Antioxidant, Antimicrobial, and Kinetic Studies of Β-Cyclodextrin Crosslinked with Lignin for Drug Delivery
Abstract
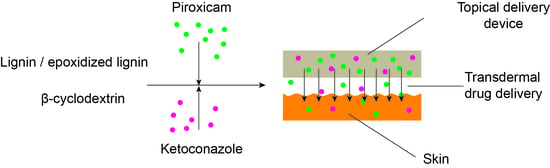
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lignin Modification
2.3. Loading of Drugs into LCD Matrix
2.4. Obtaining of Materials
2.5. Fourier Transform Infrared Spectroscopy (Ftir)
2.6. Diametral Tensile Measurements
2.7. Dynamic Vapor Sorption (Dvs)
2.8. In Vitro Release Studies
2.9. Anti-Inflammatory Activity
2.10. Antimicrobial Activity
2.11. Antioxidant Activity (DPPH Assay)
3. Results and Discussions
3.1. FTIR Analysis of Materials
3.2. Mechanical Properties
3.3. Dynamic Vapor Sorption (DVS)
3.4. In Vitro Release Studies
3.5. Anti-Inflammatory Activity
3.6. Antimicrobial Activity
3.7. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, J.; Wittmann, C. A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef]
- Li, Q.; Xie, S.; Serem, W.K.; Naik, M.T.; Liu, L.; Yuan, J.S. Quality carbon fibers from fractionated lignin. Green Chem. 2017, 19, 1628–1634. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Agrawal, R.; Satlewal, A.; Kumar, R.; Gupta, R.P.; Ramakumar, S.S.V. Next generation applications of lignin derived commodity products, their life cycle, techno-economics and societal analysis. Int. J. Biol. Macromol. 2022, 197, 179–200. [Google Scholar] [CrossRef]
- Caravaca, A.; Garcia-Lorefice, W.E.; Gil, S.; de Lucas-Consuegra, A.; Vernoux, P. Towards a sustainable technology for H2 production: Direct lignin electrolysis in a continuous-flow polymer electrolyte membrane reactor. Electrochem. Commun. 2019, 100, 43–47. [Google Scholar] [CrossRef]
- Spiridon, I. Extraction of lignin and therapeutic applications of lignin-derived compounds. A Review. Environ. Chem. Lett. 2020, 18, 771–785. [Google Scholar] [CrossRef]
- Anghel, N.; Dinu, V.M.; Verestiuc, L.; Spiridon, I.A. Transcutaneous drug delivery systems based on collagen/polyurethane composites reinforced with cellulose. Polymers 2021, 13, 1845. [Google Scholar] [CrossRef]
- Azadfar, M.; Gao, A.H.; Chen, S. Structural characterization of lignin: A potential source of antioxidants guaiacol and 4-vinylguaiacol. Int. J. Biol. Macromol. 2015, 75, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, X.; Yao, X.; Fang, Y.; Chen, H.; Ji, H. β-Cyclodextrin grafted on lignin as inverse phase transfer catalyst for the oxidation of benzyl alcohol in H2O. Tetrahedron 2016, 72, 1773–1781. [Google Scholar] [CrossRef]
- Paczkowska, M.; McDonagh, A.F.; Bialek, K.; Tajber, L.; Cielecka-Piontek, J. Mechanochemical activation with Cyclodextrins followed by compaction as an effective approach to improving dissolution of Rutin. Int. J. Pharm. 2020, 581, 119294. [Google Scholar] [CrossRef]
- Yan, T.; Ji, M.; Sun, Y.; Yan, T.; Zhao, J.; Zhang, H.; Wang, Z. Preparation and characterization of baicalein/hydroxypropyl-β-Cyclodextrin inclusion complex for enhancement of solubility, antioxidant activity and antibacterial activity using supercritical antisolvent technology. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 285–295. [Google Scholar] [CrossRef]
- Franco, P.; de Marco, I. Preparation of non-steroidal anti-inflammatory drug/β-Cyclodextrin inclusion complexes by supercritical antisolvent process. J. CO2 Utilization. 2021, 44, 101397. [Google Scholar] [CrossRef]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose-lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C. 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Culebras, M.; Barrett, A.; Pishnamazi, M.; Walker, G.M.; Collins, M.N. Wood-derived hydrogels as a platform for drug-release systems. ACS Sustain. Chem. Eng. 2021, 9, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Tanase, C.E. Design, characterization and preliminary biological evaluation of new lignin-PLA biocomposites. Int. J. Biol. Macromol. 