1. Introduction
In recent years, the number of highly active pharmaceutical ingredients (HPAPIs) has significantly increased, resulting in a high interest of pharmaceutical companies in the safe handling of these hazardous compounds [
1,
2,
3]. For example, in oncological therapy, HPAPIs are commonly used as cytostatics or peptides. Hormones and antibiotics are other examples of high potency compounds with a high potential for hazard exposure to the personnel present in the intermediate environment. Therefore, measures must be taken regarding the technical design of the facilities and the equipment for handling HPAPIs [
3,
4,
5].
The potential hazard exposures may lead to a health risk for the operators, as well as to a contamination of the outdoor environment and to cross-contaminations during manufacturing or other production processes within the facility. Therefore, the generation of dust, gases, or vapors is an important issue in the processing of dosage forms containing HPAPIs. The application of suitable containment equipment and, in some cases, additional personal protective equipment, is essential to ensure safe handling [
6,
7]. To guarantee the safe manufacture of dosage forms containing HPAPIs, special production setups are applied which are operated at a negative pressure to reduce the risk of potential airborne transfer to the immediate environment. In addition, barrier isolators, special transfer and valve systems, and high performance filters are further governance methods to reduce the risk of hazards [
3,
8].
Generally, the dustiness of formulations containing HPAPIs is a major problem concerning potential exposure. Thus, the minimization or elimination of any potential exposure is a common safety measure in the production of dosage forms containing HPAPIs [
3,
9,
10].
Dustiness is defined as the propensity of a material to generate airborne particles during its handling [
11]. Various processes in pharmaceutical manufacturing, such as processing, milling, grinding, and mixing, are associated with the generation of dust and, consequently, dustiness is a major risk of potential hazard exposure. Surprisingly, the number of studies on the dustiness of pharmaceutical powders is rather low, compared to that on the dustiness of non-pharmaceutical powders. The dustiness of a pharmaceutical powder is influenced by many factors, especially by the physicochemical and mechanical properties of the material and the method of dust determination. Therefore, the measurement of the dustiness of pharmaceutical powders is of particular importance for the assessment of the hazard exposure in pharmaceutical facilities [
9,
12,
13,
14,
15,
16]. For this reason, various systems and methods were developed to measure the dustiness of solid substances in a reproducible manner. Most of these systems are not designed for the measurements of pharmaceutical powders because the required substance quantities for these measurements are relatively high. Accordingly, because of the typically scarcely available quantities of HPAPIs, the measurements are limited and relatively expensive. The compounds used in the pharmaceutical production are exposed to a wide variety of stresses. Thus, no single test system may reproduce all conditions during handling [
17,
18,
19,
20,
21,
22,
23].
The atomization of powders results in an airborne particle distribution and is achieved by the application of energy. Therefore, atomization may be obtained by using a mechanical stimulus or by the dispersion of the pharmaceutical powder in the air. The application of too much energy may result in a fragmentation of single particles and possibly, in a greater dustiness. Each method for the determination of the dustiness includes a different technique for atomization. Thus, a comparability of the different methods for determination of the dustiness is nearly unimaginable because of the complexity of the different measurement processes. Moreover, an accurate prediction of the dustiness based on material properties such as particle size and density is not possible. However, the particle size distribution, particle shape, bulk density, humidity, and the cohesive and adhesive forces of powder particles have a major influence on the resulting dustiness. A reproducible dust generation requires a standardized method, and thus detailed specifications, regarding test duration, type, intensity of the mechanical stimulus, and the amount of test material are necessary. Therefore, a high standardization with relation to industrial manufacturing processes is essential for suitable dustiness measurements [
9,
11,
19,
24,
25,
26,
27,
28].
The assessment of containment equipment and the evaluation of systems and devices for dustiness measurements should be performed using safe surrogate substances. These substances, such as lactose, naproxen sodium, mannitol, acetaminophen, insulin, riboflavin, and sucrose, along with additional information regarding such measurements, are listed in the Good Practice Guide of the International Society for Pharmaceutical Engineering (ISPE) [
6]. The choice of the surrogates should not only depend on the physicochemical and mechanical substance properties, but also on the conditions of the analytical quantification [
29].
