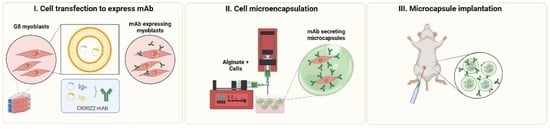
Sustained Delivery of a Monoclonal Antibody against SARS-CoV-2 by Microencapsulated Cells: A Proof-of-Concept Study
Abstract
:1. Introduction
2. Methods
2.1. Cells
2.2. mAb Expression
2.3. Western Blotting
2.4. SARS-CoV-2 ELISA
2.5. Cell Encapsulation
2.6. Animal Studies
2.7. Statistical Analysis
2.8. Role of the Funding Source
3. Results
3.1. G-8 Myoblasts Are Capable of Sustained CR3022 Expression
3.2. G-8 Microcapsules Secrete Detectable Levels of CR3022
3.3. In Vivo Microcapsule Implantation Results in Systemically Detectable CR3022 mAb
3.4. Host Anti-CR3022 Response and Per-Capsule mAb Expression Changes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nature (London, UK). COVID research: A year of scientific milestones. Nature 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/32221507/ (accessed on 28 June 2022). [CrossRef]
- World Health Organization (Geneva, Switzerland). Therapeutics and COVID-19: Living Guideline. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-therapeutics-2022.2 (accessed on 6 March 2022).
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell Encapsulation Within Alginate Microcapsules: Immunological Challenges and Outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Hernández, R.M.; Gascón, A.R.; Calafiore, R.; Chang, T.M.S.; De Vos, P.; Hortelano, G.; Hunkeler, D.; Lacík, I.; Shapiro, A.M.J.; et al. Cell encapsulation: Promise and progress. Nat. Med. 2003, 9, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Calafiore, R.; Basta, G.; Luca, G.; Lemmi, A.; Racanicchi, L.; Mancuso, F.; Montanucci, M.P.; Brunetti, P. Standard technical procedures for microencapsulation of human islets for graft into nonimmunosuppressed patients with type 1 diabetes mellitus. Transpl. Proc. 2006, 38, 1156–1157. [Google Scholar] [CrossRef] [PubMed]
- Calafiore, R.; Basta, G.; Luca, G.; Lemmi, A.; Montanucci, M.P.; Calabrese, G.; Racanicchi, L.; Mancuso, F.; Brunetti, P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: First two cases. Diabetes Care 2006, 29, 137–138. [Google Scholar] [CrossRef]
- Orive, G.; De Castro, M.; Ponce, S.; Hernández, R.M.; Gascón, A.R.; Bosch, M.; Alberch, J.; Pedraz, J.L. Long-Term Expression of Erythropoietin from Myoblasts Immobilized in Biocompatible and Neovascularized Microcapsules. Mol. Ther. 2005, 12, 283–289. [Google Scholar] [CrossRef]
- Löhr, J.M.; Haas, S.L.; Kröger, J.C.; Friess, H.M.; Höft, R.; Goretzki, P.E.; Peschel, C.; Schweigert, M.; Salmons, B.; Gunzburg, W.H. Encapsulated cells expressing a chemotherapeutic activating enzyme allow the targeting of subtoxic chemotherapy and are safe and efficacious: Data from two clinical trials in pancreatic cancer. Pharmaceutics 2014, 6, 447–466. [Google Scholar] [CrossRef]
- Aebischer, P.; Pochon, N.A.; Heyd, B.; Deglon, N.; Joseph, J.M.; Zurn, A.D.; Baetge, E.E.; Hammang, J.P.; Goddard, M.; Lysaght, M.; et al. Gene therapy for amyotrophic lateral sclerosis (ALS) using a polymer encapsulated xenogenic cell line engineered to secrete hCNTF. Hum. Gene 1996, 7, 851–860. [Google Scholar] [CrossRef]
- Luo, X.-M.; Lin, H.; Wang, W.; Geaney, M.S.; Law, L.; Wynyard, S.; Shaikh, S.B.; Waldvogel, H.; Faull, R.L.M.; Elliott, R.B.; et al. Recovery of neurological functions in non-human primate model of Parkinson’s disease by transplantation of encapsulated neonatal porcine choroid plexus cells. J. Parkinsons Dis. 2013, 3, 275–291. [Google Scholar] [CrossRef] [Green Version]