2018, 114, 855–863. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.; Crudu, A.; Rosu, D.; Bele, A. Ecofriendly wet–white leather vs. Conventional tanned wet–blue leather. A photochemical approach. J. Clean Prod. 2018, 177, 708–720. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. In Vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jan, S.; Khan, M.R.; Rashid, U.; Bokhari, J. Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public Health Res. Perspect. 2013, 4, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Alriols, M.G.; García, A.; Llano-ponte, R.; Labidi, J. Combined organosolv and ultrafiltration lignocellulosic biorefinery process. Chem. Eng. J. 2010, 157, 113–120. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Sun, L.; Yuan, Q.; Cheng, G.; Argyropoulos, D.S. Extraction and characterization of lignin from corncob residue after acid-catalyzed steam explosion pretreatment. Ind. Crops. Prod. 2019, 133, 241–249. [Google Scholar] [CrossRef]
- Dimofte, A.; Dinu, M.V.; Anghel, N.; Doroftei, F.; Spiridon, I. Xanthan and alginate-matrix used as transdermal delivery carrier for Piroxicam and Ketoconazole. Int. J. Biol. Macromol. 2022, 209, 2084–2096. [Google Scholar] [CrossRef]
- Suihko, E.; Korhonen, O.; Järvinen, T.; Ketolainen, J.; Jarho, P.; Laine, E.; Paronen, P. Complexation with Tolbutamide modifies the physicochemical and tableting properties of Hydroxypropyl-β-Cyclodextrin. Int. J. Pharm. 2001, 215, 137–145. [Google Scholar] [CrossRef]
- Eichie, F.E.; Kudehinbu, A.O. Effect of particle size of granules on some mechanical properties of Paracetamol tablets. Afr. J. Biotechnol. 2009, 8, 5913–5916. [Google Scholar] [CrossRef]
- van den Mooter, G.; Wuyts, M.; Blaton, N.; Busson, R.; Grobet, P.; Augustijns, P.; Kinget, R. Physical stabilisation of amorphous Ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur. J. Pharm. Sci. 2001, 12, 261–269. [Google Scholar] [CrossRef]
- Hirakawa, Y.; Ueda, H.; Takata, Y.; Minamihata, K.; Wakabayashi, R.; Kamiya, N.; Goto, M. Co-amorphous formation of Piroxicam-citric acid to generate supersaturation and improve skin permeation. Eur. J. Pharm. Sci. 2021, 158, 105667. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.L.; Seaton, N.A.; Day, M.A. Adsorption-based method for the characterization of pore networks containing both mesopores and macropores. Langmuir 1999, 15, 6728–6737. [Google Scholar] [CrossRef]
- Moriasi, G.; Ireri, A.; Ngugi, M. Cognitive-enhancing, Ex Vivo antilipid peroxidation and qualitative phytochemical evaluation of the aqueous and methanolic stem bark extracts of Lonchocarpus eriocalyx (Harms.). Biochem. Res. Int. 2020, 2020, 8819045. [Google Scholar] [CrossRef] [PubMed]
- Luis Espinoza-Acosta, J.; Torres-Chávez, P.I.; Ramírez-Wong, B.; María López-Saiz, C.; Montaño-Leyva, B. Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. BioResources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to Ethanol production. Ind. Crops. Prod. 2011, 34, 1629–1634. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J.; et al. Antimicrobial lignin-based Polyurethane/Ag composite foams for improving wound healing. Biomacromolecules 2022, 23, 1622–1632. [Google Scholar] [CrossRef]
- Rahouti, M.; Steiman, R.; Seigle-Murandi, F.; Christov, L.P. Growth of 1044 strains and species of fungi on 7 phenolic lignin model compounds. Chemosphere 1999, 38, 2549–2559. [Google Scholar] [CrossRef]
- Kaur, R.; Uppal, S.K.; Sharma, P. Antioxidant and antibacterial activities of sugarcane bagasse lignin and chemically modified lignins. Sugar. Tech. 2017, 19, 675–680. [Google Scholar] [CrossRef]