The applications of computational fluid dynamics (CFD) are versatile, and it is currently used as an efficient tool in the pharmaceutical industry for various purposes, such as the characterization of dry powder inhalers, the Venturi dustiness tester, and the Heubach Dustmeter [
30,
31,
32,
33]. In addition, CFD is commonly applied to assess systems for the measurement of dustiness. In this context, CFD allows a modeling of the aerodynamics within the systems to assess the homogenous distribution of dust and to identify zones in which dust accumulates at the end of the sampling process. Furthermore, these simulations are also appropriate to investigate a possible inhomogeneous or delayed powder atomization [
32]. Moreover, CFD is suitable to describe the atomization process of pharmaceutical powders and to assess their flow behavior within the systems [
33,
34].
The number of investigations regarding the dustiness of pharmaceutical powders is relatively low compared to the dustiness investigations of non-pharmaceutical powders [
21,
35]. In addition, in the literature, the description of the distribution of airborne particles in a defined environment as a function of various flows is rather vague.
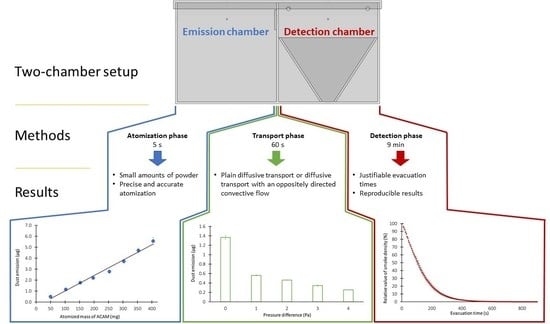
Based on the above-mentioned remarks, further research is needed to expand the knowledge on the influence of the plain diffusive transport and the diffusive transport with an oppositely directed convective flow of pharmaceutical airborne particles on the dustiness of HPAPIs. Therefore, the objective of this work was to develop a novel TCS consisting of an emission and a detection chamber to investigate the dustiness depending on different flow conditions. A further aim of this study was to confirm that the newly developed TCS ensures a reproducible and precise dustiness investigation of pharmaceutical powders. The different parameters of the atomization phase, transport phase, and detection phase may influence the investigation of the TCS. In this context, both the reproducible and precise atomization of small amounts of the surrogate substance ACAM and the accurate determination of the dust emission by an IOM sampler (Institute of Occupational Medicine) are the crucial parameters that may significantly influence the dustiness investigations in terms of the reproducibility and precision of the results.
Modern equipment for the production of dosage forms containing HPAPIs is typically operated with negative pressure to reduce potential hazard exposure [
1]. Consequentially, another objective of this study was the generation of an adjustable oppositely directed convective flow from the detection to the emission chamber within the TCS, simulating the negative pressure for reduction of the potential hazard exposure.
3. Results and Discussion
3.1. Validation of the Atomization Process
The two-chamber setup (TCS) was developed for the study of the dustiness of pharmaceutical powders by atomizing the powders with an overpressure of 50,000 Pa through the ball valve, centrally located above the emission chamber. The measurement process may be divided into three phases, the first of which is the atomization phase. Accordingly, the purpose of this study was to verify whether a reproducible and precise atomization of pharmaceutical powders is feasible using the TCS. For this purpose, various amounts of the surrogate substance acetaminophen (ACAM) were atomized in 50 mg portions. The dust emission was determined by the IOM sampler mounted in the detection chamber and by the subsequent quantification of ACAM with HPLC. In
Figure 6, a linear correlation (R
2 = 0.9842) between the dust emission and the atomized amount of ACAM can be observed within the range of 50 and 400 mg of atomized ACAM. Within this range, the maximum relative SD is 6.58%. No significant increase in dust emission (
p > 0.05) was observed within the range of 400 and 500 mg of atomized ACAM. Consequently, a reproducible atomization of ACAM is only possible within the range of 50 and 400 mg.
