Physicochemical Stability of a Novel Tacrolimus Ophthalmic Formulation for the Treatment of Ophthalmic Inflammatory Diseases
Abstract
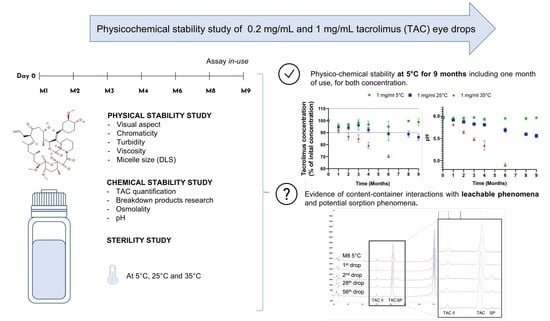
:1. Introduction
2. Materials and Methods
2.1. Preparation and Storage of TAC Formulation
2.2. Study Design
2.3. Stability of Tacrolimus in Unopened Multidose Eyedroppers
2.4. Stability of Tacrolimus in Opened Multidose Eyedroppers (Simulated Use Study)
2.5. Analyses Performed on the Tacrolimus Solution
2.5.1. Visual Inspection
2.5.2. Chromaticity and Luminance Analysis
2.5.3. Tacrolimus Quantification and Breakdown Products (BP) Research
- Chemicals and instrumentation
- Method validation
2.5.4. pH
2.5.5. Osmolality
2.5.6. Turbidity
2.5.7. Viscosity Measurements
2.5.8. Micelle Size Measurements
2.5.9. Sterility Assay
2.5.10. Determination of the Volume of an Eye Drop
2.6. Degradation Kinetics during Storage
2.7. Data Analysis—Acceptability Criteria
2.8. Complementary Study: Analysis of a Suspected Leachable Compound
3. Results
3.1. Quantification of Tacrolimus: HPLC Method Validation
3.2. Stability of Tacrolimus in Unopened Multidose Eyedroppers
3.2.1. Physical Stability
- Visual inspection and chromaticity measurements
- Turbidity
- Viscosity
- Micelle size
3.2.2. Chemical Stability
- Tacrolimus quantification and BPr
- pH
- Osmolality
3.2.3. Sterility Assay
3.2.4. Tacrolimus Degradation Kinetics during Storage
3.3. Tacrolimus Concentrations in Eye Drops during Simulated Use
3.4. Eye Drop Volume
3.5. Complementary Study: Identification of the Leachable Compound
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siekierka, J.J.; Hung, S.H.; Poe, M.; Lin, C.S.; Sigal, N.H. A Cytosolic Binding Protein for the Immunosuppressant FK506 Has Peptidyl-Prolyl Isomerase Activity but Is Distinct from Cyclophilin. Nature 1989, 341, 755–757. [Google Scholar] [CrossRef]
- Harding, M.W.; Galat, A.; Uehling, D.E.; Schreiber, S.L. A Receptor for the Immuno-Suppressant FK506 Is a Cis–Trans Peptidyl-Prolyl Isomerase. Nature 1989, 341, 758–760. [Google Scholar] [CrossRef]
- Shaw, K.T.; Ho, A.M.; Raghavan, A.; Kim, J.; Jain, J.; Park, J.; Sharma, S.; Rao, A.; Hogan, P.G. Immunosuppressive Drugs Prevent a Rapid Dephosphorylation of Transcription Factor NFAT1 in Stimulated Immune Cells. Proc. Natl. Acad. Sci. USA 1995, 92, 11205–11209. [Google Scholar] [CrossRef] [Green Version]
- Moscovici, B.K.; Holzchuh, R.; Sakassegawa-Naves, F.E.; Hoshino-Ruiz, D.R.; Albers, M.B.V.; Santo, R.M.; Hida, R.Y. Treatment of Sjögren’s Syndrome Dry Eye Using 0.03% Tacrolimus Eye Drop: Prospective Double-Blind Randomized Study. Cont. Lens Anterior Eye 2015, 38, 373–378. [Google Scholar] [CrossRef]
- Sanz-Marco, E.; Udaondo, P.; García-Delpech, S.; Vazquez, A.; Diaz-Llopis, M. Treatment of Refractory Dry Eye Associated with Graft Versus Host Disease with 0.03% Tacrolimus Eyedrops. J. Ocul. Pharmacol. Ther. 2013, 29, 776–783. [Google Scholar] [CrossRef] [Green Version]
- Zhai, J.; Gu, J.; Yuan, J.; Chen, J. Tacrolimus in the Treatment of Ocular Diseases. BioDrugs 2011, 25, 89–103. [Google Scholar] [CrossRef]
