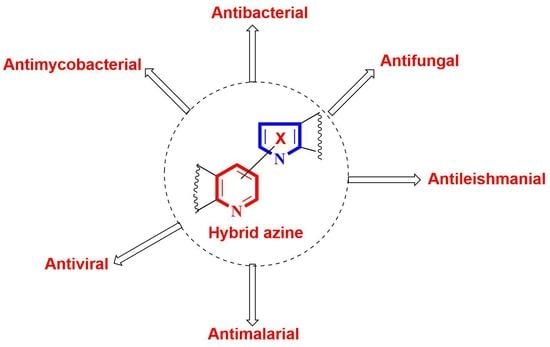
Hybrid Azine Derivatives: A Useful Approach for Antimicrobial Therapy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Six-Member Ring Azaheterocycles with One Nitrogen Atom. Hybrid Pyridine
2.2. Six-Member Ring Azaheterocycles with One Nitrogen Atom. Hybrid Quinoline and Isoquinoline
2.3. Our Group Recent Contributions
3. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMET | Absorption, Distribution, Metabolism, Excretion, Toxicity |
| ESKAPE | an acronym for the six highly virulent and antibiotic- resistant bacterial pathogens: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. |
| MIC | minimal inhibitory concentration |
| MBC | minimal bactericidal concentration |
| MRSA | methicillin-resistant Staphylococcus aureus |
| DIZ | diameter of inhibition zones |
| DNA | desoxyribonucleic acid |
| SAR | structure activity relationship |
| IC50 | inhibitory concentration at 50% |
| LORA | Low oxygen recovery assay |
| INH-R1 and INH-R2 | strains resistant to isoniazid |
| RIF-R1 and RIF-R2 | strains resistant to rifampicin |
| FQ-R1 | strain resistant to fluoroquinolone |
| NTM | nontuberculous mycobacteria |
| O-alkylation | oxigen-alkylation |
| N-alkylation | nitrogen-alkylation |
| S-alkylation | sulphur-alkylation |
| t | tert (eg, t—butyl means tert-butyl) |
| i | iso (eg, i—propyl means iso-propyl) |
| n | normal (eg, n—butyl means normal-butyl) |
| o, m, p | ortho, meta, para |
| Me | methyl |
| Et | ethyl |
| Pr | propyl |
| Bu | butyl |
| Ph | phenyl |
| OMe | methoxy |
| Bacillus cereus | B. cereus |
| Bifidobacterium animalis | B. animalis |
| Bacillus subtilis | B. subtilis |
| Enterococcus faecalis | E. faecalis |
| Lactobacillus plantarum | L. plantarum |
| Staphylococcus aureus | S. aureus |
| Staphylococcus epidermidis | S. epidermidis |
| Streptococcus pneumoniae | S. pneumoniae |
| Streptococcus mutans | S. mutans |
| Streptococcus pyogenes | S. pyogenes |
| Acinetobacter baumannii | A. baumannii |
| Escherichia coli | E. coli |
| Klebsiella pneumoniae | K. pneumoniae |
| Neisseria gonorrhoeae | N. gonorrhoeae |
| Proteus mirabilis | P. mirabilis |
| Proteus vulgaris | P. vulgaris |
| Pseudomonas aeruginosa | P. aeruginosa |
| Salmonella enterica | S. enterica |
| Salmonella typhi | S. typhi |
| Shigella flexneri | S. flexneri |
| Aspergillus clavatus | A. clavatus |
| Aspergillus niger | A. niger |
| Aspergillus flavus | A. flavus |
| Aspergillus fumigatus | A. fumigatus |
| Candida albicans | C. albicans |
| Candida parapsilosis | C. parapsilosis |
| Candida parapsilosis | C. parapsilosis |
| Cryptococcus neoformans | C. neoformans |
| Geotrichum candidum | G. candidum |
| Penicillium marneffei | P. marneffei |
| Saccharomyces boulardii | S. boulardii |
| Trichoderma viridae | T. viridae |
| Mycobacterium tuberculosis | M. tuberculosis, Mtb |
| Mycobacterium avium | M. avium |
| Mycobacterium abscessus | M. abscessus |
| WHO | World Health Organization |
References
- WHO. Global Action Plan on Antimicrobial Resistance: WHO. 2016. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 12 April 2022).
