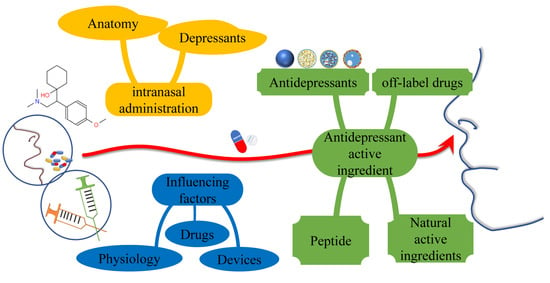
Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients
Abstract
:1. Introduction
2. Unique Advantages of Intranasal Administration in the Treatment of Depression
2.1. Direct Pathway
2.2. Indirect Pathway
3. Challenges of Intranasal Administration
4. The Delivery Carriers and Nanocarriers for Intranasal Administration
4.1. Polymer-Based Carriers
4.2. Lipid-Based Carriers
5. Intranasal Administration of Antidepressant Active Ingredients
5.1. Antidepressants
5.2. Off-Label Drugs
5.3. Peptides
5.4. Natural Active Ingredients
6. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; de Girolamo, G.; Gureje, O.; et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO World Mental Health (WMH) surveys. Psychol. Med. 2017, 48, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Panek, M.; Kawalec, P.; Pilc, A.; Lasoń, W. Developments in the discovery and design of intranasal antidepressants. Expert Opin. Drug Discov. 2020, 15, 1145–1164. [Google Scholar] [CrossRef] [PubMed]
- Depression and Other Common Mental Disorders: Global Health Estimates. Available online: https://www.who.int/publications/i/item/depression-global-health-estimates (accessed on 17 September 2022).
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Kashyap, K.; Shukla, R. Drug Delivery and Targeting to the Brain Through Nasal Route: Mechanisms, Applications and Challenges. Curr. Drug Deliv. 2019, 16, 887–901. [Google Scholar] [CrossRef]
- Mato, Y.L. Nasal route for vaccine and drug delivery: Features and current opportunities. Int. J. Pharm. 2019, 572, 118813. [Google Scholar] [CrossRef]
- Giunchedi, P.; Gavini, E.; Bonferoni, M.C. Nose-to-brain delivery. Pharmaceutics 2020, 12, 138. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Faster, better, stronger: Towards new antidepressant therapeutic strategies. Eur. J. Pharmacol. 2015, 753, 32–50. [Google Scholar] [CrossRef]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, X.; Benet, L.Z. Reliability of In Vitro and In Vivo Methods for Predicting the Effect of P-Glycoprotein on the Delivery of Antidepressants to the Brain. Clin. Pharmacokinet. 2015, 55, 143–167. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.E.; Dinan, T.G.; Griffin, B.T.; Cryan, J.F. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: Clinical significance of in vitro and in vivo findings. Br. J. Pharmacol. 2011, 165, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Brückl, T.M.; Uhr, M. ABCB1 genotyping in the treatment of depression. Pharmacogenomics 2016, 17, 2039–2069. [Google Scholar] [CrossRef] [PubMed]
- Bicker, J.; Fortuna, A.; Alves, G.; Falcão, A. Nose-to-brain delivery of natural compounds for the treatment of central nervous system disorders. Curr. Pharm. Des. 2020, 26, 594–619. [Google Scholar]
- Long, Y.; Yang, Q.; Xiang, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N.; Peng, W. Nose to brain drug delivery—A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020, 159, 104795. [Google Scholar] [CrossRef]
- Shringarpure, M.; Gharat, S.; Momin, M.; Omri, A. Management of epileptic disorders using nanotechnology-based strategies for nose-to-brain drug delivery. Expert Opin. Drug Deliv. 2020, 18, 169–185. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-brain delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Bae, J.H. Olfactory Function and Depression: A Meta-Analysis. Ear Nose Throat J. 2022. [Google Scholar] [CrossRef]
- Staszelis, A.; Mofleh, R.; Kocsis, B. The effect of ketamine on delta-range coupling between prefrontal cortex and hippocampus supported by respiratory rhythmic input from the olfactory bulb. Brain Res. 2022, 1791, 147996. [Google Scholar] [CrossRef]
- Schwartz, J.S.; Tajudeen, B.A.; Kennedy, D.W. Diseases of the nasal cavity. Handb. Clin. Neurol. 2019, 164, 285–302. [Google Scholar] [CrossRef]
- Gonçalves, J.; Alves, G.; Fonseca, C.; Carona, A.; Bicker, J.; Falcão, A.; Fortuna, A. Is intranasal administration an opportunity for direct brain delivery of lacosamide? Eur. J. Pharm. Sci. 2021, 157, 105632. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery—The potential of nanotechnology. Bioorg. Med. Chem. 2018, 26, 2888–2905. [Google Scholar] [CrossRef] [PubMed]
- Rottstädt, F.; Han, P.; Weidner, K.; Schellong, J.; Wolff-Stephan, S.; Strauß, T.; Kitzler, H.; Hummel, T.; Croy, I. Reduced olfactory bulb volume in depression-A structural moderator analysis. Hum. Brain Mapp. 2018, 39, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Rottstaedt, F.; Weidner, K.; Strauß, T.; Schellong, J.; Kitzler, H.; Wolff-Stephan, S.; Hummel, T.; Croy, I. Size matters—The olfactory bulb as a marker for depression. J. Affect. Disord. 2017, 229, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Cecon, E.; Ivanova, A.; Luka, M.; Gbahou, F.; Friederich, A.; Guillaume, J.; Keller, P.; Knoch, K.; Ahmad, R.; Delagrange, P.; et al. Detection of recombinant and endogenous mouse melatonin receptors by monoclonal antibodies targeting the C-terminal domain. J. Pineal Res. 2018, 66, e12540. [Google Scholar] [CrossRef]
- Noseda, A.C.D.; Rodrigues, L.S.; Targa, A.D.S.; Ilkiw, J.L.; Fagotti, J.; Dos Santos, P.D.; Cecon, E.; Markus, R.P.; Solimena, M.; Jockers, R.; et al. MT(2) melatonin receptors expressed in the olfactory bulb modulate depressive-like behavior and olfaction in the 6-OHDA model of Parkinson's disease. Eur. J. Pharmacol. 2021, 891, 173722. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Renner, D.B.; Svitak, A.L.; Gallus, N.J.; Ericson, M.E.; Frey, W.H., 2nd; Hanson, L.R. Intranasal delivery of insulin via the olfactory nerve pathway. J. Pharm. Pharmacol. 2012, 64, 1709–1714. [Google Scholar] [CrossRef]
- Tan, M.S.A.; Parekh, H.S.; Pandey, P.; Siskind, D.J.; Falconer, J.R. Nose-to-brain delivery of antipsychotics using nanotechnology: A review. Expert Opin. Drug Deliv. 2020, 17, 839–853. [Google Scholar] [CrossRef]
- Altner, H.; Altner-Kolnberger, I. Freeze-fracture and tracer experiments on the permeability of the zonulae occludentes in the olfactory mucosa of vertebrates. Cell Tissue Res. 1974, 154, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.A.; Kurtenbach, S.; Sargi, Z.B.; Harbour, J.W.; Choi, R.; Kurtenbach, S.; Goss, G.M.; Matsunami, H.; Goldstein, B.J. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci. 2020, 23, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Trevino, J.; Quispe, R.; Khan, F.; Novak, V. Non-Invasive Strategies for Nose-to-Brain Drug Delivery. J. Clin. Trials 2020, 10, 439. [Google Scholar] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Croy, I.; Hummel, T. Involvement of nasal trigeminal function in human stereo smelling. Proc. Natl. Acad. Sci. USA 2020, 117, 25979. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Kellohen, K.L.; Ronaldson, P.T.; Davis, T.P. Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci. Rep. 2019, 9, 2621. [Google Scholar] [CrossRef]
- Kumar, N.N.; Lochhead, J.; Pizzo, M.; Nehra, G.; Boroumand, S.; Greene, G.; Thorne, R.G. Delivery of immunoglobulin G antibodies to the rat nervous system following intranasal administration: Distribution, dose-response, and mechanisms of delivery. J. Control. Release 2018, 286, 467–484. [Google Scholar] [CrossRef]
- Pang, Y.; Fan, L.-W.; Carter, K.; Bhatt, A. Rapid transport of insulin to the brain following intranasal administration in rats. Neural Regen. Res. 2019, 14, 1046–1051. [Google Scholar] [CrossRef]
