Resina Draconis Particles Encapsulated in a Hyaluronic-Acid-Based Hydrogel to Treat Complex Burn Wounds
Abstract
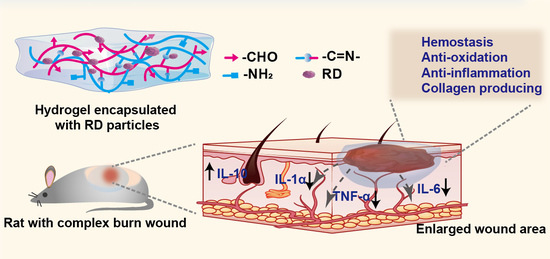
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physicochemical Properties of Resina Draconis
2.2.1. HPLC Conditions
2.2.2. Preparation of Standard Solution
2.2.3. Micronizing Resina Draconis and Characterizations of the Particles
2.3. Synthesis of OHA
2.4. Preparations of Blank Hydrogels and Resina-Draconis-Loaded Hydrogels
2.5. Characterizations of Hydrogels
2.5.1. Gelation and Physical Appearance of the Hydrogel
2.5.2. Injectable, Self-Healing Properties of the Hydrogel
2.5.3. Swelling Property of the Hydrogel
2.5.4. Rheological Characterizations
2.6. In Vitro Drug Release
2.7. The Hemocompatibility of the Hydrogel
2.8. Cell Cytotoxicity of the Hydrogel
2.9. Antioxidative Effect of the Hydrogel
2.10. Determination of Anti-Inflammatory Effect of the Hydrogel
2.11. Hemostatic Ability of the Hydrogel In Vivo
2.12. In Vitro Cell Migration Assays of the Hydrogel
2.13. Wound Healing Efficacy of the Hydrogel In Vivo
2.14. Histopathological Analysis
2.15. Statistical Analysis
3. Results and Discussions
3.1. Micronizing the Resin and Determination of the Content of Loureirin A and Loureirin B in Resina Draconis
3.2. Formation of RD-Loaded Hydrogels
3.3. Characterizations of Hydrogels
3.4. Rheological Characterizations
3.5. In Vitro Drug Release of the RD-Gel
3.6. In Vitro Biocompatibility Tests of RD-Gel
3.7. In Vitro Antioxidant Ability and Anti-Inflammatory Efficiency of RD-Gel
3.8. Hemostatic Effect of RD-Gel In Vivo
3.9. Migration-Promoting Effects of RD-Gel
3.10. Wound Healing Performance of Hydrogel In Vivo and Histological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evers, L.H.; Bhavsar, D.; Mailander, P. The biology of burn injury. Exp. Dermatol. 2010, 19, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Herndon, D.N.; Sidossis, L.S.; Borsheim, E. The impact of severe burns on skeletal muscle mitochondrial function. Burns 2013, 39, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Hop, M.J.; Polinder, S.; van der Vlies, C.H.; Middelkoop, E.; van Baar, M.E. Costs of burn care: A systematic review. Wound Repair Regen. 2014, 22, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Zhang, F.; Lineaweaver, W.C. History and Advancement of Burn Treatments. Ann. Plast. Surg. 2017, 78, S2–S8. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent trends on burn wound care: Hydrogel dressings and scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef]
- Nischwitz, S.P.; Luze, H.; Popp, D.; Winter, R.; Draschl, A.; Schellnegger, M.; Kargl, L.; Rappl, T.; Giretzlehner, M.; Kamolz, L.P. Global burn care and the ideal burn dressing reloaded—A survey of global experts. Burns 2021, 47, 1665–1674. [Google Scholar] [CrossRef]
- Seaman, S. Dressing selection in chronic wound management. J. Am. Podiatr. Med. Assoc. 2002, 92, 24–33. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Ousey, K.; Cutting, K.F.; Rogers, A.A.; Rippon, M.G. The importance of hydration in wound healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 122–130. [Google Scholar] [CrossRef]