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.B.; Ambrus, J.I.; Samosorn, S. Dual action-based approaches to antibacterial agents. Curr. Med. Chem. 2007, 14, 1459–1477. [Google Scholar] [CrossRef] [PubMed]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Chourasiya, S.S.; Kathuria, D.; Wani, A.A.; Bharatam, P.V. Azines: Synthesis, structure, electronic structure and their applications. Org. Biomol. Chem. 2019, 17, 8486–8521. [Google Scholar] [CrossRef] [PubMed]
- Amariucai-Mantu, D.; Antoci, V.; Sardaru, M.C.; Al Matarneh, C.M.; Mangalagiu, I.I.; Danac, R. Fused pyrrolo-pyridines and pyrrolo-(iso) quinoline as anticancer agents. Phys. Sci. Rev. 2022, 1. [Google Scholar] [CrossRef]
- Amariucai-Mantu, D.; Mangalagiu, V.; Danac, R.; Mangalagiu, I.I. Microwave assisted reactions of azaheterocycles for medicinal chemistry applications. Molecules 2020, 25, 716. [Google Scholar] [CrossRef]
- Luca, M.C.; Tura, V.; Mangalagiu, I.I. Considerations concerning design and mechanism of action of a new class of dual DNA intercalators. Med. Hypotheses 2010, 75, 627–629. [Google Scholar] [CrossRef]
- Eryılmaz, S.; Turkcelikoglu, E.; Idil, O.; Inkaya, I.; Kozak, Z.; Mısır, E.; Gul, M. Derivatives of pyridine and thiazole hybrid: Synthesis, DFT, biological evaluation via antimicrobial and DNA cleavage activity. Bioorg. Chem. 2020, 95, 103476. [Google Scholar] [CrossRef]
- Cinarli, M.; Ataol, C.Y.; Cinarli, E.; Idil, O. Synthesis, characterization, biological, X-ray diffraction analysis andcomputational chemistry studies of new 2-acetylpyridine derivativehydrazone and its Zn(II) complex. J. Mol. Struct. 2020, 1213, 128152. [Google Scholar] [CrossRef]
- Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Synthesis and antimycobacterial activity of thiazolidine-2,4-dione based derivatives with halogenbenzohydrazones and pyridinecarbohydrazones substituents. Eur. J. Med. Chem. 2020, 189, 112045. [Google Scholar] [CrossRef]
- Sanad, S.M.H.; Ahmed, A.A.M.; Mekky, A.E.M. Efficient synthesis and molecular docking of novel antibacterial pyrimidines and their related fused heterocyclic derivatives. J. Heterocycl.Chem. 2020, 57, 590–605. [Google Scholar] [CrossRef]
- Desai, N.C.; Bhatt, N.B.; Joshi, S.B.; Jadeja, K.A.; Khedkar, V.M. Synthesis, Antimicrobial Activity and 3D-QSAR Study of Hybrid Oxazine Clubbed Pyridine Scaffolds. ChemistrySelect 2019, 4, 7541–7550. [Google Scholar] [CrossRef]
- Sribalan, R.; Banuppriya, G.; Kirubavathi, M.; Padmini, V. Synthesis, biological evaluation and in silico studies of tetrazole-heterocycle hybrids. J. Mol. Struct. 2019, 1175, 577–586. [Google Scholar] [CrossRef]
- Kuthyala, S.; Shankar, M.K.; Nagaraja, G.K. Synthesis, Single-Crystal X-ray, Hirshfeld and Antimicrobial Evaluation of some New Imidazopyridine Nucleus Incorporated with Oxadiazole Scaffold. ChemistrySelect 2018, 3, 12894–12899. [Google Scholar] [CrossRef]
- Ahirwar, J.; Ahirwar, D.; Lanjhiyana, S.; Jha, A.K.; Dewangan, D.; Badwaik, H. Synthesis, Characterization, Molecular Modeling, and Biological Evaluation of 1,2,4-Triazole-pyridine Hybrids as Potential Antimicrobial Agents. J. Heterocycl. Chem. 2018, 55, 2598–2609. [Google Scholar] [CrossRef]
- Jaabil, G.; Ranganathan, R.; Ponnuswamy, A.; Suresh, P.; Shanmugaiah, V.; Ravikumar, C.; Murugavel, S.A. Green and Efficient Synthesis of Bioactive 1, 2, 3-Triazolyl-Pyridine/Cyanopyridine Hybrids via One-Pot Multicomponent Grinding Protocol. ChemistrySelect 2018, 3, 10388–10393. [Google Scholar] [CrossRef]
- Flefel, E.M.; El-Sofany, W.I.; El-Shahat, M.; Naqvi, A.; Assirey, E. Synthesis, molecular docking and in vitro screening of some newly synthesized triazolopyridine, pyridotriazine and pyridine-pyrazole hybrid derivatives. Molecules 2018, 23, 2548. [Google Scholar] [CrossRef]
