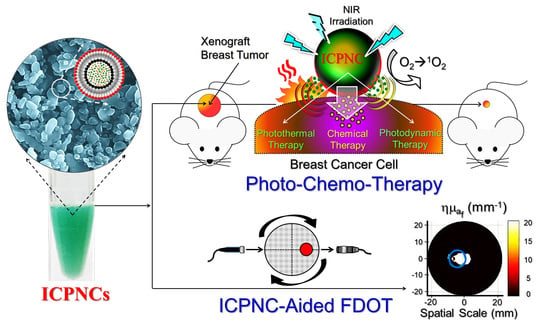
Indocyanine Green-Camptothecin Co-Loaded Perfluorocarbon Double-Layer Nanocomposite: A Versatile Nanotheranostics for Photochemotherapy and FDOT Diagnosis of Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication, Surface Modification, and Characterization of the ICPNCs
2.2. Evaluation of Stability and Drug Release Efficiency of the ICPNCs
2.3. Measurement of ICPNC-Induced Hyperthermia Effect
2.4. Measurement of Yield of ICPNC-Induced Singlet Oxygen
2.5. Cell Culture
2.6. Assessment of Cell Binding Specificity of the HICPNCs In Vitro
2.7. Cytotoxicity of the ICPNCs In Vitro
2.8. Experimental Setup of FDOT
2.9. FDOT Modeling
2.10. Assessment of Effect of ICPNC-Aided FDOT In Vitro
2.11. Animal Model
2.12. Evaluation of Retention Effect of the ICPNCs in Tumor
2.13. Evaluation of Anticancer Efficacy of the ICPNCs In Vivo
2.14. Histological Study
2.15. In Vivo Biocompatibility Evaluation
2.16. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the ICPNCs and HICPNCs
3.2. Thermal Stability of ICPNC-Encapsulated ICG and Efficiency of CPT Release
3.3. Effects of Hyperthermia and Singlet Oxygen Production of the ICPNCs
3.4. Binding Specificity of the HICPNCs In Vitro
3.5. Cytotoxicity of the ICPNCs In Vitro
3.6. Phantom Study of ICPNC-Aided FDOT Detection
3.7. Retention Effect of the ICPNCs in Xenograft Tumor
3.8. Tumoricidal Effect of the ICPNCs In Vivo
3.9. Inflammatory Response of Skin after ICPNC-Mediated Photochemotherapy
3.10. In Vivo Systematic Toxicity Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key Steps for Effective Breast Cancer Prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Corlu, A.; Choe, R.; Durduran, T.; Rosen, M.A.; Schweiger, M.; Arridge, S.R.; Schnall, M.D.; Yodh, A.G. Three-Dimensional In Vivo Fluorescence Diffuse Optical Tomography of Breast Cancer in Humans. Opt. Express 2007, 15, 6696–6716. [Google Scholar] [CrossRef]
- Intes, X.; Maloux, C.; Guven, M.; Yazici, B.; Chance, B. Diffuse Optical Tomography with Physiological and Spatial a Priori Constraints. Phys. Med. Biol. 2004, 49, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Pogue, B.W.; Poplack, S.P.; McBride, T.O.; Wells, W.A.; Osterman, K.S.; Osterberg, U.L.; Paulsen, K.D. Quantitative Hemoglobin Tomography with Diffuse Near-Infrared Spectroscopy: Pilot Results in the Breast. Radiology 2001, 218, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Penjweini, R.; Andreoni, A.; Rosales, T.; Kim, J.; Brenner, M.D.; Sackett, D.L.; Chung, J.H.; Knutson, J.R. Intracellular Oxygen Mapping Using a Myoglobin-McHerry Probe with Fluorescence Lifetime Imaging. J. Biomed. Opt. 2018, 23, 1–14. [Google Scholar]
- Pacheco-Liñán, P.J.; Bravo, I.; Nueda, M.L.; Albaladejo, J.; Garzón-Ruiz, A. Functionalized CdSe/ZnS Quantum Dots for Intracellular pH Measurements by Fluorescence Lifetime Imaging Microscopy. ACS Sens. 2020, 5, 2106–2117. [Google Scholar] [CrossRef] [PubMed]
- Tyurikova, O.; Zheng, K.; Nicholson, E.; Timofeeva, Y.; Semyanov, A.; Volynski, K.E.; Rusakov, D.A. Fluorescence Lifetime Imaging Reveals Regulation of Presynaptic Ca2+ by Glutamate Uptake and mGluRs, but Not Somatic Voltage in Cortical Neurons. J. Neurochem. 2021, 156, 48–58. [Google Scholar] [CrossRef]
- Boutet, J.; Herve, L.; Debourdeau, M.; Guyon, L.; Peltie, P.; Dinten, J.M.; Saroul, L.; Duboeuf, F.; Vray, D. Bimodal Ultrasound and Fluorescence Approach for Prostate Cancer Diagnosis. J. Biomed. Opt. 2009, 14, 064001. [Google Scholar] [CrossRef]