- Lochhead, J.; Wolak, D.J.; Pizzo, M.; Thorne, R.G. Rapid Transport within Cerebral Perivascular Spaces Underlies Widespread Tracer Distribution in the Brain after Intranasal Administration. J. Cereb. Blood Flow Metab. 2015, 35, 371–381. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Rajput, P.V.; Belgamwar, V.S.; Tekade, A.R. Formulation, optimization and evaluation of spray-dried mucoadhesive microspheres as intranasal carriers for Valsartan. J. Microencapsul. 2011, 29, 103–114. [Google Scholar] [CrossRef]
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal nose-to-brain delivery: A review on architecture, physico-chemical characteristics and mucociliary cearance of the nasal olfactory mucosa. Pharmaceutics 2018, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.; Merkel, O.M. Nose-to-brain delivery of biologics. Ther. Deliv. 2019, 10, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.D.; Bhatnagar, K.P. Anatomy of the olfactory system. Handb. Clin. Neurol. 2019, 164, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Olivares, J.; Schmachtenberg, O. An update on anatomy and function of the teleost olfactory system. PeerJ 2019, 7, e7808. [Google Scholar] [CrossRef]
- Palleria, C.; Roberti, R.; Iannone, L.F.; Tallarico, M.; Barbieri, M.A.; Vero, A.; Manti, A.; De Sarro, G.; Spina, E.; Russo, E. Clinically relevant drug interactions between statins and antidepressants. J. Clin. Pharm. Ther. 2019, 45, 227–239. [Google Scholar] [CrossRef]
- Wyska, E. Pharmacokinetic considerations for current state-of-the-art antidepressants. Expert Opin. Drug Metab. Toxicol. 2019, 15, 831–847. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Ruigrok, M.J.; de Lange, E.C. Emerging insights for translational pharmacokinetic and pharmacokinetic-pharmacodynamic studies: Towards prediction of nose-to-brain transport in humans. AAPS J. 2015, 17, 493–505. [Google Scholar] [CrossRef]
- Martins, P.P.; Smyth, H.D.; Cui, Z. Strategies to facilitate or block nose-to-brain drug delivery. Int. J. Pharm. 2019, 570, 118635. [Google Scholar] [CrossRef]
- Iwasaki, S.; Yamamoto, S.; Sano, N.; Tohyama, K.; Kosugi, Y.; Furuta, A.; Hamada, T.; Igari, T.; Fujioka, Y.; Hirabayashi, H.; et al. Direct Drug Delivery of Low-Permeable Compounds to the Central Nervous System Via Intranasal Administration in Rats and Monkeys. Pharm. Res. 2019, 36, 76. [Google Scholar] [CrossRef] [PubMed]
- Marttin, E.; Verhoef, J.C.; Merkus, F.W.H.M. Efficacy, Safety and Mechanism of Cyclodextrins as Absorption Enhancers in Nasal Delivery of Peptide and Protein Drugs. J. Drug Target. 1998, 6, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Zhang, X.; Ding, J.; Mao, S. Non-ionic surfactants as novel intranasal absorption enhancers: In vitro and in vivo characterization. Drug Deliv. 2014, 23, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-beta-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release 2015, 201, 68–77. [Google Scholar] [CrossRef]
- Akita, T.; Kimura, R.; Akaguma, S.; Nagai, M.; Nakao, Y.; Tsugane, M.; Suzuki, H.; Oka, J.-I.; Yamashita, C. Usefulness of cell-penetrating peptides and penetration accelerating sequence for nose-to-brain delivery of glucagon-like peptide-2. J. Control. Release 2021, 335, 575–583. [Google Scholar] [CrossRef]
- Ozsoy, Y.; Güngör, S. Nasal route: An alternative approach for antiemetic drug delivery. Expert Opin. Drug Deliv. 2011, 8, 1439–1453. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Silva-Abreu, M.; Clares, B.; Rodriguez-Lagunas, M.J.; Halbaut, L.; Canas, M.A.; Calpena, A.C. Formulation dtrategies to improve nose-to-brain delivery of donepezil. Pharmaceutics 2019, 11, 64. [Google Scholar] [CrossRef]
- Liu, L.; Tian, C.; Dong, B.; Xia, M.; Cai, Y.; Hu, R.; Chu, X. Models to evaluate the barrier properties of mucus during drug diffusion. Int. J. Pharm. 2021, 599, 120415. [Google Scholar] [CrossRef]
- Wu, H.; Hu, K.; Jiang, X. From nose to brain: Understanding transport capacity and transport rate of drugs. Expert Opin. Drug Deliv. 2008, 5, 1159–1168. [Google Scholar] [CrossRef]
- Graff, C.L.; Pollack, G.M. Nasal Drug Administration: Potential for Targeted Central Nervous System Delivery. J. Curr. Chem. Pharm. Sci. 2005, 94, 1187–1195. [Google Scholar] [CrossRef]
- Shingaki, T.; Hidalgo, I.J.; Furubayashi, T.; Sakane, T.; Katsumi, H.; Yamamoto, A.; Yamashita, S. Nasal Delivery of P-gp Substrates to the Brain through the Nose–Brain Pathway. Drug Metab. Pharmacokinet. 2011, 26, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd; Ii, W.H.F. Novel Vasoconstrictor Formulation to Enhance Intranasal Targeting of Neuropeptide Therapeutics to the Central Nervous System. J. Pharmacol. Exp. Ther. 2008, 328, 312–320. [Google Scholar] [CrossRef]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Bravo, L.; Mico, J.A.; Berrocoso, E. Fluoxetine: A case history of its discovery and preclinical development. Expert Opin. Drug Discov. 2014, 9, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Suwała, J.; Machowska, M.; Wiela-Hojeńska, A. Venlafaxine pharmacogenetics: A comprehensive review. Pharmacogenomics 2019, 20, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.G. Nasal Anatomy and Function. Facial Plast. Surg. 2017, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, A.N.; Jain, S.K. Nasal-nanotechnology: Revolution for efficient therapeutics delivery. Drug Deliv. 2014, 23, 671–683. [Google Scholar] [CrossRef]
- Costa, C.P.; Moreira, J.; Amaral, M.H.; Lobo, J.M.S.; Silva, A. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Quintana, D.S.; Westlye, L.T.; Rustan, G.Ø.; Tesli, N.; Poppy, C.L.; Smevik, H.; Tesli, M.; Røine, M.; Mahmoud, R.A.; Smerud, K.T.; et al. Low-dose oxytocin delivered intranasally with Breath Powered device affects social-cognitive behavior: A randomized four-way crossover trial with nasal cavity dimension assessment. Transl. Psychiatry 2015, 5, e602. [Google Scholar] [CrossRef]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2013, 21, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2012, 3, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Dong, J.; Shang, Y.; Inthavong, K.; Tu, J. Effects of nasal drug delivery device and its orientation on sprayed particle deposition in a realistic human nasal cavity. Comput. Biol. Med. 2016, 77, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Warnken, Z.N.; Smyth, H.D.; Watts, A.B.; Weitman, S.; Kuhn, J.G.; Williams, R.O. Formulation and device design to increase nose to brain drug delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 213–222. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xie, J.; Zheng, Q.; Yue, P.; Chen, L.; Hu, P.; Yang, M. Nose-to-Brain Delivery by Nanosuspensions-Based in situ Gel for Breviscapine. Int. J. Nanomed. 2020, 15, 10435–10451. [Google Scholar] [CrossRef]
- Cunha, S.; Forbes, B.; Lobo, J.M.S.; Silva, A.C. Improving drug delivery for alzheimer's disease through nose-to-brain delivery using nanoemulsions, nanostructured lipid carriers (NLC) and in situ hydrogels. Int. J. Nanomed. 2021, 16, 4373–4390. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.; et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J. Control. Release 2020, 327, 235–265. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnürch, A. In situ gelling and mucoadhesive polymers: Why do they need each other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef]