- Amperayani, K.R.; Kumar, K.N.; Parimi, U.D. Synthesis and in vitro and in silico antimicrobial studies of novel piperine–pyridine analogs. Res. Chem. Intermed. 2018, 44, 3549–3564. [Google Scholar] [CrossRef]
- Albayrak, F.; Çiçek, M.; Alkaya, D.; Kulu, I. Design, synthesis and biological evaluation of 8-aminoquinoline-1,2,3-triazole hybrid derivatives as potential antimicrobial agents. Med. Chem. Res. 2022, 31, 625–665. [Google Scholar] [CrossRef]
- Hryhoriv, H.; Mariutsa, I.; Kovalenko, S.M.; Georgiyants, V.; Perekhoda, L.; Filimonova, N.; Geyderikh, O.; Sidorenko, L. The Search for New Antibacterial Agents among 1,2,3-Triazole Functionalized Ciprofloxacin and Norfloxacin Hybrids: Synthesis, Docking Studies, and Biological Activity Evaluation. Sci. Pharm. 2022, 90, 2. [Google Scholar] [CrossRef]
- Hryhoriv, H.; Mariutsa, I.; Kovalenko, S.M.; Sidorenko, L.; Perekhoda, L.; Filimonova, N.; Geyderikh, O.; Georgiyants, V. Structural modification of ciprofloxacin and norfloxacin for searching new antibiotics to combat drug-resistant bacteria. Sci. Pharm. Sci. 2021, 5, 4–11. [Google Scholar]
- Drweesh, E.A.; Kucharova, V.; Volarevic, V.; Miloradovic, D.; Ilic, A.; Radojevic, I.D.; Rakovic, I.R.; Smolkova, R.; Vilkova, M.; Sabolov, D.; et al. Low-dimensional compounds containing bioactive ligands. Part XVII: Synthesis, structural, spectral and biological properties of hybrid organic-inorganic complexes based on [PdCl4]2− with derivatives of 8-hydroxyquinolinium. J. Inorg. Biochem. 2022, 228, 111697. [Google Scholar] [CrossRef] [PubMed]
- Nehra, N.; Tittal, R.K.; Ghule, V.D. 1,2,3-Triazoles of 8-Hydroxyquinoline and HBT: Synthesis and Studies (DNA Binding, Antimicrobial, Molecular Docking, ADME, and DFT). ACS Omega 2021, 6, 27089–27100. [Google Scholar] [CrossRef] [PubMed]
- Awolade, P.; Cele, N.; Kerru, N.; Singh, P. Synthesis, antimicrobial evaluation, and in silico studies of quinoline—1H-1,2,3-triazole molecular hybrids. Mol. Divers. 2021, 25, 2201–2218. [Google Scholar] [CrossRef]
- Ammar, Y.A.; El-Hafez, S.M.A.A.; Hessein, S.A.; Ali, A.M.; Askar, A.A.; Ragab, A. One-pot strategy for thiazole tethered 7-ethoxy quinoline hybrids: Synthesis and potential antimicrobial agents as dihydrofolate reductase (DHFR) inhibitors with molecular docking study. J. Mol. Struct. 2021, 1242, 130748. [Google Scholar] [CrossRef]
- Eissa, S.I.; Farrag, A.M.; Abbas, S.Y.; El Shehry, M.F.; Ragab, A.; Fayed, E.A.; Ammar, Y.A. Novel structural hybrids of quinoline and thiazole moieties: Synthesis and evaluation of antibacterial and antifungal activities with molecular modeling studies. Bioorg. Chem. 2021, 110, 104803. [Google Scholar] [CrossRef]
- Lagdhir, M.; Pandya, C.; Pandya, A.; Vekariya, R.H.; Rajani, D.P. Design and synthesis of new quinoline hybrid derivatives and their antimicrobial, antimalarial and antitubercular activities. Indian J. Chem. Sect. B 2021, 60, 986–998. [Google Scholar]
- Desai, N.C.; Harsora, J.P.; Mehta, H.K. 2-Pyridone quinoline hybrids as potent antibacterial and antifungal agents. Indian J. Chem. Sect. B 2021, 60, 261–266. [Google Scholar]
- Vishnuvardhan, M.; Pradeep, M.; Gangadhar, T. An efficient microwave assisted synthesis and antimicrobial activity of novel p-Tolyloxyquinoline-Triazole hybrid derivatives. Chem. Data Collect. 2021, 31, 100612. [Google Scholar] [CrossRef]
- Abdel-Rahman, I.M.; Mustafa, M.; Mohamed, S.A.; Yahia, R.; Abdel-Aziz, M.; Abuo-Rahma, G.; Hayallah, A.M. Novel Mannich bases of ciprofloxacin with improved physicochemical properties, antibacterial, anticancer activities and caspase-3 mediated apoptosis. Bioorg. Chem. 2021, 107, 104629. [Google Scholar] [CrossRef]