- Koenig, A.; Hervé, L.; Josserand, V.; Berger, M.; Boutet, J.; Da Silva, A.; Dinten, J.M.; Peltié, P.; Coll, J.L.; Rizo, P. In Vivo Mice Lung Tumor Follow-Up with Fluorescence Diffuse Optical Tomography. J. Biomed. Opt. 2008, 13, 011008. [Google Scholar] [CrossRef]
- Genevois, C.; Loiseau, H.; Couillaud, F. In Vivo Follow-up of Brain Tumor Growth via Bioluminescence Imaging and Fluorescence Tomography. Int. J. Mol. Sci. 2016, 17, 1815. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wu, L.; Li, J.; Yi, X.; Wang, X.; Lu, Y.; Chen, W.; Zhou, Z.; Zhang, L.; Zhao, H.; et al. Combined Hemoglobin and Fluorescence Diffuse Optical Tomography for Breast Tumor Diagnosis: A Pilot Study on Time-Domain Methodology. Biomed. Opt. Express 2013, 4, 331–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Ven, S.; Wiethoff, A.; Nielsen, T.; Brendel, B.; Van der Voort, M.; Nachabe, R.; Van der Mark, M.; Van Beek, M.; Bakker, L.; Fels, L.; et al. A Novel Fluorescent Imaging Agent for Diffuse Optical Tomography of the Breast: First Clinical Experience in Patients. Mol. Imaging Biol. 2010, 12, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Huang, Y.; Hou, X.; Quan, X.; Jiang, J.; Wei, X.; Liu, Y.; Li, H.; Wang, P.; Zhan, M.; et al. Two Novel Camptothecin Derivatives Inhibit Colorectal Cancer Proliferation via Induction of Cell Cycle Arrest and Apoptosis In Vitro and In Vivo. Eur. J. Pharm. Sci. 2018, 123, 546–559. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Zhao, L. Lysine-Mediated Hydroxyethyl Starch-10-Hydroxy Camptothecin Micelles for the Treatment of Liver Cancer. Drug Deliv. 2020, 27, 519–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchihashi, Y.; Abe, S.; Miyamoto, L.; Tsunematsu, H.; Izumi, T.; Hatano, A.; Okuno, H.; Yamane, M.; Yasuoka, T.; Ikeda, Y.; et al. Novel Hydrophilic Camptothecin Derivatives Conjugated to Branched Glycerol Trimer Suppress Tumor Growth without Causing Diarrhea in Murine Xenograft Models of Human Lung Cancer. Mol. Pharm. 2020, 17, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, M.; Lahr, C.A.; Kaur, I.; Shafiee, A.; Sanchez-Herrero, A.; Janowicz, P.W.; Ravichandran, A.; Howard, C.B.; Cifuentes-Rius, A.; McGovern, J.A.; et al. Targeted Camptothecin Delivery via Silicon Nanoparticles Reduces Breast Cancer Metastasis. Biomaterials 2020, 240, 119791. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Liu, X.; Zhang, W.; Xiong, X.; Han, Z.; Liang, X. Camptothecin-Based Nanodrug Delivery Systems. Cancer Biol. Med. 2017, 14, 363–370. [Google Scholar] [PubMed] [Green Version]
- Behera, A.; Padhi, S. Passive and Active Targeting Strategies for the Delivery of the Camptothecin Anticancer Drug: A Review. Environ. Chem. Lett. 2020, 18, 1557–1567. [Google Scholar] [CrossRef]
- Lane, D. Designer Combination Therapy for Cancer. Nat. Biotechnol. 2006, 24, 163–164. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging Combination Strategies with Phototherapy in Cancer Nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Rahoui, N.; Jiang, B.; Taloub, N.; Huang, Y.D. Spatio-Temporal Control Strategy of Drug Delivery Systems Based Nano Structures. J. Control Release 2017, 255, 176–201. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” Materials-Based Near-Infrared Light-Responsive Drug Delivery Systems for Cancer Treatment: A Review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Hussein, E.A.; Zagho, M.M.; Nasrallah, G.K.; Elzatahry, A.A. Recent Advances in Functional Nanostructures as Cancer Photothermal Therapy. Int. J. Nanomedicine 2018, 13, 2897–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Yang, C.; McGuckin, L.E.; Simon, J.D.; Choma, M.A.; Applegate, B.E.; Izatt, J.A. Spectral Triangulation Molecular Contrast Optical Coherence Tomography with Indocyanine Green as the Contrast Agent. Opt. Lett. 2004, 29, 2016–2018. [Google Scholar] [CrossRef] [Green Version]
- Egloff-Juras, C.; Bezdetnaya, L.; Dolivet, G.; Lassalle, H.P. NIR Fluorescence-Guided Tumor Surgery: New Strategies for the Use of Indocyanine Green. Int. J. Nanomed. 2019, 14, 7823–7838. [Google Scholar] [CrossRef] [Green Version]
- Forcione, M.; Chiarelli, A.M.; Davies, D.J.; Perpetuini, D.; Sawosz, P.; Merla, A.; Belli, A. Cerebral Perfusion and Blood-Brain Barrier Assessment in Brain Trauma Using Contrast-Enhanced Near-Infrared Spectroscopy with Indocyanine Green: A Review. J. Cereb. Blood Flow Metab. 2020, 40, 1586–1598. [Google Scholar] [CrossRef]
- Zanganeh, S.; Xu, Y.; Hamby, C.V.; Backer, M.V.; Backer, J.M.; Zhu, Q. Enhanced fluorescence diffuse optical tomography with indocyanine green-encapsulating liposomes targeted to receptors for vascular endothelial growth factor in tumor vasculature. J. Biomed. Opt. 2013, 18, 126014. [Google Scholar] [CrossRef] [Green Version]
- Solomon, M.; White, B.R.; Nothdruft, R.E.; Akers, W.; Sudlow, G.; Eggebrecht, A.T.; Achilefu, S.; Culver, J.P. Video-Rate Fluorescence Diffuse Optical Tomography for In Vivo Sentinel Lymph Node Imaging. Biomed. Opt. Express. 2011, 2, 3267–3277. [Google Scholar] [CrossRef] [Green Version]
- Waks Serra, M.V.; Grosenick, D.; Macdonald, R.; Pomarico, J.A.; Iriarte, D.I. A Systematic Study on Fluorescence Contrast in Near Infrared Diffuse Transmittance Imaging with Indocyanine Green. J. Near Infrared Spectrosc. 2019, 27, 333–344. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Yodh, A.G.; Schnall, M.; Chance, B. Concurrent MRI and Diffuse Optical Tomography of Breast After Indocyanine Green Enhancement. Proc. Natl. Acad. Sci. USA 2000, 97, 2767–2772. [Google Scholar] [CrossRef] [Green Version]
- Intes, X.; Ripoll, J.; Chen, Y.; Nioka, S.; Yodh, A.G.; Chance, B. In Vivo Continuous-Wave Optical Breast Imaging Enhanced with Indocyanine Green. Med. Phys. 2003, 30, 1039–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Xu, F.; Wang, L.; Liu, M.; Cao, X.; Shi, X.; Guo, R. Polydopamine-Coated Laponite Nanoplatforms for Photoacoustic Imaging-Guided Chemo-Phototherapy of Breast Cancer. Nanomaterials 2021, 11, 394. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Lim, S.J.; Lee, M.K. Chitosan-Coated Liposomes to Stabilize and Enhance Transdermal Delivery of Indocyanine Green for Photodynamic Therapy of Melanoma. Carbohydr. Polym. 2019, 224, 115143. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Park, J.S.; Kim, S.W.; Park, S.J.; Kim, S.K. Near-Infrared Phototherapy for Patient-Derived Orthotopic Xenograft Model of Hepatocellular Carcinoma in Combination with Indocyanine Green. J. Photochem. Photobiol. B 2020, 209, 111938. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tse, B.W.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine Green-Incorporating Nanoparticles for Cancer Theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation Kinetics of Indocyanine Green in Aqueous Solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef]
