spiteller, M. Fluoroquinolone Antibiotics in the Environment. Rev. Environ. Contam. Toxicol. 2007, 191, 131–162. [Google Scholar] [CrossRef] [PubMed]
- Ware, G. Reviews of Environmental Contamination and Toxicology 191; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-0-387-69163-3. [Google Scholar]
- Simon, J.; Guyot, A. Pefloxacin: Safety in Man. J. Antimicrob. Chemother. 1990, 26 (Suppl. B), 215–218. [Google Scholar] [CrossRef]
- List of Nationally Authorised Medicinal Products EMA/116496/2021 09 April 2021. Available online: https://www.ema.europa.eu/en/documents/psusa/pefloxacin-list-nationally-authorised-medicinal-products-psusa/00002322/202008_en.pdf (accessed on 6 August 2021).
- Rubinstein, E. History of Quinolones and Their Side Effects. Chemotherapy 2001, 47, 3–8. [Google Scholar] [CrossRef]
- Blum, M.D.; Graham, D.J.; McCloskey, C.A. Temafloxacin Syndrome: Review of 95 Cases. Clin. Infect. Dis. 1994, 18, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Takahama, H.; Tazaki, H. Tosufloxacin Tosilate-Induced Thrombocytopenic Purpura. J. Derm. 2007, 34, 465–467. [Google Scholar] [CrossRef]
- Trouchon, T.; Lefebvre, S. A Review of Enrofloxacin for Veterinary Use. Open J. Vet. Med. 2016, 6, 40–58. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, X.; Qin, W.; Zhao, H. Capillary Electrophoresis–Chemiluminescence Determination of Norfloxacin and Prulifloxacin. Anal. Chim. Acta 2008, 623, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Chaudhary, U.; Sikka, R. In the Quest of Drugs for Bad Bugs: Are Newer Fluoroquinolones Any Better? J. Lab. Physicians 2011, 3, 130–131. [Google Scholar] [CrossRef]
- Cazedey, E.C.L.; Salgado, H.R.N. Orbifloxacin: A Review of Properties, Its Antibacterial Activities, Pharmacokinetic/Pharmacodynamic Characteristics, Therapeutic Use, and Analytical Methods. Crit. Rev. Anal. Chem. 2013, 43, 79–99. [Google Scholar] [CrossRef]
- Martindale, W.; Sweetman, S.C. (Eds.) Martindale: The Complete Drug Reference, 36th ed.; Pharmaceuticale Press, PhP: London, UK; Chicago, IL, USA,, 2009; ISBN 978-0-85369-840-1. [Google Scholar]
- Nenoff, P. Acne Vulgaris and Bacterial Skin Infections: Review of the Topical Quinolone Nadifloxacin. Expert. Rev. Dermatol. 2006, 1, 643–654. [Google Scholar] [CrossRef]
- Morita, S.; Otsubo, K.; Uchida, M.; Kawabata, S.; Tamaoka, H.; Shimizu, T. Synthesis and Antibacterial Activity of the Metabolites of 9-Fluoro-6,7-Dihydro-8-(4-Hydroxy-1-Piperidyl)-5-Methyl-1-Oxo-1H,5H- Benzo[i,j]Quinolizine-2-Carboxylic Acid (OPC-7251). Chem. Pharm. Bull. 1990, 38, 2027–2029. [Google Scholar] [CrossRef] [Green Version]
- Grimshaw, W.T.; Giles, C.J.; Cooper, A.C.; Shanks, D.J. The Efficacy of Danofloxacin in the Therapy of Pneumonia Associated with Pasteurella Species in Housed Calves. Dtsch. Tierarztl. Wochenschr. 1990, 97, 529–532. [Google Scholar]
- Limberakis, C. Quinolone Antibiotics: Levofloxacin (Levaquin®), Moxifloxacin (Avelox®), Gemifloxacin (Factive®), and Garenoxacin (T-3811). In The Art of Drug Synthesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 39–69. ISBN 978-0-470-13497-9. [Google Scholar]
- Yu, Y.; Zhou, Y.F.; Sun, J.; Shi, W.; Liao, X.P.; Liu, Y.H. Pharmacokinetic and Pharmacodynamic Modeling of Sarafloxacin against Avian Pathogenic Escherichia Coli in Muscovy Ducks. BMC Vet. Res. 2017, 13, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott Laboratories’ Sarafloxacin for Poultry; Withdrawal of Approval of NADAs. Available online: https://www.federalregister.gov/documents/2001/04/30/01-10067/abbott-laboratories-sarafloxacin-for-poultry-withdrawal-of-approval-of-nadas (accessed on 8 June 2021).
- Alksne, L. Balofloxacin Choongwae. Curr. Opin. Investig. Drugs 2003, 4, 224–229. [Google Scholar] [PubMed]
- Shen, J.; Qian, J.-J.; Gu, J.-M.; Hu, X.-R. Marbofloxacin. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o998–o999. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Leggett, J. Gatifloxacin, Gemifloxacin, and Moxifloxacin: The Role of 3 Newer Fluoroquinolones. Clin. Infect. Dis. 2003, 37, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- EMA/CVMP/411755/2010-Rev.1 Summary of Opinion, Veraflox. European Medicines Agency 2011. Available online: https://www.ema.europa.eu/en/documents/smop-initial/cvmp-summary-positive-opinion-veraflox_en.pdf (accessed on 25 May 2021).
- Sykes, J.E.; Blondeau, J.M. Pradofloxacin: A Novel Veterinary Fluoroquinolone for Treatment of Bacterial Infections in Cats. Vet. J. 2014, 201, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Hanselmann, R.; Johnson, G.; Reeve, M.M.; Huang, S.-T. Identification and Suppression of a Dimer Impurity in the Development of Delafloxacin. Org. Process Res. Dev. 2009, 13, 54–59. [Google Scholar] [CrossRef]
- PubChem Delafloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/487101 (accessed on 1 June 2021).
- Markham, A. Delafloxacin: First Global Approval. Drugs 2017, 77, 1481–1486. [Google Scholar] [CrossRef] [Green Version]
- Haight, A.R.; Ariman, S.Z.; Barnes, D.M.; Benz, N.J.; Gueffier, F.X.; Henry, R.F.; Hsu, M.C.; Lee, E.C.; Morin, L.; Pearl, K.B.; et al. Synthesis of the Quinolone ABT-492: Crystallizations for Optimal Processing. Org. Process Res. Dev. 2006, 10, 751–756. [Google Scholar] [CrossRef]
- Van Bambeke, F. Delafloxacin, a Non-Zwitterionic Fluoroquinolone in Phase III of Clinical Development: Evaluation of Its Pharmacology, Pharmacokinetics, Pharmacodynamics and Clinical Efficacy. Future Microbiol. 2015, 10, 1111–1123. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, C.; Lonneman, M.; Wu, C. Polypharmacological Drug Actions of Recently FDA Approved Antibiotics. Eur. J. Med. Chem. 2021, 209, 112931. [Google Scholar] [CrossRef]
- Cervantes, L.J.; Mah, F.S. Clinical Use of Gatifloxacin Ophthalmic Solution for Treatment of Bacterial Conjunctivitis. Clin. Ophthalmol. 2011, 5, 495–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fish, D.N.; North, D.S. Gatifloxacin, an Advanced 8-Methoxy Fluoroquinolone. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Factive: Withdrawal of the Marketing Authorisation Application. Available online: https://www.ema.europa.eu/en/medicines/human/withdrawn-applications/factive (accessed on 1 June 2021).
- Totoli, E.G.; Nunes Salgado, H.R. Besifloxacin: A Critical Review of Its Characteristics, Properties, and Analytical Methods. Crit. Rev. Anal. Chem. 2018, 48, 132–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, P.G.; Stubbings, W.; Wisplinghoff, H.; Seifert, H. Activity of the Investigational Fluoroquinolone Finafloxacin against Ciprofloxacin-Sensitive and -Resistant Acinetobacter Baumannii Isolates. Antimicrob. Agents Chemother. 2010, 54, 1613–1615. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, S.; Van Bambeke, F.; Tulkens, P.M. Activity of Finafloxacin, a Novel Fluoroquinolone with Increased Activity at Acid PH, towards Extracellular and Intracellular Staphylococcus Aureus, Listeria Monocytogenes and Legionella Pneumophila. Int. J. Antimicrob. Agents 2011, 38, 52–59. [Google Scholar] [CrossRef] [PubMed]
- PubChem Finafloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11567473 (accessed on 25 May 2021).
- Hong, J.; Zhang, Z.; Lei, H.; Cheng, H.; Hu, Y.; Yang, W.; Liang, Y.; Das, D.; Chen, S.-H.; Li, G. A Novel Approach to Finafloxacin Hydrochloride (BAY35-3377). Tetrahedron Lett. 2009, 50, 2525–2528. [Google Scholar] [CrossRef]
- Takagi, H.; Tanaka, K.; Tsuda, H.; Kobayashi, H. Clinical Studies of Garenoxacin. Int. J. Antimicrob. Agents 2008, 32, 468–474. [Google Scholar] [CrossRef]
- Andersson, M.I.; MacGowan, A.P. Development of the Quinolones. J. Antimicrob. Chemother. 2003, 51 (Suppl. 1), 1–11. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Poole, R.M. Nemonoxacin: First Global Approval. Drugs 2014, 74, 1445–1453. [Google Scholar] [CrossRef]
- Kocsis, B.; Szabo, D. Zabofloxacin for Chronic Bronchitis. Drugs Today 2016, 52, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Paterson, D.L. Antibiotics in the Clinical Pipeline in October 2019. J. Antibiot. 2020, 73, 329–364. [Google Scholar] [CrossRef]
- Ghebremedhin, B. Bacterial Infections in the Elderly Patient: Focus on Sitafloxacin. Clin. Med. Insights Ther. 2012, 4, CMT-S7435. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-K.; Cheng, I.-L.; Chen, Y.-H.; Lai, C.-C. Efficacy and Safety of Sitafloxacin in the Treatment of Acute Bacterial Infection: A Meta-Analysis of Randomized Controlled Trials. Antibiotics 2020, 9, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czyrski, A. Analytical Methods for Determining Third and Fourth Generation Fluoroquinolones: A Review. Chromatographia 2017, 80, 181–200. [Google Scholar] [CrossRef] [Green Version]
- Systèmes, D. BIOVIA Draw for Academics. Available online: https://discover.3ds.com/biovia-draw-academic (accessed on 6 July 2021).
- ChemAxon-Software Solutions and Services for Chemistry & Biology. Available online: https://chemaxon.com/ (accessed on 11 June 2021).
