Understanding the Multidimensional Effects of Polymorphism, Particle Size and Processing for D-Mannitol Powders
Abstract
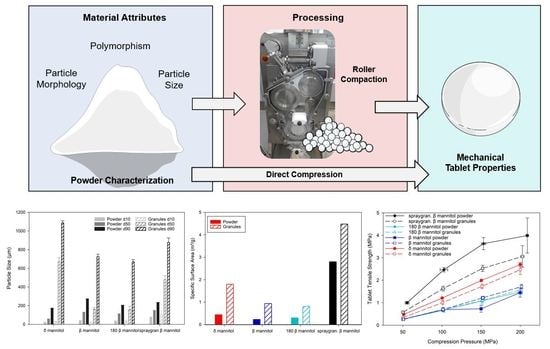
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Roller Compaction
2.2.2. Particle Size Distribution
2.2.3. Powder Densities
2.2.4. Flowability
2.2.5. Powder Compressibility
2.2.6. Specific Surface Area
2.2.7. Scanning Electron Microscopy
2.2.8. Tablet Compression
2.2.9. Tablet Characterization
Tablet Tensile Strength
Tablet Solid Fraction
Elastic Recovery
Heckel Yield Pressure
3. Results and Discussion
3.1. Impact of Polymorphism on Mechanical Material Characteristics
3.1.1. Powder and Granule Characterization
3.1.2. Mechanical Characterization
3.2. Impact of Particle Size on Mechanical Material Characteristics
3.2.1. Powder and Granule Characterization
3.2.2. Mechanical Characterization
3.3. Impact of Morphology and Preprocessing of Mannitol on Mechanical Material Characteristics
3.3.1. Powder and Granule Characterization
3.3.2. Mechanical Characterization
3.4. Direct Compression vs. Roller Compaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caira, M.R. Crystalline Polymorphism of Organic Compounds. In Design of Organic Solids. Topics in Current Chemistry; Weber, E., Ed.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Haleblian, J.; McCrone, W. Pharmaceutical Applications of Polymorphism. J. Pharm. Sci. 1969, 58, 911. [Google Scholar] [CrossRef] [PubMed]
- Hary, G. Brittain Polymorphism in Pharmaceutical Solids, 2nd ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2009; ISBN 9781420073218. [Google Scholar]
- Hilfiker, R.; von Raumer, M. Polymorphism in the Pharmaceutical Industry: Solid Form and Drug Development; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019. [Google Scholar]
- Traini, D.; Young, P.M.; Thielmann, F.; Acharya, M. The Influence of Lactose Pseudopolymorphic Form on Salbutamol Sulfate—Lactose Interactions in DPI Formulations. Drug Dev. Ind. Pharm. 2008, 34, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Joiris, E.; Di Martino, P.; Berneron, C.; Guyot-Hermann, A.-M.; Guyot, J.-C. Compression Behavior of Orthorombic Paracetamol. Pharm. Res. 1998, 15, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- International Conference on Harmonization ICH Topic Q 6 A Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances 2000. Available online: https://www.ema.europa.eu/en/ich-q6a-specifications-test-procedures-acceptance-criteria-new-drug-substances-new-drug-products%20 (accessed on 14 March 2022).
- Council of Europe European Pharmacopoeia, 10.0 (2017) 5.15. Functionality-Related Characteristics of Excipients. 793–794. Available online: https://pheur.edqm.eu/app/10-8/content/10-8/51500E.htm?highlight=on&terms=5.15 (accessed on 14 March 2022).
- Council of Europe European Pharmacopoeia. 2021, 10.4, 0316 Cellulosum Microcristallinum, 5446–5450. Available online: https://pheur.edqm.eu/app/10-8/content/10-8/0316E.htm?highlight=on&terms=0316&terms=0316 (accessed on 14 March 2022).
- Council of Europe European Pharmacopoeia 2021, 10.3, 0187 Lactosum Monohydricum, 5049–5050. Available online: https://pheur.edqm.eu/app/10-8/content/10-8/0187E.htm%20 (accessed on 14 March 2022).