- Wan, Q.; Tang, J.; Han, Y.; Wang, D.; Ye, H. Therapeutic Effect of 0.1% Tacrolimus Eye Drops in the Tarsal Form of Vernal Keratoconjunctivitis. Ophthalmic Res. 2018, 59, 126–134. [Google Scholar] [CrossRef]
- Roumeau, I.; Coutu, A.; Navel, V.; Pereira, B.; Baker, J.S.; Chiambaretta, F.; Bremond-Gignac, D.; Dutheil, F. Efficacy of Medical Treatments for Vernal Keratoconjunctivitis: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. 2021, 148, 822–834. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Zavareh, M.K.; Farzbod, F.; Mahbod, M.; Behrouz, M.J. Topical 0.005% Tacrolimus Eye Drop for Refractory Vernal Keratoconjunctivitis. Eye 2011, 25, 872–880. [Google Scholar] [CrossRef]
- Erdinest, N.; Ben-Eli, H.; Solomon, A. Topical Tacrolimus for Allergic Eye Diseases. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 535–543. [Google Scholar] [CrossRef]
- Koh, K.; Jun, I.; Kim, T.-I.; Kim, E.K.; Seo, K.Y. Long-Term Results of Topical 0.02% Tacrolimus Ointment for Refractory Ocular Surface Inflammation in Pediatric Patients. BMC Ophthalmol. 2021, 21, 247. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 445643, Tacrolimus. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/445643 (accessed on 29 November 2021).
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent Advances in the Design of Topical Ophthalmic Delivery Systems in the Treatment of Ocular Surface Inflammation and Their Biopharmaceutical Evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef]
- Senju Pharmaceutical Co., Ltd. Marketing Authorization Holder Talymus®. Ophthalmic Suspension 0.1%; Senju Pharmaceutical, Co., Ltd.: Osaka, Japan, 2018. [Google Scholar]
- Figus, M.; Agnifili, L.; Lanzini, M.; Brescia, L.; Sartini, F.; Mastropasqua, L.; Posarelli, C. Topical Preservative-Free Ophthalmic Treatments: An Unmet Clinical Need. Expert Opin. Drug Deliv. 2020, 18, 655–672. [Google Scholar] [CrossRef]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in Eyedrops: The Good, the Bad and the Ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
- Goldstein, M.H.; Silva, F.Q.; Blender, N.; Tran, T.; Vantipalli, S. Ocular Benzalkonium Chloride Exposure: Problems and Solutions. Eye 2021, 1–8. [Google Scholar] [CrossRef]
- Gauthier, A.-S.; Rival, B.; Sahler, J.; Fagnoni-Legat, C.; Limat, S.; Guillaume, Y.; Delbosc, B. Développement galénique et analytique d’un collyre à base de tacrolimus 0.06%. J. Français d’Ophtalmologie 2013, 36, 408–413. [Google Scholar] [CrossRef]
- Zulim, L.F.d.C.; Nai, G.A.; Giuffrida, R.; Pereira, C.S.G.; Benguella, H.; Cruz, A.G.; Foglia, B.T.D.; Batista, A.D.S.; Andrade, S.F. Comparison of the Efficacy of 0.03% Tacrolimus Eye Drops Diluted in Olive Oil and Linseed Oil for the Treatment of Keratoconjunctivitis Sicca in Dogs. Arq. Bras. Oftalmol. 2018, 81, 293–301. [Google Scholar] [CrossRef]
- Ghiglioni, D.G.; Martino, P.A.; Bruschi, G.; Vitali, D.; Osnaghi, S.; Corti, M.G.; Beretta, G. Stability and Safety Traits of Novel Cyclosporine A and Tacrolimus Ophthalmic Galenic Formulations Involved in Vernal Keratoconjunctivitis Treatment by a High-Resolution Mass Spectrometry Approach. Pharmaceutics 2020, 12, 378. [Google Scholar] [CrossRef]