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochem. 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of Action and Resistance of Older and Newer Fluoroquinolones. Clin. Infect. Dis. 2000, 31, S24–S28. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of Action of Antimicrobials: Focus on Fluoroquinolones. Clin. Infect. Dis. 2001, 32, S9–S15. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase Inhibitors: Fluoroquinolone Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of Action of and Resistance to Quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, K.S.; Xie, Y.; Hiasa, H.; Khodursky, A.B. Analysis of Pleiotropic Transcriptional Profiles: A Case Study of DNA Gyrase Inhibition. PLoS Genet. 2006, 2, e152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.R.; Malik, M.; Snyder, M.; Drlica, K. DNA Gyrase and Topoisomerase IV on the Bacterial Chromosome: Quinolone-Induced DNA Cleavage. J. Mol. Biol. 1996, 258, 627–637. [Google Scholar] [CrossRef]
- Drlica, K.; Malik, M.; Kerns, R.J.; Zhao, X. Quinolone-Mediated Bacterial Death. Antimicrob. Agents Chemother. 2008, 52, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Malik, M.; Zhao, X.; Drlica, K. Lethal Fragmentation of Bacterial Chromosomes Mediated by DNA Gyrase and Quinolones. Mol. Microbiol. 2006, 61, 810–825. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Somova, L.M.; Timchenko, N.F. Molecular and Genetic Characteristics of Cell Death in Prokaryotes. Mol. Genet. Microbiol. Virol. 2018, 33, 73–83. [Google Scholar] [CrossRef]
- Correia, S.; Poeta, P.; Hébraud, M.; Capelo, J.L.; Igrejas, G. Mechanisms of Quinolone Action and Resistance: Where Do We Stand? J. Med. Microbiol. 2017, 66, 551–559. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone Resistance: Mechanisms, Impact on Bacteria, and Role in Evolutionary Success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Solano-Gálvez, S.G.; Valencia-Segrove, M.F.; Prado, M.J.O.; Boucieguez, A.B.L.; Álvarez-Hernández, D.A.; Vázquez-López, R. Mechanisms of Resistance to Quinolones; IntechOpen: London, UK, 2020; ISBN 978-1-83962-433-9. [Google Scholar]
- Hong, Y.; Li, Q.; Gao, Q.; Xie, J.; Huang, H.; Drlica, K.; Zhao, X. Reactive Oxygen Species Play a Dominant Role in All Pathways of Rapid Quinolone-Mediated Killing. J. Antimicrob. Chemother. 2020, 75, 576–585. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-Stress Bacterial Cell Death Mediated by Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer, D.J.; Kohanski, M.A.; Hayete, B.; Collins, J.J. Gyrase Inhibitors Induce an Oxidative Damage Cellular Death Pathway in Escherichia Coli. Mol. Syst. Biol. 2007, 3, 91. [Google Scholar] [CrossRef]
- Pan, X.; Qin, P.; Liu, R.; Li, J.; Zhang, F. Molecular Mechanism on Two Fluoroquinolones Inducing Oxidative Stress: Evidence from Copper/Zinc Superoxide Dismutase. RSC Adv. 2016, 6, 91141–91149. [Google Scholar] [CrossRef]
- Michalak, K.; Sobolewska-Włodarczyk, A.; Włodarczyk, M.; Sobolewska, J.; Woźniak, P.; Sobolewski, B. Treatment of the Fluoroquinolone-Associated Disability: The Pathobiochemical Implications. Oxidative Med. Cell. Longev. 2017, 2017, e8023935. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rosado, A.I.; Valencia, E.Y.; Rodríguez-Rojas, A.; Costas, C.; Galhardo, R.S.; Blázquez, J.; Rodríguez-Beltrán, J. Reactive Oxygen Species Are Major Contributors to SOS-Mediated Mutagenesis Induced by Fluoroquinolones. BioRxiv 2018, 428961. [Google Scholar] [CrossRef]
- Loganathan, R.; Ganeshpandian, M.; Bhuvanesh, N.S.P.; Palaniandavar, M.; Muruganantham, A.; Ghosh, S.K.; Riyasdeen, A.; Akbarsha, M.A. DNA and Protein Binding, Double-Strand DNA Cleavage and Cytotoxicity of Mixed Ligand Copper(II) Complexes of the Antibacterial Drug Nalidixic Acid. J. Inorg. Biochem. 2017, 174, 1–13. [Google Scholar] [CrossRef]
- Dearden, J.C. The History and Development of Quantitative Structure-Activity Relationships (QSARs). Available online: www.igi-global.com/article/the-history-and-development-of-quantitative-structure-activity-relationships-qsars/144688 (accessed on 14 June 2020).
- Refat, M.S. Synthesis and Characterization of Norfloxacin-Transition Metal Complexes (Group 11, IB): Spectroscopic, Thermal, Kinetic Measurements and Biological Activity. Spectrochim. Acta Mol Biomol. Spectrosc. 2007, 68, 1393–1405. [Google Scholar] [CrossRef]
- Clardy, J.; Fischbach, M.; Currie, C. The Natural History of Antibiotics. Curr. Biol. 2009, 19, R437–R441. [Google Scholar] [CrossRef] [Green Version]
- Van, T.T.; Minejima, E.; Chiu, C.A.; Butler-Wu, S.M. Don’t Get Wound Up: Revised Fluoroquinolone Breakpoints for Enterobacteriaceae and Pseudomonas Aeruginosa. J. Clin. Microbiol. 2019, 57, e02072-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwahara, K.; Kitazawa, T.; Kitagaki, H.; Tsukamoto, T.; Kikuchi, M. Nadifloxacin, an Antiacne Quinolone Antimicrobial, Inhibits the Production of Proinflammatory Cytokines by Human Peripheral Blood Mononuclear Cells and Normal Human Keratinocytes. J. Dermatol. Sci. 2005, 38, 47–55. [Google Scholar] [CrossRef]
- Narayanan, V.; Motlekar, S.; Kadhe, G.; Bhagat, S. Efficacy and Safety of Nadifloxacin for Bacterial Skin Infections: Results from Clinical and Post-Marketing Studies. Dermatol. Ther. 2014, 4, 233–248. [Google Scholar] [CrossRef] [Green Version]
- EMA EMA/150639/2017 Nadifloxacin, List of Nationally Authorised Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/psusa/nadifloxacin-list-nationally-authorised-medicinal-products-psusa/00002102/201605_en.pdf (accessed on 11 June 2021).
- Fish, D.N.; Chow, A.T. The Clinical Pharmacokinetics of Levofloxacin. Clin. Pharm. 1997, 32, 101–119. [Google Scholar] [CrossRef]
- Keating, G.M. Levofloxacin 0.5% Ophthalmic Solution A Review of Its Use in the Treatment of External Ocular Infections and in Intraocular Surgery. Drugs 2009, 69, 1267–1286. [Google Scholar] [CrossRef]
- Rhee, C.K.; Chang, J.H.; Choi, E.G.; Kim, H.K.; Kwon, Y.-S.; Kyung, S.Y.; Lee, J.-H.; Park, M.J.; Yoo, K.H.; Oh, Y.M. Zabofloxacin versus Moxifloxacin in Patients with COPD Exacerbation: A Multicenter, Double-Blind, Double-Dummy, Randomized, Controlled, Phase III, Non-Inferiority Trial. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Al Omari, M.M.H.; Jaafari, D.S.; Al-Sou’od, K.A.; Badwan, A.A. Chapter Seven-Moxifloxacin Hydrochloride. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 39, pp. 299–431. [Google Scholar]
- Baxdela (Delafloxacin) Tablets and Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208610Orig1s000,208611Orig1s000TOC.cfm (accessed on 5 June 2021).
- Singh, C.L.; Singh, A.; Kumar, S.; Majumdar, D.K. Besifloxacin the fourth generation fluoroquinolone: A review. J. Drug Deliv. Ther. 2014, 4, 39–44. [Google Scholar] [CrossRef]
- DeCory, H.H.; Sanfilippo, C.M.; Proskin, H.M.; Blondeau, J.M. Characterization of Baseline Polybacterial versus Monobacterial Infections in Three Randomized Controlled Bacterial Conjunctivitis Trials and Microbial Outcomes with Besifloxacin Ophthalmic Suspension 0.6%. PLoS ONE 2020, 15, e0237603. [Google Scholar] [CrossRef] [PubMed]
- BESIVANCE® (Besifloxacin Ophthalmic Suspension) 0.6%, for Topical Ophthalmic Use. 9. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022308s013lbl.pdf (accessed on 28 May 2021).
- FDA Drug Approval Package Xtoro (Finafloxacin) Otic Suspension. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/206307Orig1s000TOC.cfm (accessed on 7 June 2021).
- McKeage, K. Finafloxacin: First Global Approval. Drugs 2015, 75, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.B.; Zumbrun, S.D.; Halasohoris, S.A.; Desai, P.D.; Miller, L.L.; Richards, M.I.; Russell, P.; Bentley, C.; Harding, S.V. Demonstration of the Broad-Spectrum In Vitro Activity of Finafloxacin against Pathogens of Biodefense Interest. Antimicrob. Agents Chemother. 2019, 63, e01470-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khimdas, S.; Visscher, K.L.; Hutnik, C.M.L. Besifloxacin Ophthalmic Suspension: Emerging Evidence of Its Therapeutic Value in Bacterial Conjunctivitis. Ophthalmol. Eye Dis. 2011, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.-C.; Sakya, S.M.; O’Donnell, C.J.; Flick, A.C.; Li, J. Synthetic Approaches to the 2009 New Drugs. Bioorganic. Med. Chem. 2011, 19, 1136–1154. [Google Scholar] [CrossRef]
- FDA. N. 22308/S-013 Besifloxacin Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022308lbl.pdf (accessed on 6 May 2021).
- Haas, W.; Pillar, C.M.; Zurenko, G.E.; Lee, J.C.; Brunner, L.S.; Morris, T.W. Besifloxacin, a Novel Fluoroquinolone, Has Broad-Spectrum in Vitro Activity against Aerobic and Anaerobic Bacteria. Antimicrob. Agents Chemother. 2009, 53, 3552–3560. [Google Scholar] [CrossRef] [Green Version]
- Mah, F.S.; Sanfilippo, C.M. Besifloxacin: Efficacy and Safety in Treatment and Prevention of Ocular Bacterial Infections. Ophthalmol. Ther. 2016, 5, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Besifloxacin. Available online: https://go.drugbank.com/drugs/DB06771 (accessed on 1 June 2021).