- Mohammed, A.A.M.; Suaifan, G.A.R.Y.; Shehadeh, M.B.; Okechukwu, P.N. Design, synthesis and antimicrobial evaluation of novel glycosylated-fluoroquinolones derivatives. Eur. J. Med. Chem. 2020, 202, 112513. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, T.G.; Subramanian, S.; Eswaran, S. Design, synthesis and study of antibacterial and antitubercular activity of quinoline hydrazone hybrids. Heterocycl. Commun. 2020, 26, 137–147. [Google Scholar]
- Kaur, G.; Kaur, M.; Sharad, L.; Bansal, M. Theoretical molecular predictions and antimicrobial activities of newly synthesized molecular hybrids of norfloxacin and ciprofloxacin. J. Heterocycl. Chem. 2020, 57, 225–237. [Google Scholar] [CrossRef]
- Insuasty, D.; Vidal, O.; Bernal, A.; Marquez, E.; Guzman, J.; Insuasty, B.; Quiroga, J.; Svetaz, L.; Zacchino, S.; Puerto, G.; et al. Antimicrobial activity of quinoline-based hydroxyimidazolium hybrids. Antibiotics 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baartzes, N.; Stringer, T.; Seldon, R.; Warner, D.F.; Taylor, D.; Wittlin, S.; Chibale, K.; Smith, G.S. Bioisosteric ferrocenyl aminoquinoline-benzimidazole hybrids: Antimicrobial evaluation and mechanistic insights. Eur. J. Med. Chem. 2019, 180, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Sączewski, J.; Konopacka, A.; Waleron, K.; Lejnowski, D.; Ciura, K.; Toma, T.; Skok, Z.; Savijoki, K.; Morawska, M.; et al. Synthesis and biological evaluation of hybrid quinolone-based quaternary ammonium antibacterial agents. Eur. J. Med. Chem. 2019, 179, 576–590. [Google Scholar] [CrossRef]
- Borazjani, N.; Jarrahpour, A.; Rad, J.A.; Mohkam, M.; Behzadi, M.; Ghasemi, Y.; Mirzaeinia, S.; Karbalaei-Heidari, H.R.; Ghanbari, M.M.; Batta, G.; et al. Design, synthesis and biological evaluation of some novel diastereoselective β-lactams bearing 2-mercaptobenzothiazole and benzoquinoline. Med. Chem. Res. 2019, 28, 329–339. [Google Scholar] [CrossRef]
- Berry, L.; Domalaon, R.; Brizuela, M.; Zhanel, G.G.; Schweizer, F. Polybasic peptide-levofloxacin conjugates potentiate fluoroquinolones and other classes of antibiotics against multidrug-resistant Gram-negative bacteria. MedChemComm 2019, 10, 517–527. [Google Scholar] [CrossRef]
- Mermer, M.; Faiz, O.; Demirbas, A.; Demirbas, N.; Alagumuthu, M.; Arumugam, V. Piperazine-azole-fluoroquinolone hybrids: Conventional and microwave irradiated synthesis, biological activity screening and molecular docking studies. Bioorg. Chem. 2019, 85, 308–318. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, T.; Bao, C.; Liu, Z.; Fan, J.; Yang, R.; Qin, S. Design and synthesis of new norfloxacin-1,3,4-oxadiazole hybrids as antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2019, 136, 104966. [Google Scholar] [CrossRef]
- Wang, Y.N.; Bheemanaboina, R.R.Y.; Gao, W.W.; Cang, J.; Cai, G.X.; Zhou, C.H. Discovery of Benzimidazole–Quinolone Hybrids as New Cleaving Agents toward Drug-Resistant Pseudomonas aeruginosa DNA. ChemMedChem 2018, 13, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.S.; Poojary, B.; Kumar, M.S.; Byrappa, K.; Nagananda, K.S.; Chaitanya, A.K.; Zaveri, K.; Yarla, N.S.; Shiralgi, Y.; Kudva, A.K.; et al. Design, synthesis and pharmacological studies of some new quinoline Schiff bases and 2,5-(disubstituted-[1,3,4])-oxadiazoles. New J. Chem. 2017, 41, 8568–8585. [Google Scholar] [CrossRef]
- Tahaab, M.; Ismailab, N.H.; Alic, M.; Rashidc, U.; Imranab, S.; Uddind, N.; Khane, K.M. Molecular hybridization conceded exceptionally potent quinolinyl-oxadiazole hybrids through phenyl linked thiosemicarbazide antileishmanial scaffolds: In silico validation and SAR studies. Bioorg. Chem. 2017, 71, 192–200. [Google Scholar]