- Lee, T.; Chang, G. Da Sucrose Conformational Polymorphism: A Jigsaw Puzzle withMultiple Routes to a Unique Solution. Cryst. Growth Des. 2009, 9, 3551–3561. [Google Scholar] [CrossRef]
- Kirk, J.H.; Dann, S.E.; Blatchford, C.G. Lactose: A definitive guide to polymorph determination. Int. J. Pharm. 2007, 334, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Pisklak, D.M.; Zielinska-Pisklak, M.A.; Szeleszczuk, Ł.; Wawer, I. C solid-state NMR analysis of the most common pharmaceutical excipients used in solid drug formulations, Part I: Chemical shifts assignment. J. Pharm. Biomed. Anal. 2016, 122, 81–89. [Google Scholar] [CrossRef]
- Dupont, A.; Guerain, M.; Danède, F.; Paccou, L.; Guinet, Y.; Hédoux, A.; Willart, J.F. Kinetics and mechanism of polymorphic transformation of sorbitol under mechanical milling. Int. J. Pharm. 2020, 590, 119902. [Google Scholar] [CrossRef]
- Ohrem, H.L.; Schornick, E.; Kalivoda, A.; Ognibene, R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm. Dev. Technol. 2014, 19, 257–262. [Google Scholar] [CrossRef]
- Burger, A.; Henck, J.O.; Hetz, S.; Rollinger, J.M.; Weissnicht, A.A.; Stöttner, H. Energy/temperature diagram and compression behavior of the polymorphs of D-mannitol. J. Pharm. Sci. 2000, 89, 457–468. [Google Scholar] [CrossRef]
- Yoshinari, T.; Forbes, R.T.; York, P.; Kawashima, Y. The improved compaction properties of mannitol after a moisture-induced polymorphic transition. Int. J. Pharm. 2003, 258, 121–131. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Almey, R.; De Beer, T.; Vervaet, C. Delta-mannitol to enable continuous twin-screw granulation of a highly dosed, poorly compactable formulation. Int. J. Pharm. 2020, 583, 119374. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorne, V.; Bekaert, B.; Peeters, E.; De Beer, T.; Remon, J.P.; Vervaet, C. Improved tabletability after a polymorphic transition of delta-mannitol during twin screw granulation. Int. J. Pharm. 2016, 506, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, C.M.; Pein, M.; Breitkreutz, J. Roll compaction of granulated mannitol grades and the unprocessed crystalline delta-polymorph. Powder Technol. 2015, 270, 470–475. [Google Scholar] [CrossRef]
- Wagner, C.M.; Pein, M.; Breitkreutz, J. Roll compaction of mannitol: Compactability study of crystalline and spray-dried grades. Int. J. Pharm. 2013, 453, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Leane, M.; Pitt, K.; Reynolds, G.; Anwar, J.; Charlton, S.; Crean, A.; Creekmore, R.; Davies, C.; DeBeer, T.; De-Matas, M.; et al. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm. Dev. Technol. 2015, 20, 12–21. [Google Scholar] [CrossRef]
- Jenike, A.W. Storage and flow of solids. Bull. No.123 Utah Eng. Exp. Stn. 1964, 53, 1–198. [Google Scholar] [CrossRef] [Green Version]
- Freeman, R. Measuring the flow properties of consolidated, conditioned and aerated powders—A comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007, 174, 25–33. [Google Scholar] [CrossRef]
- Escotet-espinoza, M.S.; Moghtadernejad, S.; Scicolone, J.; Wang, Y.; Pereira, G.; Schäfer, E.; Vigh, T.; Klingeleers, D.; Ierapetritou, M.; Muzzio, F.J. Using a material property library to find surrogate materials for pharmaceutical process development. Powder Technol. 2018, 339, 659–676. [Google Scholar] [CrossRef]
- Van Snick, B.; Dhondt, J.; Pandelaere, K.; Bertels, J.; Mertens, R.; Klingeleers, D.; Di, G.; Paul, J.; Vervaet, C.; Beer, T. De A multivariate raw material property database to facilitate drug product development and enable in-silico design of pharmaceutical dry powder processes. Int. J. Pharm. 2018, 549, 415–435. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Thielmann, F.; Burnett, D.J.; Heng, J.Y.Y. Determination of the Surface Energy Distributions of Different Processed Lactose Determination of the Surface Energy Distributions. Drug Dev. Ind. Pharm. 2007, 33, 1240–1253. [Google Scholar] [CrossRef]