- O’Brien, T.P. Besifloxacin Ophthalmic Suspension, 0.6%: A Novel Topical Fluoroquinolone for Bacterial Conjunctivitis. Adv. Ther. 2012, 29, 473–490. [Google Scholar] [CrossRef]
- Watanabe, R.; Nakazawa, T.; Yokokura, S.; Kubota, A.; Kubota, H.; Nishida, K. Fluoroquinolone Antibacterial Eye Drops: Effects on Normal Human Corneal Epithelium, Stroma, and Endothelium. Clin. Ophthalmol. 2010, 4, 1181–1187. [Google Scholar] [CrossRef] [Green Version]
- Kovoor, T.A.; Kim, A.S.; McCulley, J.P.; Cavanagh, H.D.; Jester, J.V.; Bugde, A.C.; Petroll, W.M. Evaluation of the Corneal Effects of Topical Ophthalmic Fluoroquinolones Using In Vivo Confocal Microscopy. Eye Contact Lens. 2004, 30, 90–94. [Google Scholar] [CrossRef]
- Kassaee, S.N.; Mahboobian, M.M. Besifloxacin-Loaded Ocular Nanoemulsions: Design, Formulation and Efficacy Evaluation. Drug Deliv. Transl. Res. 2021. [Google Scholar] [CrossRef]
- Dos Santos, G.A.; Ferreira-Nunes, R.; Dalmolin, L.F.; dos Santos Re, A.C.; Vieira Anjos, J.L.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.V.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin Liposomes with Positively Charged Additives for an Improved Topical Ocular Delivery. Sci. Rep. 2020, 10, 19285. [Google Scholar] [CrossRef]
- Polat, H.K.; Pehlivan, S.B.; Ozkul, C.; Calamak, S.; Ozturk, N.; Aytekin, E.; Firat, A.; Ulubayram, K.; Kocabeyoglu, S.; Irkec, M.; et al. Development of Besifloxacin HCl Loaded Nanofibrous Ocular Inserts for the Treatment of Bacterial Keratitis: In Vitro, Ex Vivo and In Vivo Evaluation. Int. J. Pharm. 2020, 585, 119552. [Google Scholar] [CrossRef]
- Peterson, L.R. Quinolone Molecular Structure-Activity Relationships: What We Have Learned about Improving Antimicrobial Activity. Clin. Infect. Dis. 2001, 33, S180–S186. [Google Scholar] [CrossRef]
- Asif, M. Study of Antimicrobial Quinolones and Structure Activity Relationship of Anti-Tubercular Compounds. Res. Rev. J. Chem. 2015, 4, 28–70. [Google Scholar]
- Schaumann, R.C.; Rodloff, A.C. Activities of Quinolones Against Obligately Anaerobic Bacteria. Anti-Infect. Agents Med. Chem. 2006, 6, 49–56. [Google Scholar] [CrossRef]
- PubChem Besifloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10178705 (accessed on 28 May 2021).
- PubChem Besifloxacin Hydrochloride. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10224595 (accessed on 28 May 2021).
- Besifloxacin Hydrochloride SML1608. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/sml1608 (accessed on 1 June 2021).
- Attia, A.K.; Abdel-Moety, M.M.; Abdel-Hamid, S.G. Thermal Analyses of Some Fluoroquinolone Pharmaceutical Compounds in Comparison with Molecular Orbital Calculations. New J. Chem. 2017, 41, 10189–10197. [Google Scholar] [CrossRef]
- Thomas, G. Medicinal Chemistry: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-119-96542-8. [Google Scholar]
- Lu, T.; Zhao, X.; Li, X.; Drlica-Wagner, A.; Wang, J.-Y.; Domagala, J.; Drlica, K. Enhancement of Fluoroquinolone Activity by C-8 Halogen and Methoxy Moieties: Action against a Gyrase Resistance Mutant of Mycobacterium Smegmatis and a Gyrase-Topoisomerase IV Double Mutant of Staphylococcus Aureus. Antimicrob. Agents Chemother. 2001, 45, 2703–2709. [Google Scholar] [CrossRef] [Green Version]
- Wermuth, C.G. Preface to the Third Edition. In The Practice of Medicinal Chemistry, 4th ed.; Wermuth, C.G., Aldous, D., Raboisson, P., Rognan, D., Eds.; Academic Press: San Diego, CA, USA, 2015; p. xvii. ISBN 978-0-12-417205-0. [Google Scholar]
- Haas, W.; Sanfilippo, C.M.; Hesje, C.K.; Morris, T.W. Contribution of the R8 Substituent to the in Vitro Antibacterial Potency of Besifloxacin and Comparator Ophthalmic Fluoroquinolones. Clin. Ophthalmol. 2013, 7, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Ren, Z.; Fan, L.; Wei, J.; Tang, X.; Xu, X.; Yang, D. Design, Synthesis, Biological Evaluation, Structure-Activity Relationship, and Toxicity of Clinafloxacin-Azole Conjugates as Novel Antitubercular Agents. Bioorganic Med. Chem. 2019, 27, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Salunke, N.; Kharkar, P.S.; Pandita, N. Study of Degradation Behavior of Besifloxacin, Characterization of Its Degradation Products by LC-ESI-QTOF-MS and Their in Silico Toxicity Prediction. Biomed. Chromatogr. 2019, 33, e4489. [Google Scholar] [CrossRef]
- Cambau, E.; Matrat, S.; Pan, X.-S.; Roth Dit Bettoni, R.; Corbel, C.; Aubry, A.; Lascols, C.; Driot, J.-Y.; Fisher, L.M. Target Specificity of the New Fluoroquinolone Besifloxacin in Streptococcus Pneumoniae, Staphylococcus Aureus and Escherichia Coli. J. Antimicrob. Chemother. 2009, 63, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Haas, W.; Pillar, C.M.; Hesje, C.K.; Sanfilippo, C.M.; Morris, T.W. In Vitro Time-Kill Experiments with Besifloxacin, Moxifloxacin and Gatifloxacin in the Absence and Presence of Benzalkonium Chloride. J. Antimicrob. Chemother. 2011, 66, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Hoover, R.; Alcorn, H.; Lawrence, L.; Paulson, S.K.; Quintas, M.; Cammarata, S.K. Pharmacokinetics of Intravenous Delafloxacin in Patients With End-Stage Renal Disease. J. Clin. Pharmacol. 2018, 58, 913–919. [Google Scholar] [CrossRef]
- Scott, L.J. Delafloxacin: A Review in Acute Bacterial Skin and Skin Structure Infections. Drugs 2020, 80, 1247–1258. [Google Scholar] [CrossRef]
- Tulkens, P.M.; Van Bambeke, F.; Zinner, S.H. Profile of a Novel Anionic FluoroquinoloneDelafloxacin. Clin. Infect. Dis. 2019, 68, S213–S222. [Google Scholar] [CrossRef] [Green Version]
- Mogle, B.T.; Steele, J.M.; Thomas, S.J.; Bohan, K.H.; Kufel, W.D. Clinical Review of Delafloxacin: A Novel Anionic Fluoroquinolone. J. Antimicrob. Chemother. 2018, 73, 1439–1451. [Google Scholar] [CrossRef] [Green Version]
- Meglumine-Brief Profile-ECHA. Available online: https://echa.europa.eu/brief-profile/-/briefprofile/100.025.916 (accessed on 5 June 2021).
- Meglumine Active Pharmaceutical Ingredient | Small Molecule Pharmaceuticals | Merck. Available online: https://www.merckmillipore.com/RO/ro/products/small-molecule-pharmaceuticals/formulation/solid-dosage-form/meglumine/mwub.qB.gf0AAAFSBHgEZXop,nav?ReferrerURL=https%3A%2F%2Fwww.google.com%2F&gclid=EAIaIQobChMI_rKg7pHT8QIV44ODBx1C3QiFEAMYASAAEgL54PD_BwE (accessed on 8 July 2021).
- Cho, J.C.; Crotty, M.P.; White, B.P.; Worley, M.V. What Is Old Is New Again: Delafloxacin, a Modern Fluoroquinolone. Pharmacotherapy 2018, 38, 108–121. [Google Scholar] [CrossRef]
- Anwer, M.K.; Iqbal, M.; Muharram, M.M.; Mohammad, M.; Ezzeldin, E.; Aldawsari, M.F.; Alalaiwe, A.; Imam, F. Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-Microbial Activity and Bioavailability of Delafloxacin. Pharmaceutics 2020, 12, 252. [Google Scholar] [CrossRef] [Green Version]
- Delafloxacin SML1869. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/sml1869 (accessed on 3 June 2021).
- Delafloxacin. Available online: https://go.drugbank.com/drugs/DB11943 (accessed on 3 June 2021).
- Hanselmann, R.; Reeve, M.M. Crystalline Forms of D-Glucitol, 1-Deoxy-1-(Methylamino)-, 1-(6-Amino-3,5-Difluoropyridine-2-Yl)-8-Chloro-6-Fluoro-1,4-Dihydro-7-(3-Hydroxyazetidin-1-Yl)-4-Oxo-3-Quinolinecarboxylate. U.S. Patent Application 14/773,655, 18 February 2016. [Google Scholar]
- Saravolatz, L.D.; Stein, G.E. Delafloxacin: A New Anti-Methicillin-Resistant Staphylococcus Aureus Fluoroquinolone. Clin. Infect. Dis. 2019, 68, 1058–1062. [Google Scholar] [CrossRef]
- Lemaire, S.; Tulkens, P.M.; Van Bambeke, F. Contrasting Effects of Acidic PH on the Extracellular and Intracellular Activities of the Anti-Gram-Positive Fluoroquinolones Moxifloxacin and Delafloxacin against Staphylococcus Aureus. Antimicrob. Agents Chemother. 2011, 55, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Ocheretyaner, E.R.; Park, T.E. Delafloxacin: A Novel Fluoroquinolone with Activity against Methicillin-Resistant Staphylococcus Aureus (MRSA) and Pseudomonas Aeruginosa. Expert Rev. Anti-Infect. Ther. 2018, 16, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, A.M. The Quinolones: Decades of Development and Use. J. Antimicrob. Chemother. 2003, 51, 13–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocsis, B.; Domokos, J.; Szabo, D. Chemical Structure and Pharmacokinetics of Novel Quinolone Agents Represented by Avarofloxacin, Delafloxacin, Finafloxacin, Zabofloxacin and Nemonoxacin. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taubert, M.; Lueckermann, M.; Vente, A.; Dalhoff, A.; Fuhr, U. Population Pharmacokinetics of Finafloxacin in Healthy Volunteers and Patients with Complicated Urinary Tract Infections. Antimicrob. Agents Chemother. 2018, 62, e02328-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vente, A.; Bentley, C.; Lueckermann, M.; Tambyah, P.; Dalhoff, A. Early Clinical Assessment of the Antimicrobial Activity of Finafloxacin Compared to Ciprofloxacin in Subsets of Microbiologically Characterized Isolates. Antimicrob. Agents Chemother. 2018, 62, e02325-17. [Google Scholar] [CrossRef] [Green Version]
- Wagenlehner, F.; Nowicki, M.; Bentley, C.; Lueckermann, M.; Wohlert, S.; Fischer, C.; Vente, A.; Naber, K.; Dalhoff, A. Explorative Randomized Phase II Clinical Study of the Efficacy and Safety of Finafloxacin versus Ciprofloxacin for Treatment of Complicated Urinary Tract Infections. Antimicrob. Agents Chemother. 2018, 62, e02317-17. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Kim, N.; Nam, R.H.; Kim, J.M.; Park, J.Y.; Lee, S.M.; Kim, J.S.; Lee, D.H.; Jung, H.C. High Efficacy of Finafloxacin on Helicobacter Pylori Isolates at PH 5.0 Compared with That of Other Fluoroquinolones. Antimicrob. Agents Chemother. 2015, 59, 7629–7636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, K.B.; Richards, M.; Laws, T.R.; Nunez, A.; Thwaite, J.E.; Bentley, C.; Harding, S. Finafloxacin Is an Effective Treatment for Inhalational Tularemia and Plague in Mouse Models of Infection. Antimicrob. Agents Chemother. 2021, 65, e02294-20. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, H.; Harding, S.V.; Tulkens, P.M.; Van Bambeke, F. Influence of PH on the Activity of Finafloxacin against Extracellular and Intracellular Burkholderia Thailandensis, Yersinia Pseudotuberculosis and Francisella Philomiragia and on Its Cellular Pharmacokinetics in THP-1 Monocytes. Clin. Microbiol. Infect. 2020, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.; Andresen, A.; Vente, A.; Heilmann, H.-D.; Stubbings, W.; Seiberling, M.; Lopez-Lazaro, L.; Pokorny, R.; Labischinski, H. Human Pharmacokinetics and Safety Profile of Finafloxacin, a New Fluoroquinolone Antibiotic, in Healthy Volunteers. Antimicrob. Agents Chemother. 2011, 55, 4386–4393. [Google Scholar] [CrossRef] [Green Version]
- Finafloxacin. Available online: https://go.drugbank.com/drugs/DB09047 (accessed on 7 June 2021).