- Yegorov, S.; Kadyrova, I.; Negmetzhanov, B.; Kolesnikova, Y.; Kolesnichenko, S.; Korshukov, I.; Baiken, Y.; Matkarimov, B.; Miller, M.S.; Hortelano, G.H.; et al. Sputnik-V reactogenicity and immunogenicity in the blood and mucosa: A prospective cohort study. Sci. Rep. 2022, 12, 13207. [Google Scholar] [CrossRef]
- Kadyrova, I.; Yegorov, S.; Negmetzhanov, B.; Kolesnikova, Y.; Kolesnichenko, S.; Korshukov, I.; Akhmaltdinova, L.; Vazenmiller, D.; Stupina, Y.; Kabildina, N.; et al. High SARS-CoV-2 seroprevalence in Karaganda, Kazakhstan before the launch of COVID-19 vaccination. PLoS ONE 2022, 17, e0272008. [Google Scholar] [CrossRef] [PubMed]
- Hortelano, G.; Al-Hendy, A.; Ofosu, F.A.; Chang, P.L. Delivery of Human Factor IX in Mice by Encapsulated Recombinant Myoblasts: A Novel Approach towards Allogeneic Gene Therapy of Hemophilia B. Blood 1996, 87, 5095–5103. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.L.; Hortelano, G.; Awrey, D.E.; Tse, M. Growth of recombinant fibroblasts in alginate microcapsules. Biotechnol. Bioeng. 1994, 43, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, B.; Dodd, M.; Wen, J.; Ma, S.; Marquez-Curtis, L.; Janowska-Wieczorek, A.; Hortelano, G. Encapsulation of factor IX–engineered mesenchymal stem cells in fibrinogen–alginate microcapsules enhances their viability and transgene secretion. J. Tissue Engl. 2012, 3, 2041731412462018. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Vargas, A.G.; Ofosu, F.A.; Hortelano, G. Sustained and therapeutic levels of human factor IX in hemophilia B mice implanted with microcapsules: Key role of encapsulated cells. J. Gene Med. 2006, 8, 362–369. [Google Scholar] [CrossRef]
- Thakur, A.; Sengupta, R.; Matsui, H.; Lillicrap, D.; Jones, K.; Hortelano, G. Characterization of viability and proliferation of alginate-poly-L-lysine-alginate encapsulated myoblasts using flow cytometry. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 296–304. [Google Scholar] [CrossRef]
- García-Martín, C.; Chuah, M.K.L.; Van Damme, A.; Robinson, K.E.; Vanzieleghem, B.; Saint-Remy, J.-M.; Gallardo, D.; Ofosu, F.A.; Vandendriessche, T.; Hortelano, G. Therapeutic levels of human factor VIII in mice implanted with encapsulated cells: Potential for gene therapy of haemophilia A. J. Gene Med. 2002, 4, 215–223. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Xiao, J.; Hooper, A.T.; Hamilton, J.D.; Musser, B.J.; et al. REGEN-COV Antibody Combination and Outcomes in Outpatients with COVID-19. N. Engl. J. Med. 2021, 385, e81. [Google Scholar] [CrossRef]
- Razonable, R.R.; Pawlowski, C.; O’Horo, J.C.; Arndt, L.L.; Arndt, R.; Bierle, D.M.; Borgen, M.D.; Hanson, S.N.; Hedin, M.C.; Lenehan, P.; et al. Casirivimab–Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. E Clin. Med. 2021, 40, 101102. [Google Scholar] [CrossRef]
- O’Brien, M.P.; Forleo-Neto, E.; Sarkar, N.; Isa, F.; Hou, P.; Chan, K.-C.; Musser, B.J.; Bar, K.J.; Barnabas, R.V.; Barouch, D.H.; et al. Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial. JAMA 2022, 327, 432–441. [Google Scholar] [CrossRef]
