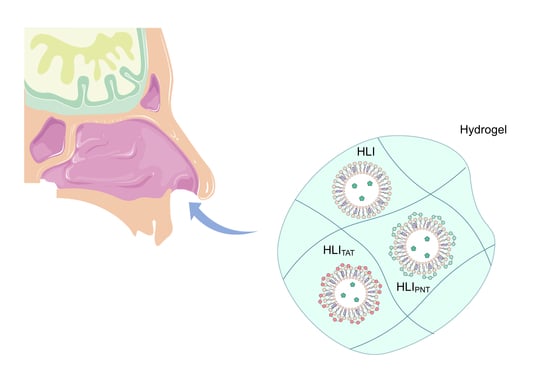
Hydroxyethylcellulose-Based Hydrogels Containing Liposomes Functionalized with Cell-Penetrating Peptides for Nasal Delivery of Insulin in the Treatment of Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Insulin-Loaded Liposomes
2.3. Functionalization of Insulin-Loaded Liposomes with TAT and Penetratin
2.4. Preparation of Hydrogel
2.5. Rheological Behavior
2.5.1. Flow Measurements
2.5.2. Oscillatory Measurements
2.6. Chromatographic Conditions for Insulin Quantification
2.7. In Vitro Release of Insulin from Hydrogels
2.8. Experimental Design for In Vivo Studies
2.8.1. Animals
2.8.2. Temporal Analysis of the Insulin Serum Levels
2.8.3. Anti-Hyperglycemic Responses in Diabetic Rats
Western Blotting Analysis
Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Provisional Report of a WHO Consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2010, 39 (Suppl. 1), S13–S22. [Google Scholar]
- Sen, S.; Chakraborty, R.; De, B. Diabetes Mellitus in 21st Century; Springer: New York, NY, USA, 2016; p. 186. [Google Scholar]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes, J.D.d.R.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: http://www.diabetesatlas.org (accessed on 3 March 2021).
- Heinemann, L. New Ways of Insulin Delivery. Int. J. Clin. Pract. Suppl. 2012, 65, 96–108. [Google Scholar] [CrossRef]
- Yadav, N.; Morris, G.; Harding, S.E.; Ang, S.; Adams, G.G. Various Non-Injectable Delivery Systems for the Treatment of Diabetes Mellitus. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 9–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.S.; Plosker, G.L. AFREZZA® (insulin human) inhalation powder: A review in diabetes mellitus. Drugs 2015, 75, 1679–1686. [Google Scholar] [CrossRef]
- Illum, L.; Jorgensen, H.; Rossing, N. Bioadhesive Microspheres as a Potential Nasal Drug Delivery System. Int. J. Pharm. 1987, 39, 189–199. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.N.; Jain, S.K. Nasal-Nanotechnology: Revolution for Efficient Therapeutics Delivery. Drug Deliv. 2016, 23, 681–693. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive Polymeric Platforms for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef]
- Ismail, R.; Csóka, I. Novel Strategies in the Oral Delivery of Antidiabetic Peptide Drugs-Insulin, GLP 1 and Its Analogs. Eur. J. Pharm. Biopharm. 2017, 115, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C.; Jelkmann, M.; Laf, F.; Bernkop-schnürch, A. Nasal Drug Delivery: Design of a Novel Mucoadhesive and in Situ Gelling Polymer. Int. J. Pharm. 2017, 517, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, S.C.D.A.; Giuberti, C.d.S.; Rocha, T.G.R.; Ferreira, D.d.S.; Leite, E.A.; Oliveira, M.C. Liposomes as carriers of anticancer drugs. In Cancer Treatment—Conventional and Innovative Approaches; Intech: Rijeka, Croatia, 2013; pp. 85–124. [Google Scholar] [CrossRef] [Green Version]
- Eloy, J.O.; de Souza, M.C.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as Carriers of Hydrophilic Small Molecule Drugs: Strategies to Enhance Encapsulation and Delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, K.; Ono, S.; Narioka, K.; Kakemi, M.; Morimoto, K.; Yamashita, S.; Namba, Y.; Oku, N. Application of surface-coated liposomes for oral delivery of peptide: Effects of coating the liposome’s surface on the GI transit of insulin. J. Pharm. Sci. 1999, 88, 248–255. [Google Scholar] [CrossRef]
- Jain, A.K.; Chalasani, K.B.; Khar, R.K.; Ahmed, F.J.; Diwan, P.V. Muco-Adhesive Multivesicular Liposomes as an Effective Carrier for Transmucosal Insulin Delivery. J. Drug Target. 2007, 15, 417–427. [Google Scholar] [CrossRef]
- Niu, M.; Lu, Y.; Hovgaard, L.; Wu, W. Liposomes Containing Glycocholate as Potential Oral Insulin Delivery Systems: Preparation, in Vitro Characterization, and Improved Protection against Enzymatic Degradation. Int. J. Nanomed. 2011, 6, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- Bitounis, D.; Fanciullino, R.; Iliadis, A.; Ciccolini, J. Optimizing Druggability through Liposomal Formulations: New Approaches to an Old Concept. ISRN Pharm. 2012, 2012, 738432. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical Liposomal Drug Delivery: A Review of New Delivery Systems and a Look at the Regulatory Landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.D.; Lee, K. On Employing a Translationally Controlled Tumor Protein-Derived Protein Transduction Domain Analog for Transmucosal Delivery of Drugs. J. Control. Release 2013, 170, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Bechara, C.; Sagan, S. Cell-Penetrating Peptides: 20 Years Later, Where Do We Stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F. Cell-Penetrating Peptides: Classes, Origin, and Current Landscape. Drug Discov. Today 2012, 17, 850–860. [Google Scholar] [CrossRef]