- Ezquer-Garin, C.; Ferriols-Lisart, R.; Alós-Almiñana, M. Stability of Tacrolimus Ophthalmic Solution. Am. J. Health Syst. Pharm. 2017, 74, 1002–1006. [Google Scholar] [CrossRef]
- Choudhury, A.K.R. Using Instruments to Quantify Colour. In Principles of Colour and Appearance Measurement; Elsevier: Cham, Switzerland, 2014; pp. 270–317. ISBN 978-0-85709-229-8. [Google Scholar]
- United States Pharmacopeia. USP <631> Color and Achromicity, USPNF 2021 Issue 2 2021; United States Pharmacopeia and National Formulary: New York, NY, USA, 2021. [Google Scholar]
- Hunt, R.W.G.; Pointer, M.R. Measuring Colour, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-119-97537-3. [Google Scholar]
- Peterka, T.R.; Lušin, T.T.; Bergles, J.; Ham, Z.; Grahek, R.; Urleb, U. Forced Degradation of Tacrolimus and the Development of a UHPLC Method for Impurities Determination. Acta Pharm. 2019, 69, 363–380. [Google Scholar] [CrossRef] [Green Version]
- European Pharmacopeia. Tacrolimus Monohydrate Monography 01/2018:2244 Corrected 10.0 2021, 10.2 ed.; European Directorate for the Quality of Medecines and Healthcare: Strasbourg, France, 2021. [Google Scholar]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER); International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use ICH Q2 (R1). Guidance for Industry: Validation of Analytical Procedures: Text and Methodology; ICH: Rockville, MD, USA, 1996.
- Hubert, P.H.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of Strategies for the Validation of Quantitative Analytical Procedures: A SFSTP Proposal—Part, I. J. Pharm. Biomed. Anal. 2004, 36, 579–586. [Google Scholar] [CrossRef]
- Hubert, P.H.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Chiap, P.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Lallier, M.; et al. Harmonization of Strategies for the Validation of Quantitative Analytical Procedures: A SFSTP Proposal—Part II. J. Pharm. Biomed. Anal. 2007, 45, 70–81. [Google Scholar] [CrossRef]
- Hubert, P.H.; Nguyen-Huu, J.-J.; Boulanger, B.; Chapuzet, E.; Cohen, N.; Compagnon, P.-A.; Dewé, W.; Feinberg, M.; Laurentie, M.; Mercier, N.; et al. Harmonization of Strategies for the Validation of Quantitative Analytical Procedures: A SFSTP Proposal–Part III. J. Pharm. Biomed. Anal. 2007, 45, 82–96. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Monography 2.6.1 Sterility 10.2 2020; European Directorate for the Quality of Medecines and Healthcare: Strasbourg, France, 2020. [Google Scholar]
- Newton, D.W. Mean Kinetic Temperature for Controlled Room Temperature Drug Storage: Official Definitions and Example Calculations. Int. J. Pharm. Compd. 2019, 23, 281–287. [Google Scholar]
- International Conference of Harmonization (ICH) Quality Guidelines: Guidelines for Stability Q1A to Q1f. Available online: http://www.ich.org/products/guidelines/%20quality/article/quality-guidelines.html (accessed on 18 June 2020).