- Irfan, M.; Alam, S.; Manzoor, N.; Abid, M. Effect of quinoline based 1,2,3-triazole and its structural analogues on growth and virulence attributes of Candida albicans. PLoS ONE 2017, 12, e0175710. [Google Scholar] [CrossRef]
- Pandya, K.M.; Battula, S.; Naik, P.J. Pd-catalyzed post-Ugi intramolecular cyclization to the synthesis of isoquinolone-pyrazole hybrid pharmacophores & discover their antimicrobial and DFT studies. Tetrahedron Lett. 2021, 81, 153353. [Google Scholar]
- Verma, V.A.; Saundane, A.R.; Meti, R.S.; Vennapu, D.R. Synthesis of novel indolo[3,2-c]isoquinoline derivatives bearing pyrimidine, piperazine rings and their biological evaluation and docking studies against COVID-19 virus main protease. J. Mol. Struct. 2021, 1229, 129829. [Google Scholar] [CrossRef]
- Ungureanu, M.; Mangalagiu, I.I.; Grosu, G.; Petrovanu, M. Antimicrobial activity of some new pyridazine derivatives. Ann. Pharm. Fr. 1997, 55, 69–72. [Google Scholar]
- Mangalagiu, I.I.; Ungureanu, M.; Mangalagiu, G.; Grosu, G.; Petrovanu, M. Antimicrobial activity of some pyrimidinium compounds. Ann. Pharm. Fr. 1998, 56, 181–183. [Google Scholar]
- Mangalagiu, G.; Ungureanu, M.; Grosu, G.; Mangalagiu, I.I.; Petrovanu, M. New pyrrolo-pyrimidine derivatives with antifungal or antibacterial properties in vitro. Ann. Pharm. Fr. 2001, 59, 139–140. [Google Scholar]
- Moldoveanu, C.; Mangalagiu, G.; Drochioiu, G.; Caprosu, M.; Petrovanu, M.; Mangalagiu, I.I. New Antituberculosis Compounds Derived from Diazine. An. Stiint. Univ. “Al. I. Cuza” Iasi Chem. 2003, 11, 367–374. [Google Scholar]
- Diaconu, D.; Antoci, V.; Mangalagiu, V.; Amariucai-Mantu, D.; Mangalagiu, I.I. Quinoline-imidazole/benzimidazole derivatives as dual- / multi- targeting hybrids inhibitors with anticancer and antimicrobial activity. Sci. Rep. 2022, in press. [Google Scholar]
- Diaconu, D.; Amariucai-Mantu, D.; Antoci, V.; Ciorteanu, R.; Mangalagiu, V.; Mangalagiu, I.I. Design and synthesis of new hybrid pyridine imidazole/benzimidazole salts with antibacterial activity. Rev. Roum. Chim. 2022, 67, 89–92. [Google Scholar]
- Antoci, V.; Cucu, D.; Zbancioc, G.; Moldoveanu, C.; Mangalagiu, V.; Amariucai-Mantu, D.; Aricu, A.; Mangalagiu, I.I. Bis-(imidazole/benzimidazole)-pyridine derivatives: Synthesis, structure and antimycobacterial activity. Part XII. Future Med. Chem. 2020, 12, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Lungu, C.N.; Bratanovici, B.I.; Grigore, M.M.; Antoci, V.; Mangalagiu, I.I. Hybrid Imidazole-Pyridine Derivatives: An Approach to Novel Anticancer DNA Intercalators. Curr. Med. Chem. 2020, 27, 154–169. [Google Scholar] [CrossRef]
- Mantu, D.; Antoci, V.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Hybrid imidazole (benzimidazole)/pyridine (quinoline) derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 96–103. [Google Scholar] [CrossRef]
- Diaconu, D.; Mangalagiu, V.; Amariucai-Mantu, D.; Antoci, V.; Giuroiu, C.L.; Mangalagiu, I.I. Hybrid Quinoline-Sulfonamide Complexes (M2+) Derivatives with Antimicrobial Activity. Molecules 2020, 25, 2946. [Google Scholar] [CrossRef]
- Al Matarneh, C.; Sardaru, M.; Apostu, M.; Rosca, I.; Ciobanu, C.; Mangalagiu, I.I.; Danac, R. Synthesis and antibacterial evaluation of new pyrrolo[3′,4′,3,4]pyrrolo[1,2a]quinoline derivatives. Studia UBB Chem. 2019, LXIV, 67–80. [Google Scholar] [CrossRef]
- Danac, R.; Al Matarneh, C.; Shova, S.; Daniloaia, T.; Balan, M.; Mangalagiu, I.I. New indolizines with phenanthroline skeleton: Synthesis, structure, antimycobacterial and anticancer properties. Bioorg. Med. Chem. 2015, 23, 2318–2327. [Google Scholar] [CrossRef]
- Danac, R.; Mangalagiu, I.I. Antituberculosis activity of nitrogen heterocycles derivatives: Bipyridine derivatives. Part III. Eur. J. Med. Chem. 2014, 74, 664–670. [Google Scholar] [CrossRef]