- Finafloxacin ≥95% (HPLC) | Sigma-Aldrich. Available online: http://www.sigmaaldrich.com/ (accessed on 7 June 2021).
- Wohlert, S.-E.; Jaetsch, T.; Gallenkamp, B.; Knops, H.J.; Lui, N.; Preiss, M.; Haebich, D.; Labischinski, H. New Fluoroquinolone Finafloxacin HCl (FIN): Route of Synthesis, Physicochemical Characteristics and Activity under Neutral and Acid Conditions. In Proceedings of the 48th Annual ICAAC/IDSA 46th Annual Meeting 2008, Washington DC, USA, 25–28 October 2008. [Google Scholar]
- Wise, R. A Review of the Clinical Pharmacology of Moxifloxacin, a New 8-Methoxyquinolone, and Its Potential Relation to Therapeutic Efficacy. Clin. Drug Investig. 1999, 17, 365–387. [Google Scholar] [CrossRef]
- Miravitlles, M. Moxifloxacin in the Management of Exacerbations of Chronic Bronchitis and COPD. Int. J. Chron. Obs. Pulmon Dis. 2007, 2, 191–204. [Google Scholar]
- Vardanyan, R.; Hruby, V. Chapter 31-Antibacterial Drugs. In Synthesis of Best-Seller Drugs; Vardanyan, R., Hruby, V., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 645–667. ISBN 978-0-12-411492-0. [Google Scholar]
- Silley, P.; Stephan, B.; Greife, H.A.; Pridmore, A. Comparative Activity of Pradofloxacin against Anaerobic Bacteria Isolated from Dogs and Cats. J. Antimicrob. Chemother. 2007, 60, 999–1003. [Google Scholar] [CrossRef]
- Tanaka, K.; Vu, H.; Hayashi, M. In Vitro Activities and Spectrum of Lascufloxacin (KRP-AM1977) against Anaerobes. J. Infect. Chemother. 2021, 27, 1265–1269. [Google Scholar] [CrossRef]
- PubChem Lascufloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/71528768 (accessed on 25 June 2021).
- THE BioTek: Recombinant Proteins, Antibodies, Antigens, Enzymes, Peptides, Inhibitors. Available online: https://www.thebiotek.com/ (accessed on 29 June 2021).
- 848416-07-9 | Lascufloxacin | 7-[(3S,4S)-3-[(Cyclopropylamino)Methyl]-4-Fluoro-1-Pyrrolidinyl]-6-Fluoro-1-(2-Fluoroethyl)-1,4-Dihydro-8-Methoxy-4-Oxo-3-Quinolinecarboxylic Acid | C₂₁H₂₄F₃N₃O₄ | TRC. Available online: https://www.trc-canada.com/product-detail/?L176205 (accessed on 29 June 2021).
- Lascufloxacin | Targetmol. Available online: https://www.targetmol.com/compound/Lascufloxacin (accessed on 29 June 2021).
- Ogihara, S. NHI Drug Price Listing and Release of Oral Quinolone Antibacterial Agent “Lasvic®Tablets 75mg.” 2. Available online: https://www.kyorin-pharm.co.jp/en/news/a329f0ae64024c1173f40660eede0efb37f1cbb0.pdf (accessed on 25 June 2021).
- Lascufloxacin-Kyorin Pharmaceutical-AdisInsight. Available online: https://adisinsight.springer.com/drugs/800035339 (accessed on 29 June 2021).
- Cornick, J.E.; Bentley, S.D. Streptococcus Pneumoniae: The Evolution of Antimicrobial Resistance to Beta-Lactams, Fluoroquinolones and Macrolides. Microbes Infect. 2012, 14, 573–583. [Google Scholar] [CrossRef]
- Koulenti, D.; Xu, E.; Yin Sum Mok, I.; Song, A.; Karageorgopoulos, D.E.; Armaganidis, A.; Lipman, J.; Tsiodras, S. Lefamulin. Comment on: “Novel Antibiotics for Multidrug-Resistant Gram-Positive Microorganisms. Microorganisms, 2019, 7, 270”. Microorganisms 2019, 7, 386. [Google Scholar] [CrossRef] [Green Version]
- Thakare, R.; Singh, S.; Dasgupta, A.; Chopra, S. Lascufloxacin Hydrochloride to Treat Bacterial Infection. Drugs Today 2020, 56, 365–376. [Google Scholar] [CrossRef]
- Kishii, R.; Yamaguchi, Y.; Takei, M. In Vitro Activities and Spectrum of the Novel Fluoroquinolone Lascufloxacin (KRP-AM1977). Antimicrob. Agents Chemother. 2017, 61, e00120-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murata, M.; Kosai, K.; Yamauchi, S.; Sasaki, D.; Kaku, N.; Uno, N.; Morinaga, Y.; Hasegawa, H.; Miyazaki, T.; Izumikawa, K.; et al. In Vitro Activity of Lascufloxacin against Streptococcus Pneumoniae with Mutations in the Quinolone Resistance-Determining Regions. Antimicrob. Agents Chemother. 2018, 62, e01971-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totsuka, K.; Sesoko, S.; Fukase, H.; Ikushima, I.; Odajima, M.; Niwayama, Y. Pharmacokinetic Study of Lascufloxacin in Non-Elderly Healthy Men and Elderly Men. J. Infect. Chemother. 2020, 26, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Furuie, H.; Tanioka, S.; Shimizu, K.; Manita, S.; Nishimura, M.; Yoshida, H. Intrapulmonary Pharmacokinetics of Lascufloxacin in Healthy Adult Volunteers. Antimicrob. Agents Chemother. 2018, 62, e02169-17. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, N.; Nakata, Y.; Yazaki, A. New Findings on the Structure-Phototoxicity Relationship and Photostability of Fluoroquinolones with Various Substituents at Position 1. Antimicrob. Agents Chemother. 2004, 48, 799–803. [Google Scholar] [CrossRef] [Green Version]
- Balfour, J.A.; Todd, P.A.; Peters, D.H. Fleroxacin. A Review of Its Pharmacology and Therapeutic Efficacy in Various Infections. Drugs 1995, 49, 794–850. [Google Scholar] [CrossRef]
- Ahmed, A.; Daneshtalab, M. Nonclassical Biological Activities of Quinolone Derivatives. J. Pharm. Pharm. Sci. 2012, 15, 52–72. [Google Scholar] [CrossRef] [Green Version]
- Domagala, J.M.; Hagen, S.E. Structure-Activity Relationships of the Quinolone Antibacterials in the New Millennium: Some Things Change and Some Do Not. In Quinolone Antimicrobial Agents, 3rd ed.; Hooper, D.C., Rubinstein, E., Eds.; ASM Press: Washington DC, USA, 2003; pp. 3–18. [Google Scholar] [CrossRef]
- Piddock, L.J.; Johnson, M.; Ricci, V.; Hill, S.L. Activities of New Fluoroquinolones against Fluoroquinolone-Resistant Pathogens of the Lower Respiratory Tract. Antimicrob. Agents Chemother. 1998, 42, 2956–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PubChem Sitafloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/461399 (accessed on 1 July 2021).
- Keating, G.M. Sitafloxacin: In Bacterial Infections. Drugs 2011, 71, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.L. Sitafloxacin Hydrate for Bacterial Infections. Drugs Today 2008, 44, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.R.; Appelbaum, P.C. Nadifloxacin: A Quinolone for Topical Treatment of Skin Infections and Potential for Systemic Use of Its Active Isomer, WCK 771. Expert Opin. Pharmacother. 2006, 7, 1957–1966. [Google Scholar] [CrossRef]
- Schofer, H.; Gollner, A.; Kusche, W.; Schwantes, U. Effectiveness and Tolerance of Topical Nadifloxacin in the Therapy of Acne Vulgaris (Grade I-II): Results of a Non-Interventional Trial in 555 Patients. J. Appl. Res. 2009, 9, 44. [Google Scholar]
- Alba, V.; Urban, E.; Angeles Dominguez, M.; Nagy, E.; Nord, C.-E.; Palacín, C.; Vila, J. In Vitro Activity of Nadifloxacin against Several Gram-Positive Bacteria and Analysis of the Possible Evolution of Resistance after 2 Years of Use in Germany. Int. J. Antimicrob. Agents 2009, 33, 272–275. [Google Scholar] [CrossRef]
- Takei, M.; Fukuda, H.; Kishii, R.; Hosaka, M. Target Preference of 15 Quinolones against Staphylococcus Aureus, Based on Antibacterial Activities and Target Inhibition. Antimicrob Agents Chemother. 2001, 45, 3544–3547. [Google Scholar] [CrossRef] [Green Version]
- PubChem Nadifloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4410 (accessed on 11 June 2021).
- [Hot Item] Manufacturers Supply Top Quality High Purity Apis Powder Nadifloxacin CAS No. 124858-35-1. Available online: https://dgpeptides.en.made-in-china.com/product/bjZnRINJksWv/China-Manufacturers-Supply-Top-Quality-High-Purity-Apis-Powder-Nadifloxacin-CAS-No-124858-35-1.html (accessed on 11 June 2021).