- French Society of Clinical Pharmacy (SFPC) and Evaluation and Research Group on Protection in Controlled Atmospher (GERPAC). Methodological Guidelines for Stability Studies of Hospital Pharmaceutical Preparations; Société Française de Pharmacie Clinique, Groupe d’Evaluation et de Recherche sur la Protection en Atmosphère Contrôlée: Paris, France, 2013. [Google Scholar]
- Hayes, R.; Ahmed, A.; Edge, T.; Zhang, H. Core–Shell Particles: Preparation, Fundamentals and Applications in High Performance Liquid Chromatography. J. Chromatogr. A 2014, 1357, 36–52. [Google Scholar] [CrossRef] [Green Version]
- Prajapati, M.; Eiriksson, F.F.; Loftsson, T. Stability Characterization, Kinetics and Mechanism of Tacrolimus Degradation in Cyclodextrin Solutions. Int. J. Pharm. 2020, 586, 119579. [Google Scholar] [CrossRef]
- Yamada, M.; Mochizuki, H.; Kawai, M.; Yoshino, M.; Mashima, Y. Fluorophotometric Measurement of PH of Human Tears in Vivo. Curr. Eye Res. 1997, 16, 482–486. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP <771> Ophthalmic Products—Quality Tests, USPNF 2021 Issue 2 2021; United States Pharmacopeia and National Formulary: New York, NY, USA, 2021. [Google Scholar]
- Chennell, P.; Delaborde, L.; Wasiak, M.; Jouannet, M.; Feschet-Chassot, E.; Chiambaretta, F.; Sautou, V. Stability of an Ophthalmic Micellar Formulation of Cyclosporine A in Unopened Multidose Eyedroppers and in Simulated Use Conditions. Eur. J. Pharm. Sci. 2017, 100, 230–237. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Amankwah, R.; Powell-Richards, A.; Dua, H.S. Sodium Hyaluronate (Hyaluronic Acid) Promotes Migration of Human Corneal Epithelial Cells in Vitro. Br. J. Ophthalmol. 2004, 88, 821–825. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Lee, S.U.; Che, C.-Y.; Lee, J.-E. Comparison of Cytotoxicity and Wound Healing Effect of Carboxymethylcellulose and Hyaluronic Acid on Human Corneal Epithelial Cells. Int. J. Ophthalmol. 2015, 8, 215–221. [Google Scholar] [CrossRef]
- Behrens-Baumann, W.; Theuring, S.; Brewitt, H. The Effect of Topical Cyclosporin A on the Rabbit Cornea. Graefe’s Arch. Clin. Exp. Ophthalmol. 1986, 224, 520–524. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yu, J.M.; Ko, J.H. Analysis of Ethanol Effects on Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3852–3856. [Google Scholar] [CrossRef] [Green Version]
- Chast, F.; Lemare, F.; Legeais, J.-M.; Batista, R.; Bardin, C.; Renard, G. Préparation d’un Collyre de Ciclosporine à 2%. J. Français d’Ophtalmologie 2004, 27, 567–576. [Google Scholar] [CrossRef]
- Kauss Hornecker, M.; Charles Weber, S.; Brandely Piat, M.-L.; Darrodes, M.; Jomaa, K.; Chast, F. Cyclosporine eye drops: A 4-year retrospective study (2009–2013). J. Français d’Ophtalmologie 2015, 38, 700–708. [Google Scholar] [CrossRef]
- United States Pharmacopeia. Tacrolimus Compounded Oral Suspension, USPNF 2021 Issue 2 2021; United States Pharmacopeia and National Formulary: New York, NY, USA, 2021. [Google Scholar]
- García-Otero, X.; Díaz-Tomé, V.; Varela-Fernández, R.; Martín-Pastor, M.; González-Barcia, M.; Blanco-Méndez, J.; Mondelo-García, C.; Bermudez, M.A.; Gonzalez, F.; Aguiar, P.; et al. Development and Characterization of a Tacrolimus/Hydroxypropyl-β-Cyclodextrin Eye Drop. Pharmaceutics 2021, 13, 149. [Google Scholar] [CrossRef]
- Badr, M.Y.; Abdulrahman, N.S.; Schatzlein, A.G.; Uchegbu, I.F. A Polymeric Aqueous Tacrolimus Formulation for Topical Ocular Delivery. Int. J. Pharm. 2021, 599, 120364. [Google Scholar] [CrossRef]