- Jain, K.K. Nanomedicine: Application of Nanobiotechnology in Medical Practice. Med. Princ. Pract. 2008, 17, 89–101. [Google Scholar] [CrossRef]
- Lowe, K.C. Perfluorochemical Respiratory Gas Carriers: Benefits to Cell Culture Systems. J. Fluor. Chem. 2002, 118, 19–26. [Google Scholar] [CrossRef]
- Sun, C.Z.; Lu, C.T.; Zhao, Y.Z.; Guo, P.; Tian, J.L.; Zhang, L.; Li, X.K.; Lv, H.F.; Dai, D.D.; Li, X. Characterization of the Doxorubicin-Pluronic F68 Conjugate Micelles and Their Effect on Doxorubicin Resistant Human Erythroleukemic Cancer Cells. J. Nanomed. Nanotechnol. 2011, 2, 1000114. [Google Scholar]
- Palmer, G.M.; Ramanujam, N. Monte Carlo-Based Inverse Model for Calculating Tissue Optical Properties. Part I: Theory and Validation on Synthetic Phantoms. Appl. Opt. 2006, 45, 1062–1071. [Google Scholar] [CrossRef]
- Davis, S.C.; Dehghani, H.; Wang, J.; Jiang, S.; Pogue, B.W.; Paulsen, K.D. Image-Guided Diffuse Optical Fluorescence Tomography Implemented with Laplacian-Type Regularization. Opt. Express 2007, 15, 4066–4082. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H. Frequency-Domain Fluorescent Diffusion Tomography: A Finite-Element-Based Algorithm and Simulations. Appl. Opt. 1998, 37, 5337–5343. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Pan, M.C.; Pan, M.C. Implementation of Edge-Preserving Regularization for Frequency-Domain Diffuse Optical Tomography. Appl. Opt. 2012, 51, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.A.; Kamil, M.; Abdurahman, N.H.; Yunus, R.M.; Awad, O.I. An Overview of Recent Advances in State-of-the-Art Techniques in the Demulsification of Crude Oil Emulsions. Processes 2019, 7, 470. [Google Scholar] [CrossRef] [Green Version]
- Walstra, P. Emulsions. In Fundamentals of Interface and Colloid Science; Lyklema, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Ferreira, B.M.S.; Ramalho, J.B.V.S.; Lucas, E.F. Demulsification of Eater-in-Crude Oil Emulsions by Microwave Radiation: Effect of Aging, Demulsifier Addition, and Selective Heating. Energy Fuels 2013, 27, 615–621. [Google Scholar] [CrossRef]
- Harush-Frenkel, O.; Debotton, N.; Benita, S.; Altschuler, Y. Targeting of Nanoparticles to the Clathrin-Mediated Endocytic Pathway. Biochem. Biophys. Res. Commun. 2007, 353, 26–32. [Google Scholar] [CrossRef]
- Ogris, M.; Steinlein, P.; Carotta, S.; Brunner, S.; Wagner, E. DNA/Polyethylenimine Transfection Particles: Influence of Ligands, Polymer Size, and PEGylation on Internalization and Gene Expression. AAPS Pharm. Sci. 2001, 3, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Cerussi, A.E.; Jakubowski, D.; Hsiang, D.; Butler, J.; Tromberg, B.J. Spatial Variations in Optical and Physiological Properties of Healthy Breast Tissue. J. Biomed. Opt. 2004, 9, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Guo, P.; Ji, L.; Zhao, Q.; Jiang, T. Improving Image Quality of Diffuse Optical Tomography with a Projection-Error-Based Adaptive Regularization Method. Opt. Express 2008, 16, 12423–12434. [Google Scholar] [CrossRef]