- Olaru, A.; Vasilache, V.; Danac, R.; Mangalagiu, I.I. Antimycobacterial activity of nitrogen heterocycles derivatives: 7-(pyridine-4-yl)- indolizine derivatives. Part VII. J. Enzym. Inhib. Med. Chem. 2017, 32, 1291–1298. [Google Scholar] [CrossRef] [Green Version]




















































| Synthesized Hybrids | Type of Reactions | Biological Activity | ||
|---|---|---|---|---|
| Antimicrobial Activity | Strains Used | Effect Observed | ||
| - thiazole-pyridine 2a–e and 4a–e | - cyclocondensation | - antibacterial; - antifungal | - B. cereus, S. aureus, E. coli, P. aeruginosa; - C. albicans | - active on B. cereus, S. aureus |
| - metal-pyridine derivatives 7 | - complexation | - antibacterial; - antifungal | - B. cereus, S. aureus, E. coli, P. aeruginosa; - C. albicans | - active on C. albicans |
| - thiazolidine-pyridine 11a–f and 12a–i | - condensation | - antitubercular | - M. tuberculosis | - active on Mtb |
| - thiophen-pyrimidin-pyridine 14–20 | - cyclocondensation | - antibacterial | - S. aureus, S. mutans, E. coli, K. pneumoniae | - active to all strains |
| -oxazino-pyridine 23a–j | - cyclocondensation, condensation | - antibacterial; - antifungal | - E. coli, P. aeruginosa, S. aureus, S. pyogenes; - C. albicans, A. niger, A. clavatus | - active on E. coli, C. albicans, A. clavatus |
| - tetrazolo-pyridine 25a–d and tetrazolo-quinoline 26a–e | - cyclocondensation | -antibacterial; - antifungal | - K. pneumoniae, P. aeruginosa, S. aureus, S. pyogenes; - C. albicans | - active on all bacterial strains |
| - oxadiazolo-imidazo-pyridine 28a–j | - cyclocondensation | - antibacterial; - antifungal | - E. coli, K. pneumoniae, S. aureus, B. subtilis - C. albicans, A. niger | - active on S. aureus, C. albicans |
| - triazolo-pyridine 30a–n and 31a–n | - cyclocondensation | - antibacterial | - S aureus, S. pyogenes, E. faecalis, E. coli, P. aeruginosa, A. baumannii | - active on all strains |
| - triazolo-pyridine 35a–r | - cyclocondensation | - antibacterial | - E. coli, S. aureus, S. typhi | - active on all strains |
| - pyrazole-pyridine 37–41, triazolo-pyridine 42–45 and triazino-pyridine 46 | - cyclocondensation | - antibacterial; - antifungal | - B. subtilis, E. coli, S. aureus, S. typhi - C. albicans, A. niger | - active on all strains |
| - piperine-pyridine 48a–h | - acylation | - antibacterial; - antifungal | - B. subtilis, Streptobacillus, S. aureus, E. coli, K. pneumoniae, P. aeruginosa, E. faecalis, S. typhi - A. niger, A. flavus, A. fumigatus, C. albicans | - active on E. coli, K. pneumoniae, E. faecalis, P. aeruginosa |
| - triazolo-quinoline 58a–j and 59a–j | - reduction, N-alkylation, cyclocondensation | - antibacterial; - antifungal | - E. coli, P. aeruginosa, K. pneumoniae, E. faecalis, S. aureus, S. pneumoniae, B. subtilis - C. albicans, C. parapsilosis | - active on all strains |
| - piperidino-quinoline 61a,b and triazolo-piperidino-quinoline 62a–k | - N-alkylation, cyclocondensation | - antibacterial; - antifungal | - E. coli, S. aureus; - C. albicans | - active on all strains |
| - metal-quinoline 64a–d | - complexation | - antibacterial; - antifungal | - B. animalis, L. plantarum, B. subtilis, S. aureus ATCC 663, S. aureus ATCC 25923, P. aeruginosa, P. mirabilis, E. coli, S. enterica; - C. albicans, S. boulardii, A. flavus, T. viridae, A. niger | - active on all strains |
| - triazole-benzothiazole-quinoline hybrids 66a–f | - cyclocondensation | - antibacterial; - antifungal | - E. coli, B. subtilis, P. aeruginosa, S. aureus; - C. albicans, A. terreus | - active on all strains |
| - triazole-quinoline hybrids 67a–u, 68a–z, 69a–n and 70a,b | - cyclocondensation | - antibacterial; - antifungal | - E. coli, A. baumannii, K. pneumoniae, S. aureus; - C. albicans, C. neoformans | - active on all strains |