- Bouattour, Y.; Chennell, P.; Wasiak, M.; Jouannet, M.; Sautou, V. Stability of an Ophthalmic Formulation of Polyhexamethylene Biguanide in Gamma-Sterilized and Ethylene Oxide Sterilized Low Density Polyethylene Multidose Eyedroppers. PeerJ 2018, 6, e4549. [Google Scholar] [CrossRef]
- United States Pharmacopeia. Tacrolimus, USPNF 2021 Issue 2 2021; United States Pharmacopeia and National Formulary: New York, NY, USA, 2021. [Google Scholar]
- Fan, Z.; Zhang, L. One- and Two-Stage Arrhenius Models for Pharmaceutical Shelf Life Prediction. J. Biopharm. Stat. 2015, 25, 307–316. [Google Scholar] [CrossRef]
- Waterman, K.C. The Application of the Accelerated Stability Assessment Program (ASAP) to Quality by Design (QbD) for Drug Product Stability. AAPS PharmSciTech 2011, 12, 932. [Google Scholar] [CrossRef]
- Waterman, K.C.; Adami, R.C. Accelerated Aging: Prediction of Chemical Stability of Pharmaceuticals. Int. J. Pharm. 2005, 293, 101–125. [Google Scholar] [CrossRef]
- Teasdale, A.; Elder, D.; Nims, R.W. ICH Quality Guidelines: An Implementation Guide; Wiley: Hoboken, NJ, USA, 2017; ISBN 9781118971116. [Google Scholar]
- Le Basle, Y.; Chennell, P.; Sautou, V. A Sorption Study between Ophthalmic Drugs and Multi Dose Eyedroppers in Simulated Use Conditions. Pharm. Technol. Hosp. Pharm. 2017, 2, 181–191. [Google Scholar] [CrossRef]
- Van Santvliet, L.; Ludwig, A. Determinants of Eye Drop Size. Surv. Ophthalmol. 2004, 49, 197–213. [Google Scholar] [CrossRef]
- Taormina, D.; Abdallah, H.Y.; Venkataramanan, R.; Logue, L.; Burckart, G.J.; Ptachcinski, R.J.; Todo, S.; Fung, J.J.; Starzl, T.E. Stability and Sorption of FK 506 in 5% Dextrose Injection and 0.9% Sodium Chloride Injection in Glass, Polyvinyl Chloride, and Polyolefin Containers. Am. J. Hosp. Pharm. 1992, 49, 119–122. [Google Scholar] [CrossRef]
- Suzuki, M.; Takamatsu, S.; Muramatsu, E.; Nakajima, S.-I.; Tanaka, M.; Kawano, K. Loss of Tacrolimus Solution Content and Leaching of Di-2-Ethylhexyl Phtalate in Practice Injection of Precision Continuous Drip Infusion. Jpn. J. Hosp. Pharm. 2000, 26, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.-E.; Jeon, S.; Byon, H.-J.; Hwang, S.-J. Evaluation of Tacrolimus Sorption to PVC- and Non-PVC-Based Tubes in Administration Sets: Pump Method vs. Drip Method. Int. J. Pharm. 2017, 528, 172–179. [Google Scholar] [CrossRef]
- Hacker, C.; Verbeek, M.; Schneider, H.; Steimer, W. Falsely Elevated Cyclosporin and Tacrolimus Concentrations over Prolonged Periods of Time Due to Reversible Adsorption to Central Venous Catheters. Clin. Chim. Acta 2014, 433, 62–68. [Google Scholar] [CrossRef]
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. Phenol Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2000; ISBN 9783527306732. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 7311, 2,4-Di-Tert-Butylphenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7311 (accessed on 3 December 2021).
- Endocrine Disruptor List. Available online: https://edlists.org/ (accessed on 29 November 2021).









| Chemical Compounds | Formulation | |
|---|---|---|
| 0.2 mg/mL = 0.02% | 1 mg/mL = 0.1% | |
| Tacrolimus monohydrate Batch 70312001218, exp 01/10/2023, Inresa, France | 200 mg | 1000 mg |
| KEL: Macrogol 35 glycerol ricinoleate (Kolliphor EL®) Batch 192835002, exp 30/04/2021, Inresa, France | 16 g | 80 g |
| Absolute ethanol Batch 20010089/B, exp 01/24, Cooper, France | 4.76 mL | 23.81 mL |