- Fell, J.T.; Newton, J.M. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Marshall, K. Use of a compaction simulator system in tabletting research. Drug Dev. Ind. Pharm. 1989, 15, 759–800. [Google Scholar] [CrossRef]
- He, X.; Secreast, P.J.; Amidon, G.E. Mechanistic Study of the Effect of Roller Compaction and Lubricant on Tablet Mechanical Strength. J. Pharm. Sci. 2007, 96, 1342–1355. [Google Scholar] [CrossRef] [PubMed]
- Herting, M.G.; Kleinebudde, P. Studies on the reduction of tensile strength of tablets after roll compaction/dry granulation. Eur. J. Pharm. Sci. 2008, 70, 372–379. [Google Scholar] [CrossRef]
- Tay, J.Y.S.; Han, Q.E.; Liew, C.V.; Sia Heng, P.W. Investigation on the effect of roller compaction on paracetamol. Pharm. Dev. Technol. 2020, 25, 100–106. [Google Scholar] [CrossRef]
- Nordström, J.; Klevan, I.; Alderborn, G. A protocol for the classification of powder compression characteristics. Eur. J. Pharm. Biopharm. 2012, 80, 209–216. [Google Scholar] [CrossRef]
- Sonnergaard, J.M. Investigation of a new mathematical model for compression of pharmaceutical powders. Eur. J. Pharm. Sci. 2001, 14, 149–157. [Google Scholar] [CrossRef]
- Wu, S.; Sun, C.C. Insensitivity of Compaction Properties of Brittle Granules to Size Enlargement by Roller Compaction. J. Pharm. Sci. 2007, 96, 1445–1450. [Google Scholar] [CrossRef]
- Klevan, I.; Nordström, J.; Tho, I.; Alderborn, G. A statistical approach to evaluate the potential use of compression parameters for classification of pharmaceutical powder materials. Eur. J. Pharm. Biopharm. 2010, 75, 425–435. [Google Scholar] [CrossRef]
- Sun, C.C. Decoding powder tabletability: Roles of particle adhesion and plasticity. J. Adhes. Sci. Technol. 2011, 25, 483–499. [Google Scholar] [CrossRef]
- Osei-yeboah, F.; Chang, S.; Sun, C.C. A critical Examination of the Phenomenon of Bonding Area—Bonding Strength Interplay in Powder Tableting. Pharm. Res. 2016, 33, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Malkowska, S.; Khan, K.A. Effect of re-compression on the properties of tablets prepared by dry granulation. Drug Dev. Ind. Pharm. 1983, 9, 331–347. [Google Scholar] [CrossRef]
- Patel, S.; Dahiya, S.; Sun, C.C.; Bansal, A.K. Understanding Size Enlargement and Hardening of Granules on Tabletability of Unlubricated Granules Prepared by Dry Granulation. J. Pharm. Sci. 2011, 100, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.; Kleinebudde, P. Mini review: Mechanisms to the loss of tabletability by dry granulation. Eur. J. Pharm. Biopharm. 2016, 106, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hardman, J.S.; Lilley, B.A. Mechanisms of compaction of powdered materials. Proc. R. Soc. London. A. Math. Phys. Sci. 1973, 333, 183–199. [Google Scholar] [CrossRef]
- Freitag, F.; Kleinebudde, P. How do roll compaction/dry granulation affect the tableting behaviour of inorganic materials? Comparison of four magnesium carbonates. Eur. J. Pharm. Sci. 2003, 19, 281–289. [Google Scholar] [CrossRef]
- Santl, M.; Ilić, I.; Vrecer, F.; Baumgartner, S. A compressibility and compactibility study of real tableting mixtures: The impact of wet and dry granulation versus a direct tableting mixture. Int. J. Pharm. 2011, 414, 131–139. [Google Scholar] [CrossRef]
- Nordström, J.; Alderborn, G. The Granule Porosity Controls the Loss of Compactibility for Both Dry- and Wet-Processed Cellulose Granules but at Different Rate. J. Pharm. Sci. 2015, 104, 2029–2039. [Google Scholar] [CrossRef]
- Mosig, J.; Kleinebudde, P. Critical Evaluation of Root Causes of the Reduced Compactability after Roll Compaction/Dry Granulation. J. Pharm. Sci. 2015, 104, 1108–1118. [Google Scholar] [CrossRef]