- Liedtke, M.; Twist, C.J.; Medeiros, B.C.; Gotlib, J.R.; Berube, C.; Bieber, M.M.; Bhat, N.M.; Teng, N.N.; Coutre, S.E. Phase I trial of a novel human monoclonal antibody mAb216 in patients with relapsed or refractory B-cell acute lymphoblastic leukemia. Haematologica 2012, 97, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Montanucci, P.; Cari, L.; Basta, G.; Pescara, T.; Riccardi, C.; Nocentini, G.; Calafiore, R. Engineered Alginate Microcapsules for Molecular Therapy through Biologic Secreting Cells. Tissue Eng. Part C Methods 2019, 25, 296–304. [Google Scholar] [CrossRef] [PubMed]
- ter Meulen, J.; van den Brink, E.N.; Poon, L.L.M.; Marissen, W.E.; Leung, C.S.W.; Cox, F.; Cheung, C.Y.; Bakker, A.Q.; Bogaards, J.A.; van Deventer, E.; et al. Human monoclonal antibody combination against SARS coronavirus: Synergy and coverage of escape mutants. PLoS Med. 2006, 3, e237. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; van Haperen, R.; Osterhaus, A.D.M.E.; van Kuppeveld, F.J.M.; Haagmans, B.L.; Grosveld, F.; Bosch, B.-J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020, 11, 2251. [Google Scholar] [CrossRef]
- Huo, J.; Zhao, Y.; Ren, J.; Zhou, D.; Duyvesteyn, H.M.E.; Ginn, H.M.; Carrique, L.; Malinauskas, T.; Ruza, R.R.; Shah, P.N.M.; et al. Neutralization of SARS-CoV-2 by Destruction of the Prefusion Spike. Cell Host Microbe 2020, 28, 445–454.e6. [Google Scholar] [CrossRef] [PubMed]
- Ghetie, V.; Ward, E.S.; Vitetta, E.S. Pharmacokinetics of Antibodies and Immunotoxins in Mice and Humans. In Handbook of Anticancer Pharmacokinetics and Pharmacodynamics; Figg, W.D., McLeod, H.L., Eds.; Cancer Drug Discovery and Development; Humana Press: Totowa, NJ, USA, 2004; pp. 475–498. ISBN 978-1-59259-734-5. [Google Scholar]
- Yuan, M.; Wu, N.C.; Zhu, X.; Lee, C.-C.D.; So, R.T.Y.; Lv, H.; Mok, C.K.P.; Wilson, I.A. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science 2020, 368, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Dubrot, J.; Portero, A.; Orive, G.; Hernández, R.M.; Palazón, A.; Rouzaut, A.; Perez-Gracia, J.L.; Hervás-Stubbs, S.; Pedraz, J.L.; Melero, I. Delivery of immunostimulatory monoclonal antibodies by encapsulated hybridoma cells. Cancer Immunol. Immunother. 2010, 59, 1621–1631. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashimova, A.; Myngbay, A.; Yegorov, S.; Negmetzhanov, B.; Kadyrova, I.; Yershova, A.; Kart, U.; Miller, M.S.; Hortelano, G. Sustained Delivery of a Monoclonal Antibody against SARS-CoV-2 by Microencapsulated Cells: A Proof-of-Concept Study. Pharmaceutics 2022, 14, 2042. https://doi.org/10.3390/pharmaceutics14102042
Ashimova A, Myngbay A, Yegorov S, Negmetzhanov B, Kadyrova I, Yershova A, Kart U, Miller MS, Hortelano G. Sustained Delivery of a Monoclonal Antibody against SARS-CoV-2 by Microencapsulated Cells: A Proof-of-Concept Study. Pharmaceutics. 2022; 14(10):2042. https://doi.org/10.3390/pharmaceutics14102042
Chicago/Turabian StyleAshimova, Assem, Askhat Myngbay, Sergey Yegorov, Baurzhan Negmetzhanov, Irina Kadyrova, Angelina Yershova, Ulpan Kart, Matthew S. Miller, and Gonzalo Hortelano. 2022. "Sustained Delivery of a Monoclonal Antibody against SARS-CoV-2 by Microencapsulated Cells: A Proof-of-Concept Study" Pharmaceutics 14, no. 10: 2042. https://doi.org/10.3390/pharmaceutics14102042
APA StyleAshimova, A., Myngbay, A., Yegorov, S., Negmetzhanov, B., Kadyrova, I., Yershova, A., Kart, U., Miller, M. S., & Hortelano, G. (2022). Sustained Delivery of a Monoclonal Antibody against SARS-CoV-2 by Microencapsulated Cells: A Proof-of-Concept Study. Pharmaceutics, 14(10), 2042. https://doi.org/10.3390/pharmaceutics14102042