- Kanwar, N.; Sinha, V.R. In Situ Forming Depot as Sustained-Release Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 93–136. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A.U. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef]
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Sen, K.K.; Gandhi, A. Alginate Based Nanocarriers for Drug Delivery Applications. Curr. Pharm. Des. 2016, 22, 3399–3410. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Severino, P.; Da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. [Google Scholar] [CrossRef]
- Thai, H.; Nguyen, C.T.; Thach, L.T.; Tran, M.T.; Mai, H.D.; Nguyen, T.T.T.; Le, G.D.; Van Can, M.; Tran, L.D.; Bach, G.L.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909–915. [Google Scholar] [CrossRef]
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2020, 170, 424–436. [Google Scholar] [CrossRef]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2016, 9, 1174–1183. [Google Scholar] [CrossRef]
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef]
- Rinaldi, F.; Oliva, A.; Sabatino, M.; Imbriano, A.; Hanieh, P.N.; Garzoli, S.; Mastroianni, C.M.; De Angelis, M.; Miele, M.C.; Arnaut, M.; et al. Antimicrobial Essential Oil Formulation: Chitosan Coated Nanoemulsions for Nose to Brain Delivery. Pharmaceutics 2020, 12, 678. [Google Scholar] [CrossRef]
- Pandey, V.; Kohli, S. Lipids and Surfactants: The Inside Story of Lipid-Based Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 99–155. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, A.J.; Eriksen, A.Z. Recent developments in liposomal drug delivery systems for the treatment of retinal diseases. Drug Discov. Today 2019, 24, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Natsheh, H.; Touitou, E. Phospholipid Magnesome—A nasal vesicular carrier for delivery of drugs to brain. Drug Deliv. Transl. Res. 2018, 8, 806–819. [Google Scholar] [CrossRef]
- Natsheh, H.; Touitou, E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959. [Google Scholar] [CrossRef]
- Touitou, E.; Duchi, S.; Natsheh, H. A new nanovesicular system for nasal drug administration. Int. J. Pharm. 2020, 580, 119243. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2018, 71, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, H.; Xu, J.; Wang, S.; Meng, D.; Geng, P.; Dai, D.; Zhou, Q.; Zhou, Y. In Vitro and In Vivo Rat Model Assessments of the Effects of Vonoprazan on the Pharmacokinetics of Venlafaxine. Drug Des. Dev. Ther. 2020, 14, 4815–4824. [Google Scholar] [CrossRef] [PubMed]
- Schoretsanitis, G.; Haen, E.; Hiemke, C.; Endres, K.; Ridders, F.; Veselinovic, T.; Gründer, G.; Paulzen, M. Pharmacokinetic correlates of venlafaxine: Associated adverse reactions. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 851–857. [Google Scholar] [CrossRef]
- Cayero-Otero, M.D.; Gomes, M.J.; Martins, C.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Sarmento, B.; Martín-Banderas, L. In vivo biodistribution of venlafaxine-PLGA nanoparticles for brain delivery: Plain vs. functionalized nanoparticles. Expert Opin. Drug Deliv. 2019, 16, 1413–1427. [Google Scholar] [CrossRef]
- Haque, S.; Md, S.; Fazil, M.; Kumar, M.; Sahni, J.K.; Ali, J.; Baboota, S. Venlafaxine loaded chitosan NPs for brain targeting: Pharmacokinetic and pharmacodynamic evaluation. Carbohydr. Polym. 2012, 89, 72–79. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Norman, T.R.; Olver, J.S. Desvenlafaxine in the treatment of major depression: An updated overview. Expert Opin. Pharmacother. 2021, 22, 1087–1097. [Google Scholar] [CrossRef]
- Tong, G.-F.; Qin, N.; Sun, L.-W. Development and evaluation of Desvenlafaxine loaded PLGA-chitosan nanoparticles for brain delivery. Saudi Pharm. J. 2016, 25, 844–851. [Google Scholar] [CrossRef]
- Ahmed, S.; Gull, A.; Aqil, M.; Ansari, M.D.; Sultana, Y. Poloxamer-407 thickened lipid colloidal system of agomelatine for brain targeting: Characterization, brain pharmacokinetic study and behavioral study on Wistar rats. Colloids Surfaces B Biointerfaces 2019, 181, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Intranasal agomelatine solid lipid nanoparticles to enhance brain delivery: Formulation, optimization and in vivo pharmacokinetics. Drug Des. Dev. Ther. 2017, 11, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Agomelatine-based in situ gels for brain targeting via the nasal route: Statistical optimization, in vitro, and in vivo evaluation. Drug Deliv. 2017, 24, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.; Vanza, J.; Pandya, N.; Tandel, H. Formulation of polymeric nanoparticles of antidepressant drug for intranasal delivery. Ther. Deliv. 2019, 10, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K. Intranasal infusion of nanostructured lipid carriers (NLC) containing CNS acting drug and estimation in brain and blood. Drug Deliv. 2013, 20, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K.; Bhatnagar, A. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014, 470, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Elsenosy, F.M.; Abdelbary, G.A.; Elshafeey, A.H.; Elsayed, I.; Fares, A.R. Brain Targeting of Duloxetine HCL via Intranasal Delivery of Loaded Cubosomal Gel: In vitro Characterization, ex vivo Permeation, and in vivo Biodistribution Studies. Int. J. Nanomed. 2020, 15, 9517–9537. [Google Scholar] [CrossRef]
- Khatoon, M.; Sohail, M.F.; Shahnaz, G.; Rehman, F.U.; Din, F.U.; Rehman, A.U.; Ullah, N.; Amin, U.; Khan, G.M.; Shah, K.U. Development and Evaluation of Optimized Thiolated Chitosan Proniosomal Gel Containing Duloxetine for Intranasal Delivery. AAPS PharmSciTech 2019, 20, 288. [Google Scholar] [CrossRef]
- Purgato, M.; Papola, D.; Gastaldon, C.; Trespidi, C.; Magni, L.R.; Rizzo, C.; Furukawa, T.A.; Watanabe, N.; Cipriani, A.; Barbui, C. Paroxetine versus other anti-depressive agents for depression. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Pandey, Y.R.; Kumar, S.; Gupta, B.K.; Ali, J.; Baboota, S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: Formulation, behavioural and biochemical estimation. Nanotechnology 2015, 27, 25102. [Google Scholar] [CrossRef]
- Silva, S.; Bicker, J.; Fonseca, C.; Ferreira, N.R.; Vitorino, C.; Alves, G.; Falcão, A.; Fortuna, A. Encapsulated Escitalopram and Paroxetine Intranasal Co-Administration: In Vitro/In Vivo Evaluation. Front. Pharmacol. 2021, 12, 751321. [Google Scholar] [CrossRef] [PubMed]
- Motaleb, M.A.; Ibrahim, I.T.; Sayyed, M.E.; Awad, G.A.S. (131)I-trazodone: Preparation, quality control and in vivo biodistribution study by intranasal and intravenous routes as a hopeful brain imaging radiopharmaceutical. Rev. Esp. Med. Nucl. Imagen Mol. 2017, 36, 371–376. [Google Scholar] [PubMed]
- Boche, M.; Pokharkar, V. Quetiapine Nanoemulsion for Intranasal Drug Delivery: Evaluation of Brain-Targeting Efficiency. AAPS PharmSciTech 2016, 18, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: Formulation, physicochemical and pharmacokinetic consideration. Eur. J. Pharm. Sci. 2016, 91, 196–207. [Google Scholar] [CrossRef]
- Naik, A.; Nair, H. Formulation and Evaluation of Thermosensitive Biogels for Nose to Brain Delivery of Doxepin. BioMed Res. Int. 2014, 2014, 847547. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, T.Q.; Angela, A.; Mateo, L.; Melanie, L.Z.; Valentina, P.F.; David, C.; Estefania, C.; Natalia, R.S.; Andrés, V.C.; Angel, R.O.; et al. Ketamine for resistant depression: A scoping review. Actas Esp. Psiquiatr. 2022, 50, 144–159. [Google Scholar] [PubMed]