- Herting, M.G.; Kleinebudde, P. Roll compaction / dry granulation: Effect of raw material particle size on granule and tablet properties. Int. J. Pharm. 2007, 338, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Grote, S.; Kleinebudde, P. Roll Compaction/Dry Granulation of Dibasic Calcium Phosphate Anhydrous—Does the Morphology of the Raw Material Influence the Tabletability of Dry Granules? J. Pharm. Sci. 2018, 107, 1104–1111. [Google Scholar] [CrossRef] [PubMed]









| Polymorph | Particle Size Distribution (µm) | Density (g/mL) | ffc | SSA (m²/g) | ||||
|---|---|---|---|---|---|---|---|---|
| d10 | d50 | d90 | ρb | ρt | ||||
| Powder | δ mannitol | 22 ± 0 | 57 ± 1 | 171 ± 2 | 0.493 ± 0.007 | 0.658 ± 0.001 | 6.21 ± 0.19 | 0.44 |
| β mannitol | 37 ± 3 | 127 ± 4 | 271 ± 3 | 0.598 ± 0.004 | 0.744 ± 0.004 | 8.40 ± 0.24 | 0.23 | |
| 180 β mannitol | 36 ± 2 | 110 ± 3 | 205 ± 1 | 0.565 ± 0.001 | 0.726 ± 0.003 | 6.56 ± 0.16 | 0.30 | |
| spray granulated β mannitol | 73 ± 5 | 149 ± 1 | 233 ± 1 | 0.542 ± 0.003 | 0.608 ± 0.003 | 37.33 ± 8.16 | 2.80 | |
| Granules | δ mannitol | 31 ± 3 | 671 ± 42 | 1087 ± 22 | 0.613 ± 0.005 | 0.776 ± 0.009 | 5.31 ± 0.22 | 1.80 |
| β mannitol | 22 ± 0 | 164 ± 18 | 725 ± 26 | 0.649 ± 0.016 | 0.799 ± 0.006 | 4.94 ± 0.20 | 0.94 | |
| 180 β mannitol | 32 ± 9 | 160 ± 34 | 671 ± 22 | 0.631 ± 004 | 0.791 ± 0.007 | 5.43 ± 0.58 | 0.81 | |
| spray granulated β mannitol | 107 ± 38 | 480 ± 41 | 879 ± 47 | 0.544 ± 0.009 | 0.647 ± 0.004 | 7.31 ± 0.16 | 4.48 | |
| Compression Pressure (kPa) | Compressibility (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Powder | Granules | |||||||
| δ man. | β man. | 180 β man. | Spray Gran β man. | δ man. | β man. | 180 β man. | Spray Gran β man. | |
| 0.5 | 7.3 ± 3.0 | 3.3 ± 0.5 | 4.3 ± 0.4 | 1.2 ± 0.1 | 4.2 ± 0.3 | 3.8 ± 0.1 | 5.2 ± 2.0 | 3.4 ± 0.3 |
| 1 | 8.5 ± 2.8 | 4.8 ± 0.4 | 5.9 ± 0.4 | 1.4 ± 0.1 | 5.5 ± 0.3 | 5.2 ± 0.1 | 6.4 ± 2.0 | 4.7 ± 0.2 |
| 2 | 13.5 ± 2.4 | 7.7 ± 0.4 | 9.5 ± 0.5 | 1.9 ± 0.1 | 8.2 ± 0.4 | 8.0 ± 0.2 | 9.2 ± 2.1 | 6.9 ± 0.1 |
| 4 | 18.0 ± 2.4 | 10.4 ± 0.4 | 12.9 ± 0.6 | 2.5 ± 0.2 | 11.0 ± 0.7 | 11.1 ± 0.2 | 12.3 ± 2.2 | 9.4 ± 0.2 |
| 6 | 20.3 ± 2.5 | 11.7 ± 0.5 | 14.5 ± 0.6 | 2.8 ± 0.2 | 12.6 ± 0.8 | 12.7 ± 0.2 | 13.9 ± 2.2 | 10.8 ± 0.2 |
| 8 | 21.8 ± 2.5 | 12.7 ± 0.5 | 15.7 ± 0.6 | 3.2 ± 0.2 | 13.6 ± 0.9 | 13.9 ± 0.1 | 15.2 ± 2.2 | 11.7 ± 0.3 |
| 10 | 22.8 ± 2.5 | 13.5 ± 0.4 | 16.7 ± 0.6 | 3.4 ± 0.2 | 14.5 ± 1.0 | 14.9 ± 0.1 | 16.1 ± 2.3 | 12.5 ± 0.3 |
| 12 | 23.7 ± 2.5 | 14.2 ± 0.4 | 17.5 ± 0.5 | 3.7 ± 0.3 | 15.2 ± 1.0 | 15.7 ± 0.2 | 16.9 ± 2.3 | 13.2 ± 0.3 |
| 15 | 24.8 ± 2.5 | 15.0 ± 0.5 | 18.4 ± 0.5 | 4.0 ± 0.3 | 16.2 ± 1.1 | 16.7 ± 0.1 | 18.0 ± 2.3 | 14.0 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mareczek, L.; Riehl, C.; Harms, M.; Reichl, S. Understanding the Multidimensional Effects of Polymorphism, Particle Size and Processing for D-Mannitol Powders. Pharmaceutics 2022, 14, 2128. https://doi.org/10.3390/pharmaceutics14102128
Mareczek L, Riehl C, Harms M, Reichl S. Understanding the Multidimensional Effects of Polymorphism, Particle Size and Processing for D-Mannitol Powders. Pharmaceutics. 2022; 14(10):2128. https://doi.org/10.3390/pharmaceutics14102128
Chicago/Turabian StyleMareczek, Lena, Carolin Riehl, Meike Harms, and Stephan Reichl. 2022. "Understanding the Multidimensional Effects of Polymorphism, Particle Size and Processing for D-Mannitol Powders" Pharmaceutics 14, no. 10: 2128. https://doi.org/10.3390/pharmaceutics14102128
APA StyleMareczek, L., Riehl, C., Harms, M., & Reichl, S. (2022). Understanding the Multidimensional Effects of Polymorphism, Particle Size and Processing for D-Mannitol Powders. Pharmaceutics, 14(10), 2128. https://doi.org/10.3390/pharmaceutics14102128