| Buffer solution (composition described below) | Q.S. 1 L | Q.S. 1 L |
| Buffer Solution | ||
| Sodium dihydrogenophosphate dihydrate (NaH2PO4) Batch 190298040, exp. 30/11/2021, Inresa, France | 500 mg | |
| Disodic monohydrogenophosphate dodecahydrate (Na2HPO4) Batch 18129611, exp. 30/04/2023, Inresa, France | 37 mg | |
| Hyaluronate sodium Batch PH13560S02, exp 01/12/2023, Inresa, France | 1500 mg | |
| Sodium chloride (NaCl) 0.9% Versylene®; Fresenius Kabi France, Louviers, France | Q.S. 1 L | |
| Studied Parameters | ||||||
|---|---|---|---|---|---|---|
| Months | Visual Aspect, pH, Osmolality, TAC quantification & BPr | Chromaticity | Viscosity | Turbidity | Micelle Size | Sterility Assay |
| 0 | X | X | X | X | X | X |
| 1 | X | X | ||||
| 2 | X | X | ||||
| 3 | X | X | X | X | X | X |
| 4 | X | |||||
| 6 | X | X | X | X | X | X |
| 8 | X | |||||
| 9 | X | X | X | X | X | X |
| Mobile Phase | ||
|---|---|---|
| Time (minutes) | A (%) | B (%) |
| 0 | 63 | 37 |
| 1 | 63 | 37 |
| 12 | 60 | 40 |
| 17 | 45 | 55 |
| 19 | 10 | 90 |
| 22.5 | 10 | 90 |
| 23 | 63 | 37 |
| 27 | 63 | 37 |
| Impurities and BP | TAC H1 | NS1 | TAC RI | Impurity A | NS2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | RRT | % | RRT | % | RRT | % | RRT | % | RRT | ||
| Initial condition | 1 mg/mL | 1.157 | 0.293 | 0.110 | 0.596 | 0.189 | 0.794 | ND | ND | ND | ND |
| 0.2 mg/mL | 0.180 | 0.292 | ND | ND | 0.211 | 0.793 | ND | ND | 0.140 | 1.069 | |
| Study endpoint | 1 mg/mL 5 °C | 0.309 | 0.299 | ND | ND | 0.243 | 0.811 | 0.230 | 0.887 | ND | ND |
| 1 mg/mL 25 °C | 1.000 | 0.252 | ND | ND | 0.259 | 0.810 | 3.126 | 0.887 | 0.198 | 1.100 | |
| 1 mg/mL 35 °C | 1.045 | 0.265 | ND | ND | 0.258 | 0.800 | 7.263 | 0.887 | 0.466 | 1.083 | |
| 0.2 mg/mL 5 °C | 0.076 | 0.269 | ND | ND | 0.261 | 0.810 | 0.328 | 0.888 | 0.446 | 1.098 | |
| 0.2 mg/mL 25 °C | 1.047 | 0.269 | ND | ND | 0.337 | 0.808 | 4.251 | 0.887 | 1.406 | 1.099 | |
| 0.2 mg/mL 35 °C | 2.346 | 0.267 | 0.196 | 0.578 | 0.374 | 0.799 | 9.418 | 0.887 | 1.890 | 1.081 | |
| Injectable tacrolimus (Prograf®) 5 mg/mL | 0.572 | 0.291 | 0.050 | 0.592 | ND | ND | 0.326 | 0.892 | ND | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrieu, M.; Chennell, P.; Yessaad, M.; Bouattour, Y.; Wasiak, M.; Jouannet, M.; Le Basle, Y.; Sautou, V. Physicochemical Stability of a Novel Tacrolimus Ophthalmic Formulation for the Treatment of Ophthalmic Inflammatory Diseases. Pharmaceutics 2022, 14, 118. https://doi.org/10.3390/pharmaceutics14010118
Barrieu M, Chennell P, Yessaad M, Bouattour Y, Wasiak M, Jouannet M, Le Basle Y, Sautou V. Physicochemical Stability of a Novel Tacrolimus Ophthalmic Formulation for the Treatment of Ophthalmic Inflammatory Diseases. Pharmaceutics. 2022; 14(1):118. https://doi.org/10.3390/pharmaceutics14010118
Chicago/Turabian StyleBarrieu, Marion, Philip Chennell, Mouloud Yessaad, Yassine Bouattour, Mathieu Wasiak, Mireille Jouannet, Yoann Le Basle, and Valérie Sautou. 2022. "Physicochemical Stability of a Novel Tacrolimus Ophthalmic Formulation for the Treatment of Ophthalmic Inflammatory Diseases" Pharmaceutics 14, no. 1: 118. https://doi.org/10.3390/pharmaceutics14010118
APA StyleBarrieu, M., Chennell, P., Yessaad, M., Bouattour, Y., Wasiak, M., Jouannet, M., Le Basle, Y., & Sautou, V. (2022). Physicochemical Stability of a Novel Tacrolimus Ophthalmic Formulation for the Treatment of Ophthalmic Inflammatory Diseases. Pharmaceutics, 14(1), 118. https://doi.org/10.3390/pharmaceutics14010118