- Hoshi, Y.; Yamada, Y. Overview of Diffuse Optical Tomography and Its Clinical Applications. J. Biomed. Opt. 2016, 21, 091312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR Effect and Beyond: Strategies to Improve Tumor Targeting and Cancer Nanomedicine Treatment Efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.S.; Al Suwayyid, A.N.; Aldarwesh, A.; Aboulwafa, O.M.; Attia, M.I. Antiestrogenic Activity and Possible Mode of Action of Certain New Nonsteroidal Coumarin-4-Acetamides. Molecules 2020, 25, 1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munn, L.L. Cancer and Inflammation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]









| NIRF luorophore | Volume Distribution of ICG in Phantom BTuM:BTiM | Concentration of ICG in BTuM (μM) | RI |
|---|---|---|---|
| Free ICG * | 0:1 | 0 | 0 |
| 3:7 | 2.3 | 30% | |
| 7:3 | 4.1 | 70% | |
| 1:0 | 5 | 100% | |
| ICPNC s † | 1:0 | 1 | 100% |
| 1:0 | 5 | 100% | |
| 1:0 | 10 | 100% |
| Reagent Type | * NIR | [ICG] (μM) | [CPT] (μM) |
|---|---|---|---|
| PBS | - | --- | --- |
| Free CPT | - | --- | †C2 |
| IPNCs | + | †C1 | --- |
| ICPNCs | - | †C1 | †C2 |
| + | †C1 | †C2 |
| Groups * Factors Area (mm2) Ratio | FDOT | DOT | ||||||
|---|---|---|---|---|---|---|---|---|
| Free ICG RI | ICPNCs (RI = 100%) [ICG] in BTuM | |||||||
| 0% | 30% | 70% | 100% | 1 μM | 5 μM | 10 μM | ||
| AT | 78.5 | 78.5 | 78.5 | 78.5 | 78.5 | 78.5 | 78.5 | 78.5 |
| AD | 580.72 | 314.29 | 122.15 | 46.50 | 42.96 | 43.74 | 51.33 | 1589.63 |
| AB | 1589.63 | 1589.63 | 1589.63 | 1589.63 | 1589.63 | 1589.63 | 1589.63 | 1589.63 |
| ANB | 1511.13 | 1511.13 | 1511.13 | 1511.13 | 1511.13 | 1511.13 | 1511.13 | 1511.13 |
| ARST | 78.5 | 78.5 | 60.37 | 30.27 | 24.73 | 31.24 | 45.33 | 78.5 |
| ARMT | 0 | 0 | 18.13 | 48.23 | 53.77 | 47.26 | 33.17 | 0 |
| ARSN | 502.22 | 235.79 | 61.78 | 16.23 | 18.23 | 12.5 | 6 | 1511.13 |
| ARMN | 1008.90 | 1275.34 | 1449.35 | 1494.89 | 1492.89 | 1498.63 | 1505.13 | 0 |
| RRST | 100% | 100% | 76.90% | 38.56% | 31.5% | 39.8% | 57.75% | 100% |
| RRMT | 0% | 0% | 23.1% | 61.44% | 68.5% | 60.2% | 42.25% | 0% |
| RRSN | 33.24% | 15.6% | 4.09% | 1.07% | 1.21% | 0.83% | 0.4% | 100% |
| RRMN | 66.76% | 84.4% | 95.91% | 98.93% | 98.79% | 99.17% | 99.6% | 0% |
| TRQI | 3.01 | 6.41 | 18.57 | 35.27 | 25.41 | 47.5 | 145.01 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Kuo, P.-W.; Chen, C.-J.; Sue, C.-J.; Hsu, Y.-F.; Pan, M.-C. Indocyanine Green-Camptothecin Co-Loaded Perfluorocarbon Double-Layer Nanocomposite: A Versatile Nanotheranostics for Photochemotherapy and FDOT Diagnosis of Breast Cancer. Pharmaceutics 2021, 13, 1499. https://doi.org/10.3390/pharmaceutics13091499
Lee Y-H, Kuo P-W, Chen C-J, Sue C-J, Hsu Y-F, Pan M-C. Indocyanine Green-Camptothecin Co-Loaded Perfluorocarbon Double-Layer Nanocomposite: A Versatile Nanotheranostics for Photochemotherapy and FDOT Diagnosis of Breast Cancer. Pharmaceutics. 2021; 13(9):1499. https://doi.org/10.3390/pharmaceutics13091499
Chicago/Turabian StyleLee, Yu-Hsiang, Po-Wei Kuo, Chun-Ju Chen, Chu-Jih Sue, Ya-Fen Hsu, and Min-Chun Pan. 2021. "Indocyanine Green-Camptothecin Co-Loaded Perfluorocarbon Double-Layer Nanocomposite: A Versatile Nanotheranostics for Photochemotherapy and FDOT Diagnosis of Breast Cancer" Pharmaceutics 13, no. 9: 1499. https://doi.org/10.3390/pharmaceutics13091499
APA StyleLee, Y. -H., Kuo, P. -W., Chen, C. -J., Sue, C. -J., Hsu, Y. -F., & Pan, M. -C. (2021). Indocyanine Green-Camptothecin Co-Loaded Perfluorocarbon Double-Layer Nanocomposite: A Versatile Nanotheranostics for Photochemotherapy and FDOT Diagnosis of Breast Cancer. Pharmaceutics, 13(9), 1499. https://doi.org/10.3390/pharmaceutics13091499