3.2. Results of the Dustiness Measurements during the Transport Phase
The TCS was constructed to study the dustiness of pharmaceutical powders affected by various flow conditions. In this study, four different pressure differences were generated between the emission and the detection chamber. The flows from the emission to the detection chamber resulting from the various pressure differences caused a reduction in the transport of airborne particles and were expected to lower the potential dust transfer from the emission to the detection chamber. The pressure differences between the chambers were obtained by compressed air flowing through the ball valve located above the detection chamber. The pressure difference was maintained for 60 s with the controllable flap open. By varying the pressure differences, resulting in corresponding flows, it was possible to determine whether a relationship exists between the air velocity and the decrease in the transfer of airborne particles from the emission to the detection chamber.
Currently, in the manufacturing of dosage forms containing HPAPIs, pharmaceutical production facilities commonly use negative pressure to reduce exposure due to potential leakage. Therefore, a convective flow from the outside to the inside occurs and prevents this leakage.
CFD was used in this study to simulate the resulting flows and flow velocities within the TCS. The air velocity within the setup is particularly crucial at the orifice (diameter 25.4 mm) between the emission and the detection chamber. In
Figure 7, the CFD simulations are shown at pressure differences of 1–4 Pa. The four simulations showed that a high air velocity was observed at the position where the compressed air passes the ball valve. It is also apparent that the extent of the airflow affected the average air velocity at the orifice between both chambers, as a function of pressure difference.
The simulated and measured average air velocities are shown in
Table 3. Accordingly, generating high pressure differences resulted in higher air velocities between both chambers.
The investigation of the effect of the air flow on dustiness was confirmed by atomizing ACAM, either with 0 Pa, for the examination of the plain diffusive transport of airborne particles, or with 1–4 Pa, for the examination of the diffusive transport with an oppositely directed convective flow. At 1–4 Pa, the oppositely directed convective air flow served as a measure to minimize dust emission. In
Figure 8, the resulting dust emissions from various flow conditions are presented. The plain diffusive transport of airborne particles (0 Pa) resulted in the highest dust emission of 1.269 ± 0.091 µg ACAM transported from the emission to the detection chamber for 60 s. A significant decrease in dust emission (
p < 0.05) was observed during the examination of the diffusive transport of airborne particles with an oppositely directed convective flow. A pressure difference of 1 Pa between the emission and the detection chamber resulted in a significant decrease in the dust emission (
p < 0.05) to a value of 0.557 ± 0.042 µg ACAM. Furthermore, a significant reduction in dust emission (
p < 0.05) was observed with increasing the pressure difference between both chambers. Compared to the examination at a pressure difference of 1 Pa, the dust emission was reduced to 0.444 ± 0.029 µg ACAM at a pressure difference of 2 Pa. An increase in the pressure difference to 3 Pa resulted in a further reduction in the dust emission to 0.342 ± 0.027 µg ACAM. The maximum pressure difference of 4 Pa showed the highest reduction in the dust emission to a value of 0.256 ± 0.023 µg ACAM, compared to that of the plain diffusive transport of airborne particles.
The results presented in
Figure 8 show that the dust emissions of ACAM resulting from the plain diffusive transport within the TCS may be reduced by an oppositely directed convective flow. Furthermore, the results also indicate that an increase in the pressure difference led to a reduction in the dust emissions. Based on the CFD, the increase in the pressure difference enhanced the airflow velocities at the orifice between the emission and the detection chamber. Consequently, the higher airflow velocities resulted in a decrease in the dust emissions. The above-mentioned investigation showed that the pressure difference set within the TCS as a closed system exerted a substantial effect on the potential transfer of dust.
CFD enables the simulation of the average air velocity depending on the pressure difference between the emission and the detection chamber. These simulated average air velocities appear to be similar to the those found in the measured data, confirming the results of the CFD simulations (
Table 3). However, the accuracy of the thermal anemometer is specified by the manufacturer with ±0.1 m/s for air velocities between 0 and 2 m/s. This raises the question of how meaningful the measured values are. In this context, it must be considered that the accuracy increases with declining air velocities and decreases with rising air velocities (for example, the accuracy within an air velocity range of 2–15 m/s is specified with ±0.3 m/s). As the measured average air velocities are very low, with about 0.1 m/s, it is therefore assumed that these values are trustworthy. A further argument for the relevance of the measured average air velocities is their low standard deviation, indicating a high reproducibility.