- Nadifloxacin | ≥99%(HPLC) | Selleck | Antibiotics Chemical. Available online: https://www.selleckchem.com/products/nadifloxacin.html (accessed on 11 June 2021).
- Shinde, U.; Pokharkar, S.; Modani, S. Design and Evaluation of Microemulsion Gel System of Nadifloxacin. Indian J. Pharm. Sci. 2012, 74, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Nadifloxacin (CAS 124858-35-1). Available online: https://www.caymanchem.com/product/20252 (accessed on 11 June 2021).
- 124858-35-1 CAS MSDS (Nadifloxacin) Melting Point Boiling Point Density CAS Chemical Properties. Available online: https://www.chemicalbook.com/ChemicalProductProperty_US_CB3301560.aspx (accessed on 11 June 2021).
- Kumar, A.; Bhashkar, B.; Bhavsar, J. New Approach for the Preparation of Key Intermediates of Nadifloxacin. J. Chem. Pharm. Res. 2016, 8, 609–613. [Google Scholar]
- Martin, Y.C. Exploring QSAR: Hydrophobic, Electronic, and Steric Constants, C. Hansch, A. Leo, and D. Hoekman. American Chemical Society, Washington, DC. 1995. Xix + 348 Pp. 22 × 28.5 Cm. Exploring QSAR: Fundamentals and Applications in Chemistry and Biology. C. Hansch and A. Leo. American Chemical Society, Washington, DC. 1995. Xvii + 557 Pp. 18.5 × 26 Cm. ISBN 0-8412-2993-7 (Set). $99.95 (Set). J. Med. Chem. 1996, 39, 1189–1190. [Google Scholar] [CrossRef]
- PubChem Ofloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4583 (accessed on 13 June 2021).
- Bhagwat, S.S.; Nandanwar, M.; Kansagara, A.; Patel, A.; Takalkar, S.; Chavan, R.; Periasamy, H.; Yeole, R.; Deshpande, P.K.; Bhavsar, S.; et al. Levonadifloxacin, a Novel Broad-Spectrum Anti-MRSA Benzoquinolizine Quinolone Agent: Review of Current Evidence. Drug Des. Devel. Ther. 2019, 13, 4351–4365. [Google Scholar] [CrossRef] [Green Version]
- Bakthavatchalam, Y.D.; Shankar, A.; Muniyasamy, R.; Peter, J.V.; Marcus, Z.; Triplicane Dwarakanathan, H.; Gunasekaran, K.; Iyadurai, R.; Veeraraghavan, B. Levonadifloxacin, a Recently Approved Benzoquinolizine Fluoroquinolone, Exhibits Potent In Vitro Activity against Contemporary Staphylococcus Aureus Isolates and Bengal Bay Clone Isolates Collected from a Large Indian Tertiary Care Hospital. J. Antimicrob. Chemother. 2020, 75, 2156–2159. [Google Scholar] [CrossRef]
- Baliga, S.; Mamtora, D.K.; Gupta, V.; Shanmugam, P.; Biswas, S.; Mukherjee, D.N.; Shenoy, S. Assessment of Antibacterial Activity of Levonadifloxacin against Contemporary Gram-Positive Clinical Isolates Collected from Various Indian Hospitals Using Disk-Diffusion Assay. Indian J. Med. Microbiol. 2020, 38, 307–312. [Google Scholar] [CrossRef]
- Rodvold, K.A.; Gotfried, M.H.; Chugh, R.; Gupta, M.; Yeole, R.; Patel, A.; Bhatia, A. Intrapulmonary Pharmacokinetics of Levonadifloxacin Following Oral Administration of Alalevonadifloxacin to Healthy Adult Subjects. Antimicrob. Agents Chemother. 2018, 62, e02297-17. [Google Scholar] [CrossRef] [Green Version]
- Lautre, C.; Sharma, S.; Sahu, J.K. Chemistry, Biological Properties and Analytical Methods of Levonadifloxacin: A Review. Crit. Rev. Anal. Chem. 2020, 2020, 1855412. [Google Scholar] [CrossRef]
- Bhatia, A.; Mastim, M.; Shah, M.; Gutte, R.; Joshi, P.; Kumbhar, D.; Periasamy, H.; Palwe, S.R.; Chavan, R.; Bhagwat, S.; et al. Efficacy and Safety of a Novel Broad-Spectrum Anti-MRSA Agent Levonadifloxacin Compared with Linezolid for Acute Bacterial Skin and Skin Structure Infections: A Phase 3, Openlabel, Randomized Study. J. Assoc. Physicians India 2020, 68, 30–36. [Google Scholar] [PubMed]
- Saxena, D.; Kaul, G.; Dasgupta, A.; Chopra, S. Levonadifloxacin Arginine Salt to Treat Acute Bacterial Skin and Skin Structure Infection Due to S. Aureus Including MRSA. Drugs Today 2020, 56, 583–598. [Google Scholar] [CrossRef]
- Kongre, V.; Bhagwat, S.; Bharadwaj, R.S. Resistance Pattern among Contemporary Gram Positive Clinical Isolates and in Vitro Activity of Novel Antibiotic, Levonadifloxacin (WCK 771). Int. J. Infect. Dis. 2020, 101, 30. [Google Scholar] [CrossRef]
- Tellis, M.; Joseph, J.; Khande, H.; Bhagwat, S.; Patel, M. In Vitro Bactericidal Activity of Levonadifloxacin (WCK 771) against Methicillin- and Quinolone-Resistant Staphylococcus Aureus Biofilms. J. Med. Microbiol. 2019, 68, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Lee, K.-Y.; Lin, S.-W.; Chen, Y.-H.; Kuo, H.-Y.; Hung, C.-C.; Hsueh, P.-R. Nemonoxacin (TG-873870) for Treatment of Community-Acquired Pneumonia. Expert Rev. Anti. Infect. Ther. 2014, 12, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy Study of TG-873870 (Nemonoxacin) in Diabetic Foot Infections. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00685698 (accessed on 21 June 2021).
- Liapikou, A.; Cilloniz, C.; Mensa, J.; Torres, A. New Antimicrobial Approaches to Gram Positive Respiratory Infections. Pulm. Pharmacol. Ther. 2015, 32, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.-W.; Hsu, M.-C.; Zhang, Y.-Y. Nemonoxacin (Taigexyn®): A New Non-Fluorinated Quinolone; IntechOpen: London, UK, 2019; ISBN 978-1-78984-473-3. [Google Scholar]
- Jean, S.-S.; Chang, L.-W.; Hsueh, P.-R. Tentative Clinical Breakpoints and Epidemiological Cut-off Values of Nemonoxacin for Streptococcus Pneumoniae and Staphylococcus Aureus Isolates Associated with Community-Acquired Pneumonia. J. Glob. Antimicrob. Resist. 2020, 23, 388–393. [Google Scholar] [CrossRef]
- Lin, L.; Chang, L.-W.; Tsai, C.-Y.; Hsu, C.-H.; Chung, D.T.; Aronstein, W.S.; Ajayi, F.; Kuzmak, B.; Lyon, R.A. Dose Escalation Study of the Safety, Tolerability, and Pharmacokinetics of Nemonoxacin (TG-873870), a Novel Potent Broad-Spectrum Nonfluorinated Quinolone, in Healthy Volunteers. Antimicrob. Agents Chemother. 2010, 54, 405–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NCATS Inxight: Drugs-Nemonoxacin Malate Hemihydrate. Available online: https://drugs.ncats.io/drug/Y97F3051FH (accessed on 22 June 2021).
- Wu, X.-J.; Zhang, J.; Guo, B.-N.; Zhang, Y.-Y.; Yu, J.-C.; Cao, G.-Y.; Yuan-cheng, C.; Zhu, D.-M.; Ye, X.-Y.; Wu, J.-F.; et al. Pharmacokinetics and Pharmacodynamics of Multiple-Dose Intravenous Nemonoxacin in Healthy Chinese Volunteers. Antimicrob. Agents Chemother. 2014, 59. [Google Scholar] [CrossRef] [Green Version]
- PubChem Nemonoxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11993740 (accessed on 22 June 2021).
- NCATS Inxight: Drugs-NEMONOXACIN. Available online: https://drugs.ncats.io/drug/P94L0PVO94 (accessed on 22 June 2021).
- Nemonoxacin. Available online: https://go.drugbank.com/drugs/DB06600 (accessed on 22 June 2021).
- Arjona, A. Nemonoxacin Quinolone Antibiotic. Drug Future 2009, 34, 196–203. [Google Scholar] [CrossRef]
- Qin, X.; Huang, H. Review of Nemonoxacin with Special Focus on Clinical Development. Drug Des. Dev. Ther. 2014, 8, 765–774. [Google Scholar] [CrossRef] [Green Version]
- Gatifloxacin. Available online: https://go.drugbank.com/drugs/DB01044 (accessed on 22 June 2021).
- Kishii, R.; Takei, M.; Fukuda, H.; Hayashi, K.; Hosaka, M. Contribution of the 8-Methoxy Group to the Activity of Gatifloxacin against Type II Topoisomerases of Streptococcus Pneumoniae. Antimicrob. Agents Chemother. 2003, 47, 77–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PubChem Balofloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/65958 (accessed on 28 June 2021).
- Ball, P.; Mandell, L.; Niki, Y.; Tillotson, G. Comparative Tolerability of the Newer Fluoroquinolone Antibacterials. Drug Saf. 1999, 21, 407–421. [Google Scholar] [CrossRef]
- Shankar, G.; Borkar, R.M.; Udutha, S.; Anagoni, S.P.; Srinivas, R. Identification and Structural Characterization of in Vivo Metabolites of Balofloxacin in Rat Plasma, Urine and Feces Samples Using Q-TOF/LC/ESI/MS/MS: In Silico Toxicity Studies. J. Pharm. Biomed. Anal. 2018, 159, 200–211. [Google Scholar] [CrossRef]
- PubChem Acorafloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11546234 (accessed on 28 June 2021).