- Karlsson, M.; Steinvall, I.; Sjoberg, F.; Olofsson, P.; Elmasry, M. Burn scar outcome at six and 12 months after injury in children with partial thickness scalds: Effects of dressing treatment. Burns 2020, 46, 546–551. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, Y.Q.; Lei, Y.; Gao, X.M.; Zhai, H.Q.; Lin, N.; Tang, S.H.; Liang, R.X.; Ma, Y.; Li, D.F.; et al. A Systems Biology-Based Approach to Uncovering the Molecular Mechanisms Underlying the Effects of Dragon’s Blood Tablet in Colitis, Involving the Integration of Chemical Analysis, ADME Prediction, and Network Pharmacology. PLoS ONE 2014, 9, e101432. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bleakley, B.; Gupta, R.K. Dragon’s blood: Botany, chemistry and therapeutic uses. J. Ethnopharmacol. 2008, 115, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.K.; Yi, H.S.; Yun, H.J.; Ko, C.H.; Choi, J.W.; Park, S.D. Ethylacetate extract from Draconis Resina inhibits LPS-induced inflammatory responses in vascular smooth muscle cells and macrophages via suppression of ROS production. Food Chem. Toxicol. 2010, 48, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhu, L.; Yi, T. Fingerprint analysis of Resina Draconis by ultra-performance liquid chromatography. Chem. Cent. J. 2017, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tiwari, S. A review on biomacromolecular hydrogel classification and its applications. Int. J. Biol. Macromol. 2020, 162, 737–747. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S.H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef]
- Mo, C.; Xiang, L.; Chen, Y. Advances in Injectable and Self-healing Polysaccharide Hydrogel Based on the Schiff Base Reaction. Macromol. Rapid Commun. 2021, 42, e2100025. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Yu, F.; Zhao, Y.X.; Mo, X.M.; Pan, J.F. In situ forming hydrogel of natural polysaccharides through Schiff base reaction for soft tissue adhesive and hemostasis. Int. J. Biol. Macromol. 2020, 147, 653–666. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [Green Version]
- Dunphy, J.E.; Udupa, K.N. Chemical and histochemical sequences in the normal healing of wounds. N. Engl. J. Med. 1955, 253, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Fazli, Y.; Shariatinia, Z. Controlled release of cefazolin sodium antibiotic drug from electrospun chitosan-polyethylene oxide nanofibrous Mats. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 641–652. [Google Scholar] [CrossRef]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014, 752, 10–24. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Li, L.; Fei, X.; Xu, L.; Wang, Y.; Tian, J.; Li, Y. A novel hydrogel with self-healing property and bactericidal activity. J. Colloid Interface Sci. 2021, 584, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, R.; Cao, N.; Sun, X.; Sui, Z.; Guo, Q. A systematical rheological study of polysaccharide from Sophora alopecuroides L. seeds. Carbohydr. Polym. 2018, 180, 63–71. [Google Scholar] [CrossRef]
- Okamoto, T.; Patil, A.J.; Nissinen, T.; Mann, S. Self-Assembly of Colloidal Nanocomposite Hydrogels Using 1D Cellulose Nanocrystals and 2D Exfoliated Organoclay Layers. Gels 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Portou, M.J.; Baker, D.; Abraham, D.; Tsui, J. The innate immune system, toll-like receptors and dermal wound healing: A review. Vascul. Pharmacol. 2015, 71, 31–36. [Google Scholar] [CrossRef]