| - thiazole-quinoline 71, 72 and 77–82, thiazolone-quinoline 73–76 | - condensation, cyclocondensation | - antibacterial; - antifungal | - S. aureus, B. faecalis, B. subtilis, E. coli, S typhi, P. aeruginosa; - C. albicans, F. oxysporum | - active on S. aureus and E. coli |
| - piperazin-quinoline 86a–l, - thiazole-quinoline 83–85a–f | - alkylation, condensation | - antibacterial; - antifungal; - antimalarial; - antitubercular | - S. aureus, S. pyogenes, E. coli, P. aeruginosa, - C. albicans, A. niger, A. clavatus; - P. falciparum; - M. tuberculosis | - active on S. aureus and E. coli |
| - pyridine-quinoline 87a–j | - condensation | - antibacterial; - antifungal | - S. aureus, S. pyogenes, E. coli, P. aeruginosa; - C. albicans, A. niger, A. clavatus | - active on S. aureus, E. coli, P. aeruginosa, C. albicans |
| - triazole-quinoline 88a–l | - cyclocondensation | - antibacterial; - antifungal | - S. aureus, S. pyogenes, E. coli, P. aeruginosa; - C. albicans, A. niger | - active on all strains |
| - piperazin-quinoline 89a–j | - cyclocondensation | - antibacterial | - S. aureus, MRSA, E. coli, P. aeruginosa | - active on all strains |
| - glycosylated-quinoline hybrids 90–94 | - hydrolysis, acylation | - antibacterial; - antifungal | - E. coli, L. monocytogenes, S. enterica, P. aeruginosa, L. monocytogenes, MRSA, MSSA; - C. albicans, A. flavus, F. solani, S. chartarum, P. chrysogenum | - active on E. coli, C. albicans, P. chrysogenum |
| - piperazine- and morpholine-quinoline 95a–e and 96a–f | - condensation, alkylation | - antibacterial; - antitubercular | - A. baumanii, E. faecium, K. pneumonia, P. aeruginosa, E. coli, S. aureus - M. tuberculosis | - active on S. aureus, E. coli, A. baumanii and M. tuberculosis |
| - piperazino-quinoline 97–104 | - acylation, alkylation | - antibacterial | - S. aureus, E. coli, P. aeruginosa, B. subtilis | - active on all strains |
| - imidazolium-quinoline 105a–h | - substitution | - antibacterial; - antifungal; - antitubercular | - S. aureus, E. coli, P. aeruginosa, B. subtilis - C. neoformans; - M. tuberculosis | - active on C. neoformans |
| - benzimidazole-quinoline and ferrocenyl-quinoline 106a–e and 107a–e | - cyclocondensation, condensation | - antimalarial; - antitubercular | - P. falciparum, P. berghei - M. tuberculosis | - active on P. falciparum, P. berghei |
| - zwiterionic pyridine-fluoroquinolone and quinoline-fluoroquinolone 108a–h and 109a–h | - Mannich-electrophilic amination | - antibacterial | - S. aureus ATCC 6538, S. aureus MRSA N315, S. epidermidis ATCC 14990, B. subtilis, E. coli, P. aeruginosa, P. vulgaris, S. aureus MRSA 6347, S. epidermidis MRSE, S. marcescens | - active on all strains |
| - benzothiazole-benzo-quinoline 110, 111a–d and 112a–m. | - cyclocondensation, condensation | - antibacterial | - S. aureus, E. coli, P. aeruginosa, B. subtilis, E. faecalis, S. typhi | - active on S. aureus, P. aeruginosa |
| - peptide-quinolone 113a–l and conjugates with ciprofloxacine, miofloxacine | - solid-phase peptide synthesis | - antibacterial | - A. baumanii, K. pneumonia, P. aeruginosa, E. coli, S. aureus, MRSA, MSSA, MSSE, E. faecalis, E. cloacae, S. maltophil | - only conjugates are active on all strains |
| - oxadiazole- and triazole-fluoroquinolone 114a,b and 115a–j | - one-pot three component Mannich reactions | - antibacterial | - S. aureus, E. coli, P. aeruginosa, E. faecalis, K. pneumoniae, A. haemolyticus | - active on P. aeruginosa, E. faecalis, A. haemolyticus |
| - oxadiazole-fluoroquinolone 116a–t | - alkylation | - antibacterial | - S. aureus, MRSA | - active on both |