- Chen, Y.-H.; Liu, C.-Y.; Lu, J.-J.; King, C.-H.R.; Hsueh, P.-R. In Vitro Activity of Nemonoxacin (TG-873870), a Novel Non-Fluorinated Quinolone, against Clinical Isolates of Staphylococcus Aureus, Enterococci and Streptococcus Pneumoniae with Various Resistance Phenotypes in Taiwan. J. Antimicrob. Chemother. 2009, 64, 1226–1229. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Wang, R.; Li, A. Antibacterial Activities of Nemonoxacin against Clinical Isolates of Staphylococcus Aureus: An in Vitro Comparison with Three Fluoroquinolones. World J. Microbiol. Biotechnol. 2014, 30, 2927–2932. [Google Scholar] [CrossRef]
- Lauderdale, T.-L.; Shiau, Y.-R.; Lai, J.-F.; Chen, H.-C.; King, C.-H.R. Comparative In Vitro Activities of Nemonoxacin (TG-873870), a Novel Nonfluorinated Quinolone, and Other Quinolones against Clinical Isolates. Antimicrob. Agents Chemother. 2010, 54, 1338–1342. [Google Scholar] [CrossRef] [Green Version]
- Adam, H.J.; Laing, N.M.; King, C.R.; Lulashnyk, B.; Hoban, D.J.; Zhanel, G.G. In Vitro Activity of Nemonoxacin, a Novel Nonfluorinated Quinolone, against 2,440 Clinical Isolates. Antimicrob. Agents Chemother. 2009, 53, 4915–4920. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-R.; Li, Y.; Li, G.-Q.; Yang, X.-Y.; Zhang, W.-X.; Lou, R.-H.; Liu, J.-F.; Yuan, M.; Huang, P.; Cen, S.; et al. In Vivo Antibacterial Activity of Nemonoxacin, a Novel Non-Fluorinated Quinolone. J. Antimicrob. Chemother. 2010, 65, 2411–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rensburg, D.J.J.; Perng, R.-P.; Mitha, I.H.; Bester, A.J.; Kasumba, J.; Wu, R.-G.; Ho, M.-L.; Chang, L.-W.; Chung, D.T.; Chang, Y.-T.; et al. Efficacy and Safety of Nemonoxacin versus Levofloxacin for Community-Acquired Pneumonia. Antimicrob. Agents Chemother. 2010, 54, 4098–4106. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.T.; Chen, Y.; Cao, Y.; Liu, X.; Huang, J.; Hu, J.; Zhao, M.; Guo, Q.; Zhang, S.; Wu, X.; et al. Pharmacokinetics and Pharmacodynamics of Nemonoxacin against Streptococcus Pneumoniae in an In Vitro Infection Model. Antimicrob. Agents Chemother. 2013, 57, 2942–2947. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Kim, S.E.; Shin, K.-H.; Lim, C.; Lim, K.S.; Yu, K.-S.; Cho, J.-Y. Comparison of Pharmacokinetics between New Quinolone Antibiotics: The Zabofloxacin Hydrochloride Capsule and the Zabofloxacin Aspartate Tablet. Curr. Med. Res. Opin. 2013, 29, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- DONG WHA PHARMACEUTICAL CO., LTD. Available online: https://www.dong-wha.co.kr/english/product/content.asp?t_idx=545&t_page=1&d=&b=10&s=11 (accessed on 24 June 2021).
- Park, H.-S.; Oh, S.-H.; Kim, H.-S.; Choi, D.-R.; Kwak, J.-H. Antimicrobial Activity of Zabofloxacin against Clinically Isolated Streptococcus Pneumoniae. Molecules 2016, 21, 1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-S.; Kim, H.-J.; Seol, M.-J.; Choi, D.-R.; Choi, E.-C.; Kwak, J.-H. In Vitro and In Vivo Antibacterial Activities of DW-224a, a New Fluoronaphthyridone. Antimicrob. Agents Chemother. 2006, 50, 2261–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.N.; Biedenbach, D.J.; Ambrose, P.G.; Wikler, M.A. Zabofloxacin (DW-224a) Activity against Neisseria Gonorrhoeae Including Quinolone-Resistant Strains. Diagn. Microbiol. Infect. Dis. 2008, 62, 110–112. [Google Scholar] [CrossRef]
- Sellarès-Nadal, J.; Burgos, J.; Falcó, V.; Almirante, B. Investigational and Experimental Drugs for Community-Acquired Pneumonia: The Current Evidence. J. Exp. Pharm. 2020, 12, 529–538. [Google Scholar] [CrossRef] [PubMed]
- PubChem Zabofloxacin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9952872 (accessed on 19 June 2021).
- Zabofloxacin. Available online: https://go.drugbank.com/drugs/DB12479 (accessed on 23 June 2021).
- Jin, H.E.; Kang, I.H.; Shim, C.K. Fluorescence Detection of Zabofloxacin, a Novel Fluoroquinolone Antibiotic, in Plasma, Bile, and Urine by HPLC: The First Oral and Intravenous Applications in a Pharmacokinetic Study in Rats. J. Pharm. Pharm. Sci. 2011, 14, 291–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benadallah, M.; Talhi, O.; Nouali, F.; Choukchou-Braham, N.; Bachari, K.; Silva, A.M.S. Advances in Spirocyclic Hybrids: Chemistry and Medicinal Actions. Curr. Med. Chem. 2018, 25, 3748–3767. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-T.; Zhu, J.-F.; Liao, G.; Xu, H.-J.; Yu, B. The Development of Biologically Important Spirooxindoles as New Antimicrobial Agents. Curr. Med. Chem. 2018, 25, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, J.; Shan, T.; Cai, X.; Peng, Y. Spirobisnaphthalenes from Fungi and Their Biological Activities. Mini Rev. Med. Chem. 2010, 10, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; He, L. Advances in Research of Spirodienone and Its Derivatives: Biological Activities and Synthesis Methods. Eur. J. Med. Chem. 2020, 203, 112577. [Google Scholar] [CrossRef]
- Fan, Y.-L.; Wu, J.-B.; Cheng, X.-W.; Zhang, F.-Z.; Feng, L.-S. Fluoroquinolone Derivatives and Their Anti-Tubercular Activities. Eur. J. Med. Chem. 2018, 146, 554–563. [Google Scholar] [CrossRef]
- Shah, P.; Westwell, A.D. The Role of Fluorine in Medicinal Chemistry: Review Article. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [Green Version]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Jain, S. Fluoroquinolone Antibacterials: A Review on Chemistry, Microbiology and Therapeutic Prospects. Acta Pol. Pharm. 2009, 66, 587–604. [Google Scholar]
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The New Fluoroquinolones: A Critical Review. Canadian J. Infect. Dis. 1999, 10, 207–238. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Matsukawa, Y.; Suematsu, H.; Mikamo, H. In Vitro Activity of Lascufloxacin, a Novel Fluoroquinolone Antibacterial Agent, against Various Clinical Isolates of Anaerobes and Streptococcus Anginosus Group. Anaerobe 2018, 54, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Suaifan, G.A.R.Y.; Mohammed, A.A.M. Fluoroquinolones Structural and Medicinal Developments (2013–2018): Where Are We Now? Bioorganic Med. Chem. 2019, 27, 3005–3060. [Google Scholar] [CrossRef]
- Jones, T.M.; Johnson, S.W.; DiMondi, V.P.; Wilson, D.T. Focus on JNJ-Q2, a Novel Fluoroquinolone, for the Management of Community-Acquired Bacterial Pneumonia and Acute Bacterial Skin and Skin Structure Infections. Infect. Drug Resist. 2016, 9, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalhoff, A. Global Fluoroquinolone Resistance Epidemiology and Implictions for Clinical Use. Interdiscip. Perspect. Infect. Dis. 2012, 2012, e976273. [Google Scholar] [CrossRef] [Green Version]
- Yamane, K.; Wachino, J.; Suzuki, S.; Kimura, K.; Shibata, N.; Kato, H.; Shibayama, K.; Konda, T.; Arakawa, Y. New Plasmid-Mediated Fluoroquinolone Efflux Pump, QepA, Found in an Escherichia Coli Clinical Isolate. Antimicrob. Agents Chemother. 2007, 51, 3354–3360. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-Z.; Nikaido, H. Efflux-Mediated Drug Resistance in Bacteria: An Update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Grkovic, S.; Brown, M.H.; Skurray, R.A. Regulation of Bacterial Drug Export Systems. Microbiol. Mol. Biol. Rev. 2002, 66, 671–701. [Google Scholar] [CrossRef] [Green Version]
- Blondeau, J.M. Fluoroquinolones: Mechanism of Action, Classification, and Development of Resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef]
- Park, H.S.; Jung, S.J.; Kwak, J.-H.; Choi, D.-R.; Choi, E.-C. DNA Gyrase and Topoisomerase IV Are Dual Targets of Zabofloxacin in Streptococcus Pneumoniae. Int. J. Antimicrob. Agents 2010, 36, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.M.; Heaton, V.J. Dual Activity of Fluoroquinolones against Streptococcus Pneumoniae. J. Antimicrob. Chemother. 2003, 51, 463–464. [Google Scholar] [CrossRef] [Green Version]
- Remy, J.M.; Tow-Keogh, C.A.; McConnell, T.S.; Dalton, J.M.; Devito, J.A. Activity of Delafloxacin against Methicillin-Resistant Staphylococcus Aureus: Resistance Selection and Characterization. J. Antimicrob. Chemother. 2012, 67, 2814–2820. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.J.; Nichol, K.A.; Hoban, D.J.; Zhanel, G.G. Dual Activity of Fluoroquinolones against Streptococcus Pneumoniae: The Facts behind the Claims. J. Antimicrob. Chemother. 2002, 49, 893–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanfilippo, C.M.; Hesje, C.K.; Haas, W.; Morris, T.W. Topoisomerase Mutations That Are Associated with High-Level Resistance to Earlier Fluoroquinolones in Staphylococcus Aureus Have Less Effect on the Antibacterial Activity of Besifloxacin. Chemotherapy 2011, 57, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Makin, K.; Twinem, T.; Leunk, R.; Hsu, M.C. In Vitro Resistance Development to Nemonoxacin in Streptococcus Pneumoniae: A Unique Profile for a Novel Nonfluorinated Quinolone. Microb. Drug Resist. 2016, 22, 578–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinabarger, D.L.; Zurenko, G.E.; Hesje, C.K.; Sanfilippo, C.M.; Morris, T.W.; Haas, W. Evaluation of the Effect of Bacterial Efflux Pumps on the Antibacterial Activity of the Novel Fluoroquinolone Besifloxacin. J. Chemother. 2011, 23, 80–86. [Google Scholar] [CrossRef]
- Avalos, E.; Catanzaro, D.; Catanzaro, A.; Ganiats, T.; Brodine, S.; Alcaraz, J.; Rodwell, T. Frequency and Geographic Distribution of GyrA and GyrB Mutations Associated with Fluoroquinolone Resistance in Clinical Mycobacterium Tuberculosis Isolates: A Systematic Review. PLoS ONE 2015, 10, e0120470. [Google Scholar] [CrossRef] [Green Version]
- Deekshit, V.K.; Jazeela, K.; Chakraborty, G.; Rohit, A.; Chakraborty, A.; Karunasagar, I. Mismatch Amplification Mutation Assay-Polymerase Chain Reaction: A Method of Detecting Fluoroquinolone Resistance Mechanism in Bacterial Pathogens. Indian J. Med. Res. 2019, 149, 146–150. [Google Scholar] [CrossRef]
- Zhang, B. Quinolone Derivatives and Their Antifungal Activities: An Overview. Arch. Pharm. 2019, 352, e1800382. [Google Scholar] [CrossRef]
- Morrow, B.J.; He, W.; Amsler, K.M.; Foleno, B.D.; Macielag, M.J.; Lynch, A.S.; Bush, K. In Vitro Antibacterial Activities of JNJ-Q2, a New Broad-Spectrum Fluoroquinolone. Antimicrob. Agents Chemother. 2010, 54, 1955–1964. [Google Scholar] [CrossRef] [Green Version]
- Davenport, J.M.; Covington, P.; Gotfried, M.; Medlock, M.; Watanalumlerd, P.; McIntyre, G.; Turner, L.; Almenoff, J. Summary of Pharmacokinetics and Tissue Distribution of a Broad-Spectrum Fluoroquinolone, JNJ-Q2. Clin. Pharm. Drug Dev. 2012, 1, 121–130. [Google Scholar] [CrossRef]
- Avarofloxacin (Furiex), Has Been Granted QIDP and Fast-Track Designations for Treatment of Acute Bacterial Skin and Skin-Structure Infections. Formulary 2013, 48, 135.