- Denzinger, M.; Held, M.; Scheffler, H.; Haag, H.; Nussler, A.K.; Wendel, H.P.; Schlensak, C.; Daigeler, A.; Krajewski, S. Hemocompatibility of different burn wound dressings. Wound Repair Regen. 2019, 27, 470–476. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Moins-Teisserenc, H.; Cordeiro, D.J.; Audigier, V.; Ressaire, Q.; Benyamina, M.; Lambert, J.; Maki, G.; Homyrda, L.; Toubert, A.; Legrand, M. Severe Altered Immune Status After Burn Injury Is Associated With Bacterial Infection and Septic Shock. Front. Immunol. 2021, 12, 586195. [Google Scholar] [CrossRef] [PubMed]
- Cagiola, M.; Giulio, S.; Miriam, M.; Katia, F.; Paola, P.; Macri, A.; Pasquali, P. In vitro down regulation of proinflammatory cytokines induced by LPS tolerance in pig CD14+ cells. Vet. Immunol. Immunopathol. 2006, 112, 316–320. [Google Scholar] [CrossRef]
- Han, S.; Gao, H.; Chen, S.; Wang, Q.; Li, X.; Du, L.J.; Li, J.; Luo, Y.Y.; Li, J.X.; Zhao, L.C.; et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-kappaB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef]
- Hubner, G.; Brauchle, M.; Smola, H.; Madlener, M.; Fassler, R.; Werner, S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 1996, 8, 548–556. [Google Scholar] [CrossRef]
- Friedl, P.; Mayor, R. Tuning Collective Cell Migration by Cell-Cell Junction Regulation. Cold Spring Harb. Perspect. Biol. 2017, 9, a029199. [Google Scholar] [CrossRef]
- Traversa, B.; Sussman, G. The Role of Growth Factors, Cytokines and Proteases in Wound Management. Prim. Intent. Aust. J. Wound Manag. 2001, 9, 161–167. [Google Scholar]
- DiPietro, L.A. Wound healing: The role of the macrophage and other immune cells. Shock 1995, 4, 233–240. [Google Scholar] [CrossRef]
- Campos, A.C.; Groth, A.K.; Branco, A.B. Assessment and nutritional aspects of wound healing. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Arif, S.; Attiogbe, E.; Moulin, V.J. Granulation tissue myofibroblasts during normal and pathological skin healing: The interaction between their secretome and the microenvironment. Wound Repair Regen. 2021, 29, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Howdieshell, T.R.; Callaway, D.; Webb, W.L.; Gaines, M.D.; Procter, C.D., Jr.; Sathyanarayana; Pollock, J.S.; Brock, T.L.; McNeil, P.L. Antibody neutralization of vascular endothelial growth factor inhibits wound granulation tissue formation. J. Surg. Res. 2001, 96, 173–182. [Google Scholar] [CrossRef]
- Nagaraja, S.; Wallqvist, A.; Reifman, J.; Mitrophanov, A.Y. Computational approach to characterize causative factors and molecular indicators of chronic wound inflammation. J. Immunol. 2014, 192, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Zhou, Z.; Chen, Y.; Lu, H.; Hu, P. Resina Draconis Particles Encapsulated in a Hyaluronic-Acid-Based Hydrogel to Treat Complex Burn Wounds. Pharmaceutics 2022, 14, 2087. https://doi.org/10.3390/pharmaceutics14102087
Xu L, Zhou Z, Chen Y, Lu H, Hu P. Resina Draconis Particles Encapsulated in a Hyaluronic-Acid-Based Hydrogel to Treat Complex Burn Wounds. Pharmaceutics. 2022; 14(10):2087. https://doi.org/10.3390/pharmaceutics14102087
Chicago/Turabian StyleXu, Lijun, Ziqiang Zhou, Yuying Chen, Huangjie Lu, and Ping Hu. 2022. "Resina Draconis Particles Encapsulated in a Hyaluronic-Acid-Based Hydrogel to Treat Complex Burn Wounds" Pharmaceutics 14, no. 10: 2087. https://doi.org/10.3390/pharmaceutics14102087
APA StyleXu, L., Zhou, Z., Chen, Y., Lu, H., & Hu, P. (2022). Resina Draconis Particles Encapsulated in a Hyaluronic-Acid-Based Hydrogel to Treat Complex Burn Wounds. Pharmaceutics, 14(10), 2087. https://doi.org/10.3390/pharmaceutics14102087