| - benzimidazole-quinoline 117a–g, 118a,b and 119a–f | - alkylation | - antibacterial; - antifungal | - K. pneumonia, P. aeruginosa, E. coli, S. aureus, S. aureus ATCC25923, S. aureus ATCC29213, E. faecalis, K. pneumonia, P. aeruginosa ATCC27853, E. coli ATCC25922, A. baumanii, A. fumigatus, C. tropicalis, C. albicans, C. albicans ATCC90023, C. parapsilosis ATCC22019 | - active on S. aureus, K. pneumonia |
| - oxadiazole-quinoline 120a–g | - cyclocondensation | - antibacterial; - antifungal | - S. aureus, E. coli, B. cereus, S. marcescens; - A. niger, T. mentagrophytes, C. albicans, C. parapsilosis | - active on B. cereus |
| - oxadiazole-quinoline 121a–r | - acylation, cyclocondensation, condensation | - leishmanicidal | - Leishmania major | - active |
| - triazole-quinoline 122a–c | - cyclocondensation | - antifungal | - C. albicans clinical strains and laboratory | - active |
| - pyrazole-isoquinoline 123a–g | - cyclocondensation | - antibacterial; - antifungal; - antitubercular | - S. aureus, E. coli, E. faecalis, S. pyogens, V. cholera; - C. albicans, C. tropicalis, C. parapsilosis, C. krusei, C. glabrata; - M. tuberculosis | - active on S. aureus, V. cholera, M. tuberculosis |
| - piperazine- and pyrimidine-isoquinoline 126a–h and 127a–h | - N-alkylation, O-alkylation, S-alkylation | - antibacterial; - antifungal; - antitubercular | - S. aureus, E. coli, K. pneumonia, B. subtilis; - C. albicans, A. niger, A. oryzae, P. chrysogenum; - M. tuberculosis | - active on all strains |
| - imidazole- and benzimidazole-quinoline 128–134 | - cycloaddition, N-alkylation | - antibacterial; - antifungal | - S. aureus, E. coli; - C. albicans | - active on all strains |
| - imidazole- and benzimidazole-pyridine 135–138 | - cycloaddition, N-alkylation | - antibacterial; - antifungal | - S. aureus, E. coli; - C. albicans | - active on S. aureus, E. coli |
| - bis(imidazole)- and bis(benzimidazole)-pyridine 139–143 | - N-alkylation | - antitubercular | - M. tuberculosis | - active |
| - imidazole- and benzimidazole-pyridine and quinoline 144–147 | - N-alkylation | - antitubercular | - M. tuberculosis | - no activity |
| - quinoline-sulfonamide complexes 149–153 | - acylation, complexation | - antibacterial; - antifungal | - S. aureus, E. coli; - C. albicans | - active on all strains |
| - pyrrolo-quinoline and pyrrolo-isoquinoline 154a–c and 155a–c | - cycloaddition | - antibacterial; - antifungal | - S. aureus, E. coli; - C. albicans | - no activity |
| - pyrrolo-phenanthroline 156a–c | - cycloaddition | - antitubercular | - M. tuberculosis | - active |
| - mono-indolizine-pyridine 157a–e, salts of mono-indolizine-pyridine 159a–l and bis-indolizine-pyridine 160a–d | - N-alkylation, cycloaddition | - antitubercular | - M. tuberculosis | - active |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amariucai-Mantu, D.; Mangalagiu, V.; Bejan, I.; Aricu, A.; Mangalagiu, I.I. Hybrid Azine Derivatives: A Useful Approach for Antimicrobial Therapy. Pharmaceutics 2022, 14, 2026. https://doi.org/10.3390/pharmaceutics14102026
Amariucai-Mantu D, Mangalagiu V, Bejan I, Aricu A, Mangalagiu II. Hybrid Azine Derivatives: A Useful Approach for Antimicrobial Therapy. Pharmaceutics. 2022; 14(10):2026. https://doi.org/10.3390/pharmaceutics14102026
Chicago/Turabian StyleAmariucai-Mantu, Dorina, Violeta Mangalagiu, Iustinian Bejan, Aculina Aricu, and Ionel I. Mangalagiu. 2022. "Hybrid Azine Derivatives: A Useful Approach for Antimicrobial Therapy" Pharmaceutics 14, no. 10: 2026. https://doi.org/10.3390/pharmaceutics14102026
APA StyleAmariucai-Mantu, D., Mangalagiu, V., Bejan, I., Aricu, A., & Mangalagiu, I. I. (2022). Hybrid Azine Derivatives: A Useful Approach for Antimicrobial Therapy. Pharmaceutics, 14(10), 2026. https://doi.org/10.3390/pharmaceutics14102026