- Darehkordi, A.; Javanmiri, M.; Ghazi, S.; Assar, S. Synthesis of N-Aryl-2,2,2-Trifluoroacetimidoyl Piperazinylquinolone Derivatives and Their Antibacterial Evaluations. J. Fluor. Chem. 2011, 132, 263–268. [Google Scholar] [CrossRef]
- Swellmeen, L.; Uzrail, A.; Shahin, R.; AL-Hiari, Y. Synthesis of Fluoroquinolones Derivatives as Antimicrobial Agents. Orient. J. Chem. 2019, 35, 1248–1253. [Google Scholar] [CrossRef] [Green Version]
- Lapointe, G.; Skepper, C.K.; Holder, L.M.; Armstrong, D.; Bellamacina, C.; Blais, J.; Bussiere, D.; Bian, J.; Cepura, C.; Chan, H.; et al. Discovery and Optimization of DNA Gyrase and Topoisomerase IV Inhibitors with Potent Activity against Fluoroquinolone-Resistant Gram-Positive Bacteria. J. Med. Chem. 2021, 64, 6329–6357. [Google Scholar] [CrossRef] [PubMed]
- Chugunova, E.; Akylbekov, N.; Bulatova, A.; Gavrilov, N.; Voloshina, A.; Kulik, N.; Zobov, V.; Dobrynin, A.; Syakaev, V.; Burilov, A. Synthesis and Biological Evaluation of Novel Structural Hybrids of Benzofuroxan Derivatives and Fluoroquinolones. Eur. J. Med. Chem. 2016, 116, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Sączewski, J. Modifications of Quinolones and Fluoroquinolones: Hybrid Compounds and Dual-Action Molecules. Mon. Chem.Chem. Mon. 2018, 149, 1199–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordeev, M.F.; Hackbarth, C.; Barbachyn, M.R.; Banitt, L.S.; Gage, J.R.; Luehr, G.W.; Gomez, M.; Trias, J.; Morin, S.E.; Zurenko, G.E.; et al. Novel Oxazolidinone-Quinolone Hybrid Antimicrobials. Bioorgan. Med. Chem. 2003, 13, 4213–4216. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Senchani, G.; Banerjee, D. Newer Tetracycline Derivatives: Synthesis, Anti-HIV, Antimycobacterial Activities and Inhibition of HIV-1 Integrase. Bioorganic. Med. Chem. Lett. 2007, 17, 2372–2375. [Google Scholar] [CrossRef]
- Pokrovskaya, V.; Belakhov, V.; Hainrichson, M.; Yaron, S.; Baasov, T. Design, Synthesis, and Evaluation of Novel Fluoroquinolone−Aminoglycoside Hybrid Antibiotics. J. Med. Chem. 2009, 52, 2243–2254. [Google Scholar] [CrossRef]
- Gorityala, B.K.; Guchhait, G.; Goswami, S.; Fernando, D.M.; Kumar, A.; Zhanel, G.G.; Schweizer, F. Hybrid Antibiotic Overcomes Resistance in P. Aeruginosa by Enhancing Outer Membrane Penetration and Reducing Efflux. J. Med. Chem. 2016, 59, 8441–8455. [Google Scholar] [CrossRef] [PubMed]
- Shavit, M.; Pokrovskaya, V.; Belakhov, V.; Baasov, T. Covalently Linked Kanamycin-Ciprofloxacin Hybrid Antibiotics as a Tool to Fight Bacterial Resistance. Bioorganic Med. Chem. 2017, 25, 2917–2925. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-N.; Bheemanaboina, R.R.Y.; Gao, W.-W.; Kang, J.; Cai, G.-X.; Zhou, C.-H. Discovery of Benzimidazole-Quinolone Hybrids as New Cleaving Agents toward Drug-Resistant Pseudomonas Aeruginosa DNA. Chem. Med. Chem. 2018, 13, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Bertino, J.; Fish, D. The Safety Profile of the Fluoroquinolones. Clin. Ther. 2000, 22, 798–817. [Google Scholar] [CrossRef]
- Briasoulis, A.; Agarwal, V.; Pierce, W.J. QT Prolongation and Torsade de Pointes Induced by Fluoroquinolones: Infrequent Side Effects from Commonly Used Medications. Cardiology 2011, 120, 103–110. [Google Scholar] [CrossRef]
- Daneman, N.; Lu, H.; Redelmeier, D.A. Fluoroquinolones and Collagen Associated Severe Adverse Events: A Longitudinal Cohort Study. BMJ Open 2015, 5, e010077. [Google Scholar] [CrossRef] [Green Version]
- Raini, M. Fluoroquinolones Antibiotics: Benefit and Side Effects. Media Penelit. Pengemb. Kesehat. 2016, 26, 163–174. [Google Scholar]
- Roberts, J.R. InFocus: Fluoroquinolone Side Effects Just Got Scarier. Emerg. Med. News 2018, 40, 26–27. [Google Scholar] [CrossRef]
- Tandan, M.; Cormican, M.; Vellinga, A. Adverse Events of Fluoroquinolones vs. Other Antimicrobials Prescribed in Primary Care: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Antimicrob. Agents 2018, 52, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Lewis, T.; Cook, J. Fluoroquinolones and Tendinopathy: A Guide for Athletes and Sports Clinicians and a Systematic Review of the Literature. J. Athl. Train. 2014, 49, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Gorelik, E.; Masarwa, R.; Perlman, A.; Rotshild, V.; Abbasi, M.; Muszkat, M.; Matok, I. Fluoroquinolones and Cardiovascular Risk: A Systematic Review, Meta-Analysis and Network Meta-Analysis. Drug Saf. 2019, 42, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Haiping, L.; Ziqiang, J.; Qina, Z.; Yuhua, D. Adverse Reactions of Fluoroquinolones to Central Nervous System and Rational Drug Use in Nursing Care. Pak. J. Pharm. Sci. 2019, 32, 427–432. [Google Scholar] [PubMed]
- Gatti, M.; Bianchin, M.; Raschi, E.; De Ponti, F. Assessing the Association between Fluoroquinolones and Emerging Adverse Drug Reactions Raised by Regulatory Agencies: An Umbrella Review. Eur. J. Intern. Med. 2020, 75, 60–70. [Google Scholar] [CrossRef] [PubMed]
- FDA. Approves Safety Labeling Changes for Fluoroquinolones. FDA. 2019. Available online: https://www.fda.gov/drugs/information-drug-class/fda-approves-safety-labeling-changes-fluoroquinolones (accessed on 12 August 2021).
- Aschenbrenner, D.S. The FDA Revises Boxed Warning For Fluoroquinolones-Again. Am. J. Nurs. 2016, 116, 22–23. [Google Scholar] [CrossRef] [PubMed]
- FDA Updates Warnings for Fluoroquinolone Antibiotics. Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics (accessed on 12 August 2021).
- FDA. Drug Safety Communication: FDA Advises Restricting Fluoroquinolone Antibiotic Use for Certain Uncomplicated Infections. Warns about Disabling Side Effects That Can Occur Together. FDA. 2019. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-advises-restricting-fluoroquinolone-antibiotic-use-certain (accessed on 12 August 2021).
- FDA Warns about Increased Risk of Ruptures or Tears in the Aorta Blood Vessel with Fluoroquinolone Antibiotics in Certain Patients.7. Available online: https://www.fda.gov/media/119532/download (accessed on 12 August 2021).
- FDA Updates Warnings for Fluoroquinolone Antibiotics on Risks of Mental Health and Low Blood Sugar Adverse Reactions. Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse (accessed on 12 August 2021).
- Francisco, E.M. Fluoroquinolone and Quinolone Antibiotics: PRAC Recommends New Restrictions on Use Following Review of Disabling Potentially Long-Lasting Side Effects. Available online: https://www.ema.europa.eu/en/news/fluoroquinolone-quinolone-antibiotics-prac-recommends-new-restrictions-use-following-review (accessed on 12 August 2021).
- Francisco, E.M. Meeting Highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 1-4 October 2018. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-1-4-october-2018 (accessed on 12 August 2021).
- Quinolone- and Fluoroquinolone-Containing Medicinal Products. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products (accessed on 12 August 2021).
- EMA/175398/2019, Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone and Fluoroquinolone Antibiotics. 2019. Available online: https://www.ema.europa.eu/en/documents/referral/quinolone-fluoroquinolone-article-31-referral-disabling-potentially-permanent-side-effects-lead_en.pdf (accessed on 12 August 2021).
- EMA/413844/2018, Summary of the EMA Public Hearing on Quinolone and Fluoroquinolone Antibiotics. 2018. Available online: https://www.ema.europa.eu/en/documents/report/ summary-ema-public-hearing-quinolone-luoroquinolone-antibiotics_en.pdf (accessed on 12 August 2021).