- Yavi, M.; Lee, H.; Henter, I.D.; Park, L.T.; Zarate, C.A., Jr. Ketamine treatment for depression: A review. Discov. Ment. Health 2022, 2, 9. [Google Scholar] [CrossRef]
- Yao, W.; Cao, Q.; Luo, S.; He, L.; Yang, C.; Chen, J.; Qi, Q.; Hashimoto, K.; Zhang, J.C. Microglial ERK-NRBP1-CREB-BDNF signaling in sustained antidepressant actions of (R)-ketamine. Mol. Psychiatry 2022, 27, 1618–1629. [Google Scholar] [CrossRef]
- Buddenberg, T.E.; Topic, B.; Mahlberg, E.D.; Silva, M.A.D.S.; Huston, J.P.; Mattern, C. Behavioral Actions of Intranasal Application of Dopamine: Effects on Forced Swimming, Elevated Plus-Maze and Open Field Parameters. Neuropsychobiology 2008, 57, 70–79. [Google Scholar] [CrossRef]
- Chemuturi, N.V.; Donovan, M.D. Metabolism of Dopamine by the Nasal Mucosa. J. Pharm. Sci. 2006, 95, 2507–2515. [Google Scholar] [CrossRef]
- Brown, V.; Liu, F. Intranasal Delivery of a Peptide with Antidepressant-Like Effect. Neuropsychopharmacology 2014, 39, 2131–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangani, C.; Giordano, B.; Stein, H.; Bonora, S.; D’Agostino, A.; Ostinelli, E.G. Efficacy of amisulpride for depressive symptoms in individuals with mental disorders: A systematic review and meta-analysis. Hum. Psychopharmacol. Clin. Exp. 2021, 36, e2801. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.C.; Wilkinson, S.; Corriveau, T.J.; Nishikawa, H.; Nakamichi, K.; Loebel, A.; Koblan, K.S. Discovery of Nonracemic Amisulpride to Maximize Benefit/Risk of 5-HT7 and D2 Receptor Antagonism for the Treatment of Mood Disorders. Clin. Pharmacol. Ther. 2021, 110, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Hagi, K.; Kurokawa, S.; Kane, J.M.; Correll, C.U. Efficacy and safety/tolerability of antipsychotics in the treatment of adult patients with major depressive disorder: A systematic review and meta-analysis. Psychol. Med. 2022, 1–19. [Google Scholar] [CrossRef]
- Yuan, B.; Yuan, M. Changes of Mental State and Serum Prolactin Levels in Patients with Schizophrenia and Depression after Receiving the Combination Therapy of Amisulpride and Chloroprothixol Tablets. Comput. Math. Methods Med. 2022, 2022, 6580030. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Tagalpallewar, A.A.; Kokare, C.R. Agranulocytosis-Protective Olanzapine-Loaded Nanostructured Lipid Carriers Engineered for CNS Delivery: Optimization and Hematological Toxicity Studies. AAPS PharmSciTech 2019, 20, 22. [Google Scholar] [CrossRef]
- El Assasy, A.E.I.; Younes, N.F.; Makhlouf, A.I.A. Enhanced oral absorption of amisulpride via a nanostructured lipid carrier-based capsules: Development, optimization applying the desirability function approach and in vivo pharmacokinetic study. AAPS PharmSciTech 2019, 20, 82. [Google Scholar] [CrossRef]
- Gadhave, D.; Tupe, S.; Tagalpallewar, A.; Gorain, B.; Choudhury, H.; Kokare, C. Nose-to-brain delivery of amisulpride-loaded lipid-based poloxamer-gellan gum nanoemulgel: In vitro and in vivo pharmacological studies. Int. J. Pharm. 2021, 607, 121050. [Google Scholar] [CrossRef]
- Lenze, E.J.; Mulsant, B.H.; Blumberger, D.M.; Karp, J.F.; Newcomer, J.W.; Anderson, S.; Dew, M.A.; Butters, M.A.; Stack, J.A.; Begley, A.E.; et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: A randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 2404–2412. [Google Scholar] [CrossRef]
- Nelson, J.C.; Papakostas, G.I. Atypical Antipsychotic Augmentation in Major Depressive Disorder: A Meta-Analysis of Placebo-Controlled Randomized Trials. Am. J. Psychiatry 2009, 166, 980–991. [Google Scholar] [CrossRef]
- Kirschbaum, K.M.; Uhr, M.; Holthoewer, D.; Namendorf, C.; Pietrzik, C.; Hiemke, C.; Schmitt, U. Pharmacokinetics of acute and sub-chronic aripiprazole in P-glycoprotein deficient mice. Neuropharmacology 2010, 59, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Landucci, E.; Urru, M.; Chiarugi, A.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Enhanced dissolution, permeation and oral bioavailability of aripiprazole mixed micelles: In vitro and in vivo evaluation. Int. J. Pharm. 2020, 583, 119361. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, S.A.; Kokare, C.R.; Shrivastava, B.; Gorain, B.; Choudhury, H. Antipsychotic Potential and Safety Profile of TPGS-Based Mucoadhesive Aripiprazole Nanoemulsion: Development and Optimization for Nose-To-Brain Delivery. J. Pharm. Sci. 2021, 110, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Sawant, K.; Pandey, A.; Patel, S. Aripiprazole loaded poly(caprolactone) nanoparticles: Optimization and in vivo pharmacokinetics. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 230–243. [Google Scholar] [CrossRef]
- Taymouri, S.; Shahnamnia, S.; Mesripour, A.; Varshosaz, J. In vitro and in vivo evaluation of an ionic sensitive in situ gel containing nanotransfersomes for aripiprazole nasal delivery. Pharm. Dev. Technol. 2021, 26, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Rashid, M.; Hallan, S.; Mehra, N.K.; Prakash, A.; Mishra, N. Pharmacological evaluation of nasal delivery of selegiline hydrochloride-loaded thiolated chitosan nanoparticles for the treatment of depression. Artif. Cells Nanomed. Biotechnol. 2015, 44, 865–877. [Google Scholar] [CrossRef]
- Sridhar, V.; Gaud, R.; Bajaj, A.; Wairkar, S. Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in Parkinson's disease. Nanomedicine 2018, 14, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, V.; Wairkar, S.; Gaud, R.; Bajaj, A.; Meshram, P. Brain targeted delivery of mucoadhesive thermosensitive nasal gel of selegiline hydrochloride for treatment of Parkinson's disease. J. Drug Target 2018, 26, 150–161. [Google Scholar] [CrossRef]
- Rukmangathen, R.; Yallamalli, I.M.; Yalavarthi, P.R. Biopharmaceutical potential of selegiline loaded chitosan nanoparticles in the management of parkinson's disease. Curr. Drug Discov. Technol. 2019, 16, 417–425. [Google Scholar] [CrossRef]
- Kumar, S.; Ali, J.; Baboota, S. Design Expert® supported optimization and predictive analysis of selegiline nanoemulsion via the olfactory region with enhanced behavioural performance in Parkinson’s disease. Nanotechnology 2016, 27, 435101. [Google Scholar] [CrossRef]
- Kumar, S.; Dang, S.; Nigam, K.; Ali, J.; Baboota, S. Selegiline nanoformulation in attenuation of oxidative stress and upregulation of dopamine in the brain for the treatment of parkinson's disease. Rejuvenation Res. 2018, 21, 464–476. [Google Scholar] [CrossRef]
- Mishra, N.; Sharma, S.; Deshmukh, R.; Kumar, A.; Sharma, R. Development and Characterization of Nasal Delivery of Selegiline Hydrochloride Loaded Nanolipid Carriers for the Management of Parkinson’s Disease. Central Nerv. Syst. Agents Med. Chem. 2019, 19, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Garg, T.; Vaidya, B.; Prakash, A.; Rath, G.; Goyal, A.K. Brain delivery of intranasal in situ gel of nanoparticulated polymeric carriers containing antidepressant drug: Behavioral and biochemical assessment. J. Drug Target 2015, 23, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.S.; Lim, H.K.; Wang, S.-M.; Bahk, W.-M. Title Clinical Evidence of Antidepressant Effects of Insulin and Anti-Hyperglycemic Agents and Implications for the Pathophysiology of Depression—A Literature Review. Int. J. Mol. Sci. 2020, 21, 6969. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.A.; Testani, D.; Mansur, R.B.; Lee, Y.; Subramaniapillai, M.; McIntyre, R.S. Brain insulin resistance: A treatment target for cognitive impairment and anhedonia in depression. Exp. Neurol. 2019, 315, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.H.; Sun, L.H.; Yang, W.; Li, B.J.; Cui, R.J. Potential role of insulin on the pathogenesis of depression. Cell Prolif. 2020, 53, e12806. [Google Scholar] [CrossRef]