3.3. Determination of the Evacuation Time during the Detection Phase
The detection chamber is an essential part of the TCS, and it has a major impact on the detection phase of the measurement course. Because of the pyramidal design and the diagonal arrangement of the orifices for pressure compensation, most of the airborne ACAM particles should be detected within a reasonable time period. After the transport phase of the measuring course, which lasted for 1 min, the controllable flap was closed to detect the airborne ACAM particles that were transported by plain diffusive transport or by diffusive transport with oppositely directed convective flow from the emission to the detection chamber. For this purpose, the detection chamber containing the air with the airborne particles was evacuated, as previously described. The airborne ACAM particles were retained in the filter and were quantified by HPLC after extraction with the mobile phase.
To ensure that the airborne particles are collected in a reasonable time, CFD, flow visualization using smoke, and dustiness measurements with various evacuation times were performed. CFD was also used to evaluate the airflow within the detection chamber during evacuation.
A particle trace analysis was also performed with CFD, and the results are displayed in
Figure 9. The negative pressure generated by the air sampling pump was compensated for by incoming air flowing through the diagonally arranged orifices (
Figure 9a,b). Initially, the air flows were parallel to the walls of the detection chamber. Because of the diagonal arrangement of the orifices and the impingement of the airflow onto the walls opposite to the orifices, a rotation of the airflow occurred. The rotating airflow also shifted downwards within the detection chamber towards the IOM sampler attached to the bottom of the chamber (
Figure 9c). The highest velocity of the airflow was measured directly at the orifices. After the incoming air passed the orifices, the velocity of the airflow within the chamber decreased.
The residence time of the airborne particles within the detection chamber within different time periods is presented in
Figure 10. The zones of the airborne particles, with a residence time between 540 and 900 s, are illustrated in
Figure 10d. Compared to
Figure 10a–c, which comprised three time periods of 180 s each up to the time point of 540 s, these zones are relatively small. These zones are located exclusively on the walls at the upper part of the detection chamber.
In
Figure 10a–c, the time course of the evacuation of the detection chamber is shown in time periods of 180 s. Every 180 s, a 15 L volume was evacuated through the glass microfiber filter of the detection chamber as a result of the flow rate of 5.0 L/min. Simultaneously, 15 L of particle-free air were introduced through the diagonally arranged orifices. The residence times of the airborne particles are shown in
Figure 10. They indicate that after a three-fold evacuation of the detection chamber, which takes 540 s, the majority of the particles were already collected by the IOM sampler and could be quantified by HPLC.
The data obtained by CFD was analyzed by the ParaView application, and the total volume of the zones within the different time periods was determined. However, in the simulations, it should be considered that the diffusion coefficient was set to a low value of 1 × 10
−8 m
2/s to accurately determine potential zones of airborne particles with a high residence time. These zones are especially perceptible at the transition from the upper part of the detection chamber to the pyramidal part of the chamber. The relative fraction of the zone within the time interval of 0–180 s was 61.92%. After an additioanl 180 s, 99.87% of the airborne particles within the TCS passed through the outlet of the detection chamber. As shown in
Figure 10c, the zones for the time interval of 360–540 s are substantially smaller. After an evacuation time of 540 s, a volume of 99.99% was achieved.
An additional investigation was performed using smoke to visualize the flow within the detection chamber. For this purpose, smoke was passed through the two orifices for 10 s for pressure compensation to create a homogeneous smoke density. Subsequently, digital camera images were taken while the air sampling pump removed the smoke from the detection chamber at a flow rate of 5.0 L/min. The images were then analyzed for the gray value within the three square fields. The reason for this investigation was to quantify the smoke density and thus, the amount of smoke removed based on the gray value. Therefore, the mean of the gray values of each square field was calculated. The evacuation time-dependent gray values were converted to relative values by defining the highest value as 100% and the lowest value of the resulting exponential decrease at the evacuation time of infinity as 0%. The removal of the smoke from the detection chamber resulted in a decrease in the relative values of the smoke density, as shown in
Figure 11. Consequently, the obtained relative values of the exponential decrease in the smoke density were converted into the corresponding values defining the amount of removed smoke.