- Majalekar, P.P.; Shirote, P.J. Fluoroquinolones: Blessings or Curses. Curr. Drug Targets 2020, 21, 1354–1370. [Google Scholar] [CrossRef]
- Richards, G.A.; Brink, A.J.; Feldman, C. Rational Use of the Fluoroquinolones. SAMJ S. Afr. Med. J. 2019, 109, 378–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| 1 Year | Generation | Antibacterial QNs | Producer | Use and Status | References |
|---|---|---|---|---|---|
| 1962 | 1st | Nalidixic acid | Lappin/Sterling Drug | Human and veterinary | [14,15] |
| 1966 | 1st | Oxolinic acid | Warner-Lambert | Withdrawn | [14,15] |
| 1967 | 1st | Piromidic acid | Dainippon | Withdrawn | [14,15] |
| 1970 | 1st | Cinoxacin | Eli Lilly | Withdrawn | [14] |
| 1973 | 1st | Flumequine | Riker | Veterinary | [14,15] |
| 1974 | 1st | Pipemidic acid | Dainippon | Withdrawn | [14] |
| 1978 | 2nd | Norfloxacin | Kyorin | Human and veterinary Approved by the FDA in 1986 | [14] |
| 1979 | 2nd | Pefloxacin | Roger Bellan (Rhône Poulenc) | Approved in France since 1985; approved in some EU countries | [14,15,16,17] |
| 1980 | 2nd | Enoxacin | Dainippon | Withdrawn | [3,14,15] |
| 1981 | 2nd | Fleroxacin | Kyorin | Introduced in therapy in 1987, withdrawn in 1990 | [14,15,18] |
| 1982 | 2nd | Ofloxacin 2 | Daiichi | Human and veterinary, Approved by the FDA in 1990 | [14,15] |
| 1984 | 2nd | Temafloxacin | Abbott Laboratories | Approved by the FDA and also withdrawn in 1992 | [8,18,19] |
| 1985 | 2nd | Lomefloxacin 2 | Hokuriku Pharm. | Approved by the FDA in 1992, then withdrawn | [3] |
| 1985 | 2nd | Tosufloxacin | Taisho-Toyama Chemistry, Abbott | Veterinary (Japan) | [8,15,20] |
| 1987 | 2nd | Ciprofloxacin | Bayer AG | Human and veterinary, Approved by the FDA in 1987 | [14,15] |
| 1987 | 3rd | Enrofloxacin | Bayer AG | Veterinary | [14,15,21] |
| 1987 | 3rd | Sparfloxacin 3 | Dainippon | Approved by the FDA in 1996, then withdrawn | [3,14,15] |
| 1987 | 4th | Prulifloxacin | Nippon | Approved only in Japan | [14,22,23] |
| 1987 | 3rd | Orbifloxacin 3 | Dainippon/Schering | Veterinary | [14,24] |
| 1989 | 2nd | Nadifloxacin 2 | Otsuka Pharmaceuticals | Approved in Japan in 1993 and 1998, approved by the EMA in 2000, topical use | [25,26,27] |
| 1989 | 3rd | Grepafloxacin 2 | Warner Lambert/Glaxo Wellcome | Withdrawn in 1999 | [3,15] |
| 1990 | 3rd | Clinafloxacin | Parke-Davis Pharmaceutical | Withdrawn in 1999 | [3,14] |
| 1991 | 3rd | Danofloxacin | Pfizer | Veterinary | [14,15,28] |
| 1992 | 4th | Trovafloxacin | Pfizer | Withdrawn in 2001 | [3,14,15] |
| 1994 | 3rd | Levofloxacin 4 | Daiichi | Approved by the FDA in 1996 | [14,15,29] |
| 1994 | 2nd | Sarafloxacin | Abbott Laboratories/Fort Dodge | Veterinary, withdrawn in 2001 | [14,15,30,31] |
| 1995 | 3rd | Balofloxacin | Choongwae Pharma | Approved by the Korean FDA in 2001 | [23,32] |
| 1995 | 3rd | Marbofloxacin | Vetoquinol/Pfizer | Veterinary | [14,15,33] |
| 1995 | 4th | Moxifloxacin 4 | Bayer AG | Approved by the FDA in 1999 | [3,29,34] |
| 1996 | 2nd | Difloxacin | Fort Dodge | Veterinary | [14] |
| 1998 | 3rd | Pradofloxacin | Bayer Animal Health GmbH | Approved by the EMA in 2011, and FDA in 2013; veterinary use | [35,36] |
| 1999 | 4th | Delafloxacin | Abbott Laboratories, Melinta Therapeutics (former Rib-X Pharmaceuticals) | Approved by the FDA in 2017 | [37,38,39,40,41,42] |
| 1999 | 3rd | Gatifloxacin 2 | Kyorin/Bristol-Myers Squibb | Approved by the FDA in 1999, withdrawn in 2006 | [3,25,43,44] |
| 1999 | 4th | Gemifloxacin | Smith-Kline Beecham | Approved by the FDA in 2003 Withdrawn in 2009 (by EMA) | [3,25,29,45] |
| 2000 | 4th | Besifloxacin 4 | SSP Co. Ltd., Japonia | Approved by the FDA in 2009 | [46] |
| 2000 | 4th | Finafloxacin 3 | Bayer HealthCare Pharmaceuticals, Byk Gulden, MerLion Pharmaceuticals | Approved by the FDA in 2017 | [42,47,48,49,50] |
| 2003 | 4th | Garenoxacin | Toyama Chemical Co., Ltd./Schering Plough | Approved by the FDA and EMA in 2006; withdrawn in 2007 | [3,29,51,52] |
| 2004 | 4th | Nemonoxacin | TaiGen Biotechnology | Approved in Taiwan in 2014 | [53,54] |
| - | 4th | Zabofloxacin | Dong Wha Pharm. Co. Ltd. | Approved in Soth Korea in 2015 | [55,56] |
| - | 4th | Sitafloxacin | Daiichi Sankyo Co., Japan | Approved in Japan in 2008, in Thailand in 2012 | [57,58,59] |
| Generation | Compounds | Antibacterial Spectrum | Therapeutic Indications/Pharmacokinetics, Administration | Ref. |
|---|---|---|---|---|
| 1st | Nalidixic acid | Gram-negative pathogens—Enterobacteriaceae (without Pseudomonas species) | Uncomplicated urinary tract infections; Oral administration, low serum and tissue concentrations, renal elimination, short half-life. | [3,8,82,83] |
| 2nd | Norfloxacin Ciprofloxacin Ofloxacin Pefloxacin | Enterobacteriaceae; Some atypical pathogens; Pseudomonas aeruginosa (ciprofloxacin only); Some Gram-positive pathogens (including Streptococcus pneumoniae), moderate activity on Staphylococcus aureus. | Uncomplicated and complicated urinary tract infections, pyelonephritis, sexually transmitted diseases, prostatitis, skin and tissue infections; Oral administration, low serum and tissue concentrations (only for norfloxacin); Oral and parenteral administration, high concentrations in serum and tissues, longer half-life. | [3,5,83,84,85,86] |
| 2nd | Nadifloxacin (topical use) | Gram-positive (including methicillin-resistant Staphylococcus aureus (MRSA) and coagulase-negative staphylococci), aerobic Gram-negative, and anaerobic pathogens. | Treatment of acne vulgaris and other skin infections. Topical use, 1% cream. | [26,87,88,89] |
| 3rd | Levofloxacin | Enterobacteriaceae; Atypical pathogens; Penicillin-resistant Streptococcus pneumoniae. | Acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial); Oral and parenteral administration, high serum and tissue concentrations, long half-life (6–8 h). Ophthalmic use (0.5% ophthalmic solution). | [29,86,90,91] |
| 3rd | Gatifloxacin (ophthalmic use) | Broad-spectrum including Staphylococcus aureus, Streptococcus species, and Gram-negative pathogens | Bacterial conjunctivitis, ophthalmic use (0.3% or 0.5% ophthalmic solution). | [43] |
| 4th | Moxifloxacin | Enterobacteriaceae; Atypical pathogens; Pseudomonas aeruginosa; Streptococci; MRSA; Anaerobic pathogens. Others: Chlamydophila pneumoniae, Mycoplasma pneumonia | Sexually transmitted diseases, prostatitis, skin and tissue infections, acute and chronic bronchitis, exacerbated forms, acquired pneumonia (nosocomial), intra-abdominal infections, gynecological infections; bacterial conjunctivitis. Oral, parenteral, and ophthalmic administration (0.5%), high serum and tissue concentrations, long half-life (8–16 h). | [34,92,93] |
| 4th | Delafloxacin | Gram-positive (including methicillin-resistant Staphylococcus aureus) and Gram-negative pathogens | Bacterial skin and skin structure infections. Oral and intravenous administration, oral bioavailability 58.8%, plasma protein binding 84%, mean half-life 4.2–8.5 h (oral), and 3.7 h (intravenous). | [39,41,94] |
| 4th | Besifloxacin (topical, ophtalmic administration) | Streptococcus pneumonia, Staphylococcus epidermidis, Staphylococcus aureus, Haemophilus influenza, Moraxella catarrhalis, Corynebacterium spp | Bacterial conjunctivitis. Ophthalmic suspension (0.6%). | [46,95,96,97] |
| 4th | Finafloxacin (topical, otic administration) | Broad-spectrum activity (very active against Pseudomonas aeruginosa, and Staphylococcus aureus) | Acute otitis externa. Otic suspension (0.3%) | [98,99,100] |
| Position (Atom) | Substituent | Moieties’ Chemical Structure | Antibacterial QN |
|---|---|---|---|
| 1 (C) | Cyclopropyl |  | Besifloxacin Finafloxacin Nemonoxacin Zabofloxacin |
| 6-Amino-3,5-difluoropyridinyl |  | Delafloxacin | |
| 6 (C) | Fluor | –F | All compounds, except nemonoxacin |
| 7 (C) | 3-Aminoazepane |  | Besifloxacin |
| 3-Hydroxyazetidinyl |  | Delafloxacin | |
| Pyrrolo-oxazinyl |  | Finafloxacin | |
| 3-Amino-5-methylpiperidinyl |  | Nemonoxacin | |
| 2,6-Diazaspiro[3.4]octanyl |  | Zabofloxacin | |
| [(Cyclopropylamino)methyl]-4-fluoropyrrolidinyl |  | Lascufloxacin | |
| 8 (7 1) (C) | 4-Hydroxypiperidinyl |  | Nadifloxacin 2 and levonadifloxacin |
| 8 (C) | Chloride | –Cl | Besifloxacin Delafloxacin |
| Cyano group | –CN | Finafloxacin | |
| Methoxy | –O–CH3 | Nemonoxacin | |
| 8 (N) | Lack of substituent | none | Zabofloxacin |
| 9 (8 3) | Condensation of quinolone nucleus with methylpiperidine |  | Nadifloxacin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusu, A.; Lungu, I.-A.; Moldovan, O.-L.; Tanase, C.; Hancu, G. Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation. Pharmaceutics 2021, 13, 1289. https://doi.org/10.3390/pharmaceutics13081289
Rusu A, Lungu I-A, Moldovan O-L, Tanase C, Hancu G. Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation. Pharmaceutics. 2021; 13(8):1289. https://doi.org/10.3390/pharmaceutics13081289
Chicago/Turabian StyleRusu, Aura, Ioana-Andreea Lungu, Octavia-Laura Moldovan, Corneliu Tanase, and Gabriel Hancu. 2021. "Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation" Pharmaceutics 13, no. 8: 1289. https://doi.org/10.3390/pharmaceutics13081289
APA StyleRusu, A., Lungu, I. -A., Moldovan, O. -L., Tanase, C., & Hancu, G. (2021). Structural Characterization of the Millennial Antibacterial (Fluoro)Quinolones—Shaping the Fifth Generation. Pharmaceutics, 13(8), 1289. https://doi.org/10.3390/pharmaceutics13081289