- Nedelcovych, M.T.; Gadiano, A.J.; Wu, Y.; Manning, A.A.; Thomas, A.G.; Khuder, S.S.; Yoo, S.-W.; Xu, J.; McArthur, J.C.; Haughey, N.J.; et al. Pharmacokinetics of Intranasal versus Subcutaneous Insulin in the Mouse. ACS Chem. Neurosci. 2017, 9, 809–816. [Google Scholar] [CrossRef]
- Ren, G.; Xue, P.; Wu, B.; Yang, F.; Wu, X. Intranasal treatment of lixisenatide attenuated emotional and olfactory symptoms via CREB-mediated adult neurogenesis in mouse depression model. Aging 2021, 13, 3898–3908. [Google Scholar] [CrossRef]
- Sasaki-Hamada, S.; Nakamura, R.; Nakao, Y.; Akimoto, T.; Sanai, E.; Nagai, M.; Horiguchi, M.; Yamashita, C.; Oka, J.-I. Antidepressant-like effects exerted by the intranasal administration of a glucagon-like peptide-2 derivative containing cell-penetrating peptides and a penetration-accelerating sequence in mice. Peptides 2017, 87, 64–70. [Google Scholar] [CrossRef]
- Nakao, Y.; Horiguchi, M.; Nakamura, R.; Sasaki-Hamada, S.; Ozawa, C.; Funane, T.; Ozawa, R.; Oka, J.-I.; Yamashita, C. LARETH-25 and β-CD improve central transitivity and central pharmacological effect of the GLP-2 peptide. Int. J. Pharm. 2016, 515, 37–45. [Google Scholar] [CrossRef]
- Dickens, M.J.; Pawluski, J.L. The HPA Axis During the Perinatal Period: Implications for Perinatal Depression. Endocrinology 2018, 159, 3737–3746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riem, M.; Kunst, L.; Bekker, M.; Fallon, M.; Kupper, N. Intranasal oxytocin enhances stress-protective effects of social support in women with negative childhood experiences during a virtual Trier Social Stress Test. Psychoneuroendocrinology 2019, 111, 104482. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, Y.-K. Possible oxytocin-related biomarkers in anxiety and mood disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 116, 110531. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhuang, J.; Zuo, R.; Zheng, H.; Dang, J.; Wang, Z. Exploring associations between postpartum depression and oxytocin levels in cerebrospinal fluid, plasma and saliva. J. Affect. Disord. 2022, 315, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.; Cole, C.; Smith, Y.; Neumann, I.; Landgraf, R.; Murphy, A.; Young, L. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience 2009, 162, 892–903. [Google Scholar] [CrossRef]
- Zhao, J.L.; Jiang, W.T.; Wang, X.; Cai, Z.D.; Liu, Z.H.; Liu, G.R. Exercise, brain plasticity, and depression. CNS Neurosci. Ther. 2020, 26, 885–895. [Google Scholar] [CrossRef]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef]
- Ma, X.-C.; Liu, P.; Zhang, X.-L.; Jiang, W.-H.; Jia, M.; Wang, C.-X.; Dong, Y.-Y.; Dang, Y.-H.; Gao, C.-G. Intranasal Delivery of Recombinant AAV Containing BDNF Fused with HA2TAT: A Potential Promising Therapy Strategy for Major Depressive Disorder. Sci. Rep. 2016, 6, 22404. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Y.; Liu, F.; Gao, C.; Ji, C.; Dang, Y.; Ma, X.; Liu, Y. A Study of Antidepressant Effect and Mechanism on Intranasal Delivery of BDNF-HA2TAT/AAV to Rats with Post-Stroke Depression. Neuropsychiatr. Dis. Treat. 2020, 16, 637–649. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.-P.; Lei, G.; Liu, P.; Chu, Z.; Gao, C.-G.; Dang, Y.-H. Antidepressant effect of recombinant NT4-NAP/AAV on social isolated mice through intranasal route. Oncotarget 2016, 8, 10103–10113. [Google Scholar] [CrossRef]
- Ma, X.-C.; Chu, Z.; Zhang, X.-L.; Jiang, W.-H.; Jia, M.; Dang, Y.-H.; Gao, C.-G. Intranasal Delivery of Recombinant NT4-NAP/AAV Exerts Potential Antidepressant Effect. Neurochem. Res. 2016, 41, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Peng, Y.; Liang, X.; Li, S.; Chang, X.; Li, L.; Chang, M. Centrally administered cortistation-14 induces antidepressant-like effects in mice via mediating ghrelin and GABA(A) receptor signaling pathway. Front. Pharmacol. 2018, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Serova, L.; Laukova, M.; Alaluf, L.; Pucillo, L.; Sabban, E. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur. Neuropsychopharmacol. 2014, 24, 142–147. [Google Scholar] [CrossRef]
- Oh, J.-Y.; Liu, Q.F.; Hua, C.; Jeong, H.J.; Jang, J.-H.; Jeon, S.; Park, S.J.A.H.-J. Intranasal Administration of Melanin-Concentrating Hormone Reduces Stress-Induced Anxiety- and Depressive-Like Behaviors in Rodents. Exp. Neurobiol. 2020, 29, 453–469. [Google Scholar] [CrossRef]
- Shi, C.-G.; Wang, L.-M.; Wu, Y.; Wang, P.; Gan, Z.-J.; Lin, K.; Jiang, L.-X.; Xu, Z.-Q.; Fan, M. Intranasal Administration of Nerve Growth Factor Produces Antidepressant-Like Effects in Animals. Neurochem. Res. 2010, 35, 1302–1314. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef]
- Obermanns, J.; Krawczyk, E.; Juckel, G.; Emons, B. Analysis of cytokine levels, T regulatory cells and serotonin content in patients with depression. Eur. J. Neurosci. 2021, 53, 3476–3489. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Comai, S.; Melloni, E.; Lorenzi, C.; Bollettini, I.; Vai, B.; Zanardi, R.; Colombo, C.; Valtorta, F.; Benedetti, F.; Poletti, S. Selective association of cytokine levels and kynurenine/tryptophan ratio with alterations in white matter microstructure in bipolar but not in unipolar depression. Eur. Neuropsychopharmacol. 2021, 55, 96–109. [Google Scholar] [CrossRef]
- Rengasamy, M.; Brundin, L.; Griffo, A.; Panny, B.; Capan, C.; Forton, C.; Price, R.B. Cytokine and Reward Circuitry Relationships in Treatment-Resistant Depression. Biol. Psychiatry Glob. Open Sci. 2021, 2, 45–53. [Google Scholar] [CrossRef]
- Markova, E.V.; Shevela, E.Y.; Knyazeva, M.A.; Savkin, I.V.; Serenko, E.V.; Rashchupkin, I.M.; Amstislavskaya, T.G.; Ostanin, A.A.; Chernykh, E.R. Effect of M2 Macrophage-Derived Soluble Factors on Behavioral Patterns and Cytokine Production in Various Brain Structures in Depression-Like Mice. Bull. Exp. Biol. Med. 2022, 172, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Wang, J.-X.; Hu, X.-X.; Chen, L.; Qiu, Z.-K.; Zhao, N.; Yu, Z.-D.; Sun, S.-Z.; Xu, Y.-Y.; Guo, Y.; et al. Antidepressant-like effects of albiflorin extracted from Radix paeoniae Alba. J. Ethnopharmacol. 2015, 179, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-X.; Fu, J.; Ma, S.-R.; Peng, R.; Yu, J.-B.; Cong, L.; Pan, L.-B.; Zhang, Z.-G.; Tian, H.; Che, C.-T.; et al. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics 2018, 8, 5945–5959. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2016, 76, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Qiao, T.; Wang, Y.; Wang, Q.-S.; Cui, Y.-L. Alginate nanogels-based thermosensitive hydrogel to improve antidepressant-like effects of albiflorin via intranasal delivery. Drug Deliv. 2021, 28, 2137–2149. [Google Scholar] [CrossRef]