After determination of the evacuation times of the detection chamber with both CFD simulations and smoke visualizations, additional experiments were performed with the surrogate substance ACAM to investigate whether a significant difference exists between the two evaluation methods. The results of the two evaluation methods revealed that within 900 s a large portion of the airborne particles has already passed through the glass microfiber filter, again in an exponential manner (
Figure 11). An exponential decrease in the amount of tracer particles or surrogate substances over time was also observed using other devices for dustiness testing [
29]. To ensure that most of the airborne particles were indeed collected within a reasonable time period, the detection chamber was evacuated for 3600 s. The ACAM dust emission measured at this time point was defined as the maximum achievable emission. The results obtained with this evacuation time were compared to those obtained with shorter evacuation times of 180, 360, 540, 720, and 900 s. With the data of the evacuation time-dependent CFD simulations and smoke visualizations, relative values were again calculated for comparative purposes. In
Table 4, an overview of the evacuation times determined using CFD, smoke visualization, and dustiness measurements, using ACAM as a surrogate substance, is provided.
For the evacuation time of 180 s, it was observed that the detection of airborne particles was insufficient using all three methods. Moreover, no significant difference (p > 0.05) between the relative values obtained by smoke visualization and dustiness measurements was found. However, a significant increase (p < 0.05) in the relative values obtained with all three methods was observed by extending the evacuation time to 360 s. The data obtained within the time period of 0–540 s showed that relative values over 99.00% were reached with all three methods. Prolonging the evacuation time to 720 or 900 s did not result in a significant increase (p > 0.05) in the relative values. The relatively large deviation of the relative values obtained with CFD from those obtained with the other two methods may likely be explained by the low diffusion coefficient of 1 × 10−8 m2/s. Therefore, increasing the evacuation time to 720 or 900 s did not result in the detection of higher amounts of dust.
3.4. Powder Characterization
As shown in previous studies, the dustiness of powders is influenced by various factors, such as true density, bulk density, and particle size [
8,
23]. Accordingly, the powder properties of the investigated ACAM were determined. The resulting values for the true density, the bulk density, the tapped density, the residual moisture content, and the particle size, with the respective standard deviations, are listed in
Table 5. These values are comparable to other well-known and frequently used APIs.
4. Conclusions
The two-chamber setup (TCS) presented in this study is a newly developed containment system created especially for the examination of the dustiness of pharmaceutical powders. A reproducible dustiness investigation of even small amounts of pharmaceutical powders is feasible with this setup, as was shown with the surrogate substance acetaminophen (ACAM). The ACAM used in the dustiness experiments exhibits powder properties comparable to other commonly used pharmaceutical powders. A high reproducibility of the dust emission was observed, as confirmed by low standard deviations and a linear correlation found between the atomized amount of up to 400 mg of ACAM and the resulting dust emissions. The deviation from the linearity for atomized amounts above 400 mg was possibly caused by a saturation effect within the system. Furthermore, the TCS was proven to be suitable for the investigation of the dust emission at different flow conditions (plain diffusive transport and diffusive transport with the oppositely directed convective flow of airborne particles). Particularly, the influence of the oppositely directed convective flow on the dust emissions was investigated, and the results indicate that an increase in the pressure difference between the emission and the detection chamber led to a major reduction in dust emissions. Moreover, a time period of 9 min for the evacuation of the detection chamber was sufficient for the detection of most airborne ACAM particles within the detection chamber. An extension of the evacuation time did not result in a significant increase in the detected particles. Computational fluid dynamics (CFD) simulations revealed that the air flowing through the orifices of the detection chamber for pressure compensation caused a rotating and downward flow towards an IOM sampler. The relative values resulting from the examinations using either atomized ACAM or smoke for the determination of the evacuation time indicate that both methods are comparable. The deviation from the values simulated with CFD may be explained by the low diffusion coefficient of the simulation.
From the results of this study, it may be concluded that the presented TCS allowed for a containment investigation of small amounts of pharmaceutical powders, with regard to their dustiness, within a justifiable time. In future studies, other powder blends will be investigated in regards to their dustiness and their particle flow properties. Moreover, it is of interest to determine whether an additional increase in the pressure difference between the emission and the detection chamber may finally lead to a complete interruption of dust emission.