- Rocca, D.M.; Vanegas, J.P.; Fournier, K.; Becerra, M.C.; Scaiano, J.C.; Lanterna, A.E. Biocompatibility and photo-induced antibacterial activity of lignin-stabilized noble metal nanoparticles. RSC Adv. 2018, 8, 40454–40463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiridon, I.; Andrei, I.M.; Anghel, N.; Dinu, M.V.; Ciubotaru, B.I. Development and characterization of novel cellulose composites obtained in 1-Ethyl-3-Methylimidazolium Chloride used as drug delivery systems. Polymers 2021, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef]
- Lourençon, T.V.; de Lima, G.G.; Ribeiro, C.S.P.; Hansel, F.A.; Maciel, G.M.; da Silva, K.; Winnischofer, S.M.B.; de Muniz, G.I.B.; Magalhães, W.L.E. Antioxidant, antibacterial and antitumoural activities of kraft lignin from hardwood fractionated by acid precipitation. Int. J. Biol. Macromol. 2021, 166, 1535–1542. [Google Scholar] [CrossRef]
- Aadil, K.R.; Barapatre, A.; Sahu, S.; Jha, H.; Tiwary, B.N. Free radical scavenging activity and reducing power of Acacia nilotica wood lignin. Int. J. Biol. Macromol. 2014, 67, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Smith, C.; Arnstein, H.R.; Halliwell, B.; Cannon, M. The antioxidant action of Ketoconazole and related Azoles: Comparison with Tamoxifen and cholesterol. Chem. Biol. Interact. 1991, 79, 229–243. [Google Scholar] [CrossRef]
- Tongul, B.; Tarhan, L. Oxidant and antioxidant status in Saccharomyces cerevisiae exposed to antifungal Ketoconazole. Process Biochem. 2016, 51, 1984–1991. [Google Scholar] [CrossRef]






| Material | Condensation Index |
|---|---|
| LIG | 0.5167 |
| Lep | 0.5215 |
| LCD | 0.6164 |
| Sample | Sorption Capacity, % d.b. | rpm, nm | BET | |
|---|---|---|---|---|
| Area (S), m2 × g−1 | Monolayer (Wm), g × g−1 | |||
| LepCD | 20.0 | 1.44 | 279 | 0.0795 |
| LepCD-P | 10.5 | 1.18 | 178 | 0.0508 |
| LepCD-K | 14.5 | 1.09 | 268 | 0.0765 |
| LCD | 17.7 | 2.64 | 134.5 | 0.0390 |
| LCD-P | 15.0 | 2.32 | 108.9 | 0.0310 |
| LCD-K | 15.4 | 1.87 | 165.2 | 0.0470 |
| Sample | Korsmeyer–Peppas | ||
|---|---|---|---|
| k, min−n | n | R2 | |
| LepCD-P | 1.117 | 0.642 | 0.982 |
| LepCD-K | 1.789 | 0.298 | 0.979 |
| LCD-K | 2.210 | 0.446 | 0.993 |
| LCD-P | 4.824 | 0.206 | 0.998 |
| Material | Anti-Inflammatory Activity, % ± SD |
|---|---|
| LepCD-P | 57.25 ± 3.987 |
| LCD-P | 78.73 ± 2.764 |
| Sample | Inhibition, % ± SD |
|---|---|
| LIG | 42.06 ± 1.075825 |
| Lep | 26.33 ± 2.540643 |
| LepCD | 11.82 ± 2.995274 |
| LepCD-K | 55.39 ± 4.138269 |
| LepCD-P | 13.82 ± 1.417604 |
| LCD-K | 14.73 ± 3.538766 |
| LCD-P | 47.65 ± 3.274945 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anghel, N.; Melinte, V.; Spiridon, I.; Pertea, M. Antioxidant, Antimicrobial, and Kinetic Studies of Β-Cyclodextrin Crosslinked with Lignin for Drug Delivery. Pharmaceutics 2022, 14, 2260. https://doi.org/10.3390/pharmaceutics14112260
Anghel N, Melinte V, Spiridon I, Pertea M. Antioxidant, Antimicrobial, and Kinetic Studies of Β-Cyclodextrin Crosslinked with Lignin for Drug Delivery. Pharmaceutics. 2022; 14(11):2260. https://doi.org/10.3390/pharmaceutics14112260
Chicago/Turabian StyleAnghel, Narcis, Violeta Melinte, Iuliana Spiridon, and Mihaela Pertea. 2022. "Antioxidant, Antimicrobial, and Kinetic Studies of Β-Cyclodextrin Crosslinked with Lignin for Drug Delivery" Pharmaceutics 14, no. 11: 2260. https://doi.org/10.3390/pharmaceutics14112260
APA StyleAnghel, N., Melinte, V., Spiridon, I., & Pertea, M. (2022). Antioxidant, Antimicrobial, and Kinetic Studies of Β-Cyclodextrin Crosslinked with Lignin for Drug Delivery. Pharmaceutics, 14(11), 2260. https://doi.org/10.3390/pharmaceutics14112260