- Zhu, W.-Q.; Wu, H.-Y.; Sun, Z.-H.; Guo, Y.; Ge, T.-T.; Li, B.-J.; Li, X.; Cui, R.-J. Current Evidence and Future Directions of Berberine Intervention in Depression. Front. Pharmacol. 2022, 13, 824420. [Google Scholar] [CrossRef]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Berberine alleviates symptoms of anxiety by enhancing dopamine expression in rats with post-traumatic stress disorder. Korean J. Physiol. Pharmacol. 2018, 22, 183–192. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, K.; Jin, Y.; Li, B.; Gao, S.; Zhu, J.; Cui, R. Pharmacological effects of berberine on mood disorders. J. Cell. Mol. Med. 2018, 23, 21–28. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Li, K.; Gao, L.-N.; Zhang, Y.; Lin, K.-M.; Cui, Y.-L. Intranasal delivery of berberine via in situ thermoresponsive hydrogels with non-invasive therapy exhibits better antidepressant-like effects. Biomater. Sci. 2020, 8, 2853–2865. [Google Scholar] [CrossRef]
- Xu, D.; Qiu, C.; Wang, Y.; Qiao, T.; Cui, Y.-L. Intranasal co-delivery of berberine and evodiamine by self-assembled thermosensitive in-situ hydrogels for improving depressive disorder. Int. J. Pharm. 2021, 603, 120667. [Google Scholar] [CrossRef]
- Zhang, K.; Lei, N.; Li, M.; Li, J.; Li, C.; Shen, Y.; Guo, P.; Xiong, L.; Xie, Y. Cang-Ai Volatile Oil Ameliorates Depressive Behavior Induced by Chronic Stress Through IDO-Mediated Tryptophan Degradation Pathway. Front. Psychiatry 2021, 12, 791991. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, J.; Xie, Y.; Ming, X.; Li, G.; Wang, J.; Li, M.; Li, X.; Xiong, L. Cang-ai volatile oil improves depressive-like behaviors and regulates DA and 5-HT metabolism in the brains of CUMS-induced rats. J. Ethnopharmacol. 2019, 244, 112088. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, Z.; Shi, J. Pharmacological effects of icariin. Adv. Pharmacol. 2020, 87, 179–203. [Google Scholar] [PubMed]
- Xu, S.; Yu, J.; Zhan, J.; Yang, L.; Guo, L.; Xu, Y. Pharmacokinetics, Tissue Distribution, and Metabolism Study of Icariin in Rat. BioMed Res. Int. 2017, 2017, 4684962. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, Y.-R.; Kou, N.; Hu, M.-J.; Wang, Q.-S.; Cui, Y.-L. Intranasal delivery of icariin via a nanogel-thermoresponsive hydrogel compound system to improve its antidepressant-like activity. Int. J. Pharm. 2020, 586, 119550. [Google Scholar] [CrossRef]
- Hu, W.; Xie, G.; Zhou, T.; Tu, J.; Zhang, J.; Lin, Z.; Zhang, H.; Gao, L. Intranasal administration of white tea alleviates the olfactory function deficit induced by chronic unpredictable mild stress. Pharm. Biol. 2020, 58, 1230–1237. [Google Scholar] [CrossRef]





| Types | Ingredients | Dosage Form | Characterization | Ex Vivo/In Vivo Studies | Relevant Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Antidepressants | Venlafaxine | Poly lactic-co-glycolic acid nanoparticles (PLGA-NPs); Peptide-modified nanoparticles | PS = 206.3 ± 3.7 nm; PI = 0.041 ± 0.017; ZP = −26.5 ± 0.5 mV; around 200 nm after lyophilization process; DL = 10–12%; EE = 48–50%. | In vitro cell viability and cellular uptake (hCMEC/D3 cells); Permeability assay and transport studies; Biodistribution studies (C57/bl6 mice). | Cell viability of h-CMEC/D3 cells is more than 85% in the MTT assay. In vivo biodistribution studies showed higher concentrations of plain fluorescent NPs than functionalized NPs in the brain after 30 min of administration. | [107] |
| Chitosan nanoparticles (CN-NPs) | PS = 167 ± 6.5 nm; PI = 0.367 ± 0.045; ZP = +23.83 ± 1.76 mV; DL = 32.25 ± 1.63%; EE = 79.3 ± 2.6%; Yield = 71.42 ± 3.24%. | Ex vivo permeation studies using porcine nasal mucosal membrane (Franz cells); Pharmacodynamic studies in Wistar rats (modified forced swim test, locomotor activity test); Qualitative localization and biodistribution studies by confocal laser scanning microscopy; Pharmacokinetic analysis. | The cumulative drug permeability after 24 h in VLF CN-NPs was nearly 3 times compared with VLF solution. VLF CN-NPs showed a more significant antidepressant effect than VLF solution on chronic depression rats by forced swimming method. DTE (%)/DTP (%): NPs = 508.59/80.34 | [108] | ||
| Desvenlafaxine | Chitosan-coated poly lactic-co-glycolic acid nanoparticles (PLGA-CN-NPs) | PS = 172.5 ± 10.2 nm; PI = 0.254 ± 0.02; ZP = +35.63 ± 8.25 mV; DL = 30.8 ± 3.1%; EE = 76.4 ± 4.2%; Release (24 h) = 77.21 ± 3.87% (pH 7.4) and 76.32 ± 3.54% (pH 6.0). | Ex vivo permeation studies on porcine nasal mucosa; Pharmacodynamic studies (Wistar rats); Stress-induced model (forced swimming test); Drug-induced model (reserpine reversal test); Biochemical estimation of serotonin, noradrenaline, and dopamine; Blood and brain pharmacokinetic studies. | In a rodent model of depression, compared with intranasal DVF solution and oral administration, increased levels of 5-HT and NE in the brain showed a more pronounced antidepressant effect. Pharmacokinetic parameters such as concentration, half-life, and AUC in the brain after intranasal administration were higher than those of through intravenous. DTE (%)/DTP (%) = 544.23/81.62 (DVF-NP s) DTE (%)/DTP (%) = 202.41/50.59 (DVF) | [111] | |
| Agomelatine | Nanoemulsion thermosensitive in situ gel + 0.5% Chitosan | Gelling point = 28 ± 1 °C; Mucoadhesive strength = 6246.27 dynes/cm2; NEs: PS = 206.3 ± 3.7 nm; Micelles of P-407: PS = 142.58 ± 4.21 nm; Ago-NE-gel + 0.5%chitosan: Viscosity = 2439 ± 23 cP (35 ± 1 °C); pH = 5.8 ± 0.2. | In vitro gel erosion study; Ex vivo drug permeation through the bovine nasal mucosa; Nasal toxicity study; Pharmacokinetic analysis: DTE (%) and DTP (%); Pharmacodynamic studies (Behavioral test; modified forced swim test and tail suspension test). | Pharmacokinetic study in Wistar rats showed plasma concentration in the brain was 2.82 times higher than that of the intravenous suspension via the intranasal route. DTE (%)/DTP (%) = 344.9/71.0 | [112] | |
| Solid lipid nanoparticles (SLNs) | PS = 167.70 ± 0.42 nm; PI = 0.12 ± 0.10; ZP = −17.90 ± 2.70 mV; EE = 91.25 ± 1.70%; Release (1 h)/(8 h) = 35.40 ± 1.13%/80.87 ± 5.16%. | Pharmacokinetic study (rats): assay of agM in plasma and brain; Pharmacokinetic analysis: DTE (%) and DTP (%). | The nasal solid lipid nanoparticles prepared by Ahmed et al. were superior to the oral suspension in brain concentration, AUC0–360min, and absolute bioavailability (44.44%) DTE (%)/DTP (%) = 190.02/47.37 | [113] | ||
| Duloxetine | Nanostructured lipid carriers (NLCs) | PS = 137.2 ± 2.88 nm; ZP = -31.53 ± 11.21 mV; DL = 9.73 ± 3.22%; EE = 79.15 ± 4.17%. | Biodistribution studies (Wistar rats); Pharmacokinetic study; Gamma-imaging study. | Intranasal DLX-NLCs showed higher concentrations in blood and brain compared with DLX solution and oral route, which showed the same results in behavioral tests in mice. Intranasal NLCs were 8-fold higher in brain concentrations than intravenous DLX. DTE (%)/DTP (%) = 757.74/86.80 (DLX-NLC) DTE (%)/DTP (%) = 287.34/65.12 (DLX) | [116,117] | |
| Thiomer gel loaded with proniosomes | 20% w/v PF127, 5% w/v PF68; 3.76 lipid ratio; PS = 265.13 ± 9.85 nm; GT = 32 ± 0.05 °C; EE = 98.13 ± 0.50%; Release (3 h) = 33%. | Pharmacokinetic analysis: DTE (%) and DTP (%); Stability study. | Thiomer gel loaded with duloxetine proniosomes increased the retention time and sustained release and penetration of DLX in the nasal mucosa (1.96 times that of duloxetine proniosomes). DTE (%)/DTP (%) = 137.77/10.5 | [118] | ||
| Paroxetine | Nanoemulsion (NEs) | PS = 58.47 ± 3.02 nm; PDI = 0.339 ± 0.007; ZP = −33 mV; Transmittance = 100.60 ± 0.577%; Refractive index = 1.412 ± 0.003. | Ex vivo permeation studies using porcine nasal mucosal membrane (Franz cells); Pharmacodynamic studies (Wistar rats; forced swimming test, locomotor activity test); Biochemical estimation: GSH and TBARS. | The permeability of paroxetine NEs was 2.57 times higher than that of its suspension via permeation studies. Results of behavioral studies in rats showed that intranasal administration of paroxetine NEs significantly improved behavioral activity in depressed rats compared with the oral suspension of paroxetine. | [121] | |
| Trazodone | Microemulsion | labelling yield = 91.23 ± 2.12%; In vitro stability of 131I-TZ = 6 h; Droplet size = 16.4 ± 2.5 nm; PDI = 0.11 ± 0.02; ZP = 3.83 ± 0.36; Viscosity (25 °C) = 261.7 ± 3.0; Viscosity (37 °C) = 157.3 ± 7.5. | Biodistribution of 131I-TZ; The 131I-TZ uptake in organs and body fluids. | Sayyed et al. radiolabeled trazodone and compared the pharmacokinetic parameters of intranasal delivery of 131I-TZ solution, 131I-TZ microemulsion, and intravenous injection of 131I-TZ solution. Intranasal 131I-TZ microemulsion had sustained and higher brain uptake at any time tested than the other two formulations and routes. In addition, the blood exposure of intranasal 131I-TZ microemulsion was lower than that of intravenous injection, reducing systemic toxicity. | [123] | |
| Quetiapine fumarate | Microemulsion Chitosan microemulsion (CH-ME) methyl-β-cyclodextrin microemulsion (MeβCD-ME) | PS: QF-ME = 29.75 ± 0.99 nm; CH-ME = 35.31 ± 1.71 nm; MeβCD-ME = 46.55 ± 1.9 nm with; PDI: QF-ME = 0.221 ± 0.01; CH-ME = 0.249 ± 0.03; MeβCD-ME = 0.233 ± 0.02; ZP: QF-ME = 2.77 ± 0.51; CH-ME = 20.29 ± 1.23 MeβCD-ME = 8.43 ± 0.7; Viscosity: QF-ME = 17.5 ± 0.69 cP; CH-ME = 38.5 ± 1.26 cP; MeβCD-ME = 33.3 ± 0.93 cP. | Ex vivo mucoadhesive strength; Ex vivo nasal and intestinal diffusion study (goat nasal mucosa and small intestine); Nasal mucosal toxicity test; Pharmacokinetic analysis: DTE (%) and DTP (%). | The brain bioavailability of quetiapine fumarate of chitosan-coated microemulsion was 3.8-fold and 2.7-fold higher than that of drug solution and chitosan-free microemulsion, respectively. DTE (%)/DTP (%) = 371.20 ± 12.02/ 68.66 ± 6.84 (QF-ME) DTE (%)/DTP (%) = 453.69 ± 10.17/80.51 ± 6.46 (CH-ME) | [125] | |
| Doxepin hydrochloride | Thermoreversible biogels | Gelation temperature = 37.4 °C; Gelation time = 7.32 min pH = 6.93. | In vitro penetration test on sheep nasal mucosa; Stress-induced model (forced swimming test). | Compared with doxepin hydrochloride solution, the thermoreversible biogel showed more advantages in immobility time and swimming activity count in mice after 13 days of drug administration. | [126] | |
| Off-label drugs | Ketamine/Esketamine | Nasal spray | N/A | N/A | Ketamine, whether administered intravenously or intranasally, has a higher bioavailability than the oral route, and has a more rapid and significant effect than traditional antidepressants with delayed onset of action. Due to the plasma elimination half-life of ketamine of 2–4 h and the discomfort associated with invasive administration, delivery of ketamine directly to the brain via the nasal cavity is a more advantageous strategy. | [127,128] |
| Amisulpride | Lipid-based poloxamer-gellan gum nanoemulgel AMS nanoemulsion (AMS-NE) AMS in situ nanoemulgel (AMS-NG) | AMS-NE: PS = 92.15 ± 0.42 nm; PI = 0.46 ± 0.03; ZP = −18.22 mV; Transmittance = 99.57%; Mucoadhesive strength = 1.24 g; Release (4 h) = 99.99%; AMS-NG: PS = 106.11 ± 0.26 nm; PI = 0.51 ± 0.01; ZP = −16.01 mV; Transmittance = 98.47%; Mucoadhesive strength = 8.90 g; Release (4 h) = 98.96%. | Ex vivo drug permeation study on freshly isolated sheep nasal mucosa; In vivo animal experiments (pharmacokinetic study, AMS in brain and blood plasma samples); Animal behavioral studies (induced locomotor activity test, paw test); In vivo safety assessment. | Pharmacokinetic studies in Wister rats showed that the intranasal C(max) of the brain was 3.39 times higher than that of the intravenous administration and intranasal administration within one month did not affect blood leukocyte and granulocyte counts. DTE (%)/DTP (%) = 314.08/76.13 (AMS-NE) DTE (%)/DTP (%) = 1821.72/275.09 (AMS-NG) | [139] | |
| Aripiprazole | Mucoadhesive nanoemulsion | PS = 121.8 ± 1.5 nm; PI = 0.248 ± 0.05; ZP = −18.89 ± 3.47 mV; Viscosity = 187.79 ± 5.35 cP (25% Carbopol); Viscosity = 626.32 ± 8.63 cP (1% Carbopol); Release (8 h) = 84.92%. | Ex vivo permeation test and nasal ciliotoxicity on sheep nasal mucosa; In vitro cytotoxicity study (Vero cells, PC12 cells); In vivo pharmacokinetic study (DTE (%) and DTP (%)); Locomotor activity study. | Pharmacokinetic studies with single-dose administration showed that the plasma concentration in the brain of intranasal ARP-MNE was 1.44 and 6.03 times higher than that of intranasal and intravenous ARP-NE, respectively, and the Tmax was smaller than that of intravenously administered ARP-NE. DTE (%)/ DTP (%) = 96.90/89.73 | [144] | |
| Poly(caprolactone) nanoparticles | PS = 199.2 ± 5.65 nm; ZP = -21.4 ± 4.6 mV; EE = 69.2 ± 2.34%; Release (8 h) = 90 ± 2.69%. | Ex vivo diffusion studies on goat nasal mucosa; Nasal toxicity study (goat nasal mucosa); In vivo pharmacokinetics study (DTE (%) and DTP (%)). | The AUC0–8h of Aripiprazole in the rat brain administered by the intranasal route of APNPs was approximately twice that of the intravenous route. DTE (%)/DTP (%) = 64.11/74.34 | [145] | ||
| Selegiline | Chitosan nanoparticle | PS = 341.6 ± 56.91 nm; PI = 0.317 ± 0.29; ZP = −13.4 ± 0.04 mV; EE = 92.20 ± 7.15%; Release (8 h) = 90 ± 2.69%. | Ex vivo drug diffusion on sheep nasal mucosa; Pharmacokinetics and pharmacodynamics studies; Behavioral testing; Biochemical analyses: dopamine level, catalase activity, reduced glutathione (GSH) content. | The Cmax of plain solution of selegiline in the brain and plasma by intranasal administration (Tmax = 5 min) was 20 and 12 times higher, respectively, compared with oral administration (Tmax = 15 min). Furthermore, intranasal administration of selegiline-loaded CN-NPs and mucoadhesive thermosensitive gel showed superior formulation advantages compared with the AUC0–24h of plain solution. | [148,149] | |
| Peptides | Insulin | N/A | N/A | Pharmacokinetics study (insulin concentrations in brain and plasma via different delivery routes); AUCbrain: plasma ratio; Repeated in insulin administration. | The study found intranasal delivery of insulin showed a 2000-fold increased AUCbrain: plasma ratio compared with subcutaneous administration, with no apparent effect on blood glucose levels. | [158] |
| Lixisenatide | N/A | N/A | Chronic unpredictable mild stress depression model (rats); Behavioral studies (forced swim test, tail suspension test, open field test); Cells were labeled with BrdU and neurogenesis in the olfactory bulb and hippocampus was observed. | Intranasal lixisenatide not only improved depressive and anxious behaviors in a chronic unpredictable mild stress model, but also improved olfactory system function. In addition, intranasal lixisenatide was demonstrated to play an antidepressant role by regulating cyclic-AMP response binding protein (CREB)-mediated neurogenesis. | [159] | |
| GLP-2 | PAS-CPPs-GLP-2 | N/A | Behavioral studies (forced swim test, tail suspension test, open field test); Distribution test (rats’ brain). | Studies have found that intranasal PAS-CPP-GLP-2 exhibited antidepressant effects similar to intracerebroventricular injection in mouse models, but not intravenous injection. | [56,160] | |
| BDNF | BDNF-HA2TAT/AAV | Each step was qualified by specific restriction enzyme reactions and AGE; High expression of BDNF in infected Hela cells. | Chronic unpredictable mild stress depression model (rats); Behavioral assessment (forced swim test, sucrose preference test, open field test); Body weight; Western-blotting analysis; Expression of BDNF mRNA. | Western-blotting analysis showed that the content of BDNF in the hippocampus increased via intranasal administration. Compared with the control group and the AVV group, the BDNF-HA2TAT/AAV group significantly reversed the depressive behavior of the rats. | [169,170] | |
| NAP | NT4-NAP/AAV | Each step was qualified by specific restriction enzyme reactions and AGE; Expression of BDNF in infected PC12 cells. | Behavioral assessment (forced swim test, sucrose preference test, open field test); Effect on plasma CORT; Expression of 5-HT and BNDF in hippocampus. | Experiments have shown that the depressive symptoms of female mice are improved after ten days of administration. Although the effect is not significant, it also proves that intranasal administration from different targets, such as microtubules, provide new ideas for the treatment of depression. | [171,172] | |
| NPY/LCG-17/MCH/CST-14/NGF | N/A | N/A | Behavioral assessment (forced swim test, sucrose preference test, open field test); Biochemical studies. | These peptides bypass the blood–brain barrier via a non-invasive intranasal route of administration, improving bioavailability and brain targeting. The peptides both improve anxiety and depression behavior in animal models. The peptides also promote neuroplasticity in the central nervous system, especially the hippocampus and prefrontal cortex. | [173,174,175,176] | |
| Natural active ingredients | Albiflorin | Alginate nanogels | PS = 45.6 ± 5.2 nm; PI < 0.20; ZP = −19.8 ± 0.9 mV; EE = ±7.15%; Release (12 h) = 99%; Gelling temperature = 28 °C. | In vivo fluorescence distribution analysis of alginate nanogels (rats); Pharmacodynamic study; Antidepressant behavioral studies: tail suspension test; Transcriptome studies: cAMP, calcium ion, and cGMP PKG signal pathway. | Fluorescent labeling showed that albiflorin could quickly reach the brain for distribution after intranasal administration (≤30 min). The authors observed through tail suspension experiments in mice that low-dose intranasal administration significantly shortened the chronic unpredictable mild stress model of mice compared with intragastric gavage and intravenous injection of albiflorin solution. Do not move time. The reduction of pro-inflammatory cytokine levels and the repair of neuronal damage in CUMS rats further suggest that albiflorin has an excellent potential for rapid antidepressant effects. | [186] |
| Berberine | Cyclodextrin + thermosensitive hydrogel | The berberine /HP-β-CD inclusion complex (1H-NMR-NMR showed good degree of inclusion); Gelling temperature = 30 °C; Release (6 h) = 83.29 ± 3.98%; Loading efficiency = 22.86%. | Brain targeting of berberine study (Radioactive tracer of 125I); Pharmacokinetic analysis: berberine in hippocampus; Monoamine neurotransmitters in rats (reserpine-induced model). | The relative intracerebral bioavailability of berberine showed that the intranasal formulation of berberine was 110 times higher than the oral inclusion complex of berberine–cyclodextrin. Pharmacological studies have found that the intranasal route, in addition to increasing the levels of monoamine neurotransmitters in the hippocampus compared with oral administration, exhibits a potential antidepressant mechanism by restoring sphingolipid and phospholipid abnormalities and mitochondrial dysfunction. | [190] | |
| Berberine and Evodiamine | Thermosensitive in situ hydrogels | P407/P188/HP-β-CD/PEG 8000 = 20/0/8/1; Release = 93% (berberine); Release = 43% (evodiamine); Gelling temperature = 28 °C. | Pharmacokinetic study (plasma and hippocampus); Antidepressant behavioral studies (open field test, tail suspension test); Monoamine neurotransmitters studies in rats. | The bioavailability of intranasal hydrogels was more than 135- and 112-fold higher than that of gavage berberine and evodiamine solutions. The intranasal formulation significantly improved behavioral despair by modulating monoamine levels and related metabolic pathways in mice. | [191] | |
| Cang-ai volatile oil | Intranasal inhaler | N/A | Chronic unpredictable mild stress depression model (rats); Behavioral studies (open field test, forced swim test, and sucrose preference test); Expression of pro-inflammatory cytokines and monoamine neurotransmitters studies in prefrontal cortex. | Studies have shown that Cang-ai volatile oil can inhibit microglia activation and kynurenine pathway to regulate 5-HT and play an antidepressant effect. The forced swim test, open field test, sucrose preference test, etc. confirmed that intranasal delivery of Cang-ai volatile oil can effectively regulate the metabolism of dopamine and 5-HT in the brain of CUMS rats and improve depressive behavior. | [192,193] | |
| Icariin | Nanogel loaded thermosensitive hydrogel (NGSTH) | PS = 73.80 ± 2.34 nm; PI < 0.15; ZP = −19.2 ± 1.14 mV; Loading efficiency = 2.03%; Release (36 h) = 70% (nanogel); Gelling temperature = 30 °C; Release (36 h) = 100% (NGSTH). | In vivo distribution fluorescently labeled nanogels Behavioral testing (tail suspension test, forced swim test); Expression of pro-inflammatory cytokines and morphological changes in the hippocampus. | ICA-NGSTH could be distributed in the brain in about half an hour and showed zero order kinetic release within 10 h. By comparing the oral route of ICA, intranasal ICA-NGSTH showed better behavior improvement ability in an animal model of depression. | [196] | |
| White tea | N/A | N/A | Chronic unpredictable mild stress depression model (rats); Behavioral testing (open-field test, sucrose preference test, buried food pellet test); Olfactory sensitivity test. | High and low levels of white tea extracts could effectively reverse depressive behavior in mice. Olfactory avoidance tests and olfactory sensitivity tests showed its relief of olfactory dysfunction. Pharmacological studies found that white tea reduced mitochondrial and synaptic damage in the olfactory bulb and enhanced the content of BDNF. | [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Han, Y.; Zhang, D.; Li, Z.; Jing, Y.; Hu, B.; Sun, S. Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients. Pharmaceutics 2022, 14, 2070. https://doi.org/10.3390/pharmaceutics14102070
Jin Z, Han Y, Zhang D, Li Z, Jing Y, Hu B, Sun S. Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients. Pharmaceutics. 2022; 14(10):2070. https://doi.org/10.3390/pharmaceutics14102070
Chicago/Turabian StyleJin, Zhiyu, Yu Han, Danshen Zhang, Zhongqiu Li, Yongshuai Jing, Beibei Hu, and Shiguo Sun. 2022. "Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients" Pharmaceutics 14, no. 10: 2070. https://doi.org/10.3390/pharmaceutics14102070
APA StyleJin, Z., Han, Y., Zhang, D., Li, Z., Jing, Y., Hu, B., & Sun, S. (2022). Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients. Pharmaceutics, 14(10), 2070. https://doi.org/10.3390/pharmaceutics14102070