- Tashima, T. Intelligent Substance Delivery into Cells Using Cell-Penetrating Peptides. Bioorg. Med. Chem. Lett. 2016, 27, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bolhassani, A.; Jafarzade, B.S.; Mardani, G. In Vitro and in Vivo Delivery of Therapeutic Proteins Using Cell Penetrating Peptides. Peptides 2016, 87, 50–63. [Google Scholar] [CrossRef]
- Koren, E.; Torchilin, V.P. Cell-Penetrating Peptides: Breaking through to the Other Side. Trends Mol. Med. 2012, 18, 385–393. [Google Scholar] [CrossRef]
- Ramsey, J.D.; Flynn, N.H. Cell-Penetrating Peptides Transport Therapeutics into Cells. Pharmacol. Ther. 2015, 154, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Thorén, P.E.G.; Persson, D.; Karlsson, M.; Nordén, B. The Antennapedia Peptide Penetratin Translocates across Lipid Bilayers-The First Direct Observation. FEBS Lett. 2000, 482, 265–268. [Google Scholar] [CrossRef]
- Khafagy, E.S.; Morishita, M.; Isowa, K.; Imai, J.; Takayama, K. Effect of Cell-Penetrating Peptides on the Nasal Absorption of Insulin. J. Control. Release 2009, 133, 103–108. [Google Scholar] [CrossRef]
- Ahwany, A.M.D.E.; Mohamed, E.A.H. Enzymatic Hydrolysis of Pseudoplastic Paint Thickener (hydroxyethyl cellulose) by a Local Isolate of Aspergillus Niger. Afr. J. Biotechnol. 2008, 7, 3765–3770. [Google Scholar]
- Bemiller, J.N.; Whistler, R.L. Industrial Gums: Polysaccharides and Their Derivatives; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Clasen, C.; Kulicke, W. Determination of Viscoelastic and Rheo-Optical Material Functions of Water-Soluble Cellulose Derivatives. Prog. Polym. Sci. 2001, 26, 1839–1919. [Google Scholar] [CrossRef]
- Goodwin, D.J.; Picout, D.R.; Ross-murphy, S.B.; Holland, S.J.; Martini, L.G.; Lawrence, M.J. Ultrasonic Degradation for Molecular Weight Reduction of Pharmaceutical Cellulose Ethers. Carbohydr. Polym. 2011, 83, 843–851. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water Soluble Polymers for Pharmaceutical Applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef] [Green Version]
- Zuben, E.D.S.V.; Eloy, J.O.; Araujo, V.H.S.; Gremião, M.P.D.; Chorilli, M. Insulin-Loaded Liposomes Functionalized with Cell-Penetrating Peptides: Influence on Drug Release and Permeation through Porcine Nasal Mucosa. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126624. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel Methods for Liposome Preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Hosny, K.M. Optimization of gatifloxacin liposomal hydrogel for enhanced transcorneal permeation. J. Liposome Res. 2010, 20, 31–37. [Google Scholar] [CrossRef]
- Petrilli, R.; Eloy, J.O.; Praça, F.S.G.; Ciampo, J.O.D.; Fantini, M.A.C.; Fonseca, M.J.V.; Bentley, M.V.L.B. Liquid Crystalline Nanodispersions Functionalized with Cell-Penetrating Peptides for Topical Delivery of Short-Interfering RNAs: A Proposal for Silencing a pro-Inflammatory Cytokine in Cutaneous Diseases. J. Biomed. Nanotechnol. 2016, 12, 1063–1075. [Google Scholar] [CrossRef]
- Mourtas, S.; Fotopoulou, S.; Duraj, S.; Sfika, V.; Tsakiroglou, C.; Antimisiaris, S.G. Liposomal Drugs Dispersed in Hydrogels. Effect of Liposome, Drug and Gel Properties on Drug Release Kinetics. Colloids Surf. B Biointerfaces 2007, 55, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Nemen, D.; Lemon-Sena, E. Preparação e caracterização de suspensões coloidais de nanocarreadores lipídicos contendo resveratrol destinados à administração cutânea. Quim. Nova 2011, 34, 408–413. [Google Scholar] [CrossRef]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Cvetkovska, M.; Tsvetanov, C. Hydroxyethylcellulose Cryogels Used for Entrapment of Saccharomyces Cerevisiae Cells. React. Funct. Polym. 2009, 69, 688–693. [Google Scholar] [CrossRef]
- Mourtas, S.; Haikou, M.; Theodoropoulou, M.; Tsakiroglou, C.; Antimisiaris, S.G. The Effect of Added Liposomes on the Rheological Properties of a Hydrogel: A Systematic Study. J. Colloid Interface Sci. 2008, 317, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.A. A Handbook of Elementary Rheology; Intitute of non-Newtonian Fluid Mechanics, University of Wales: Aberystwyth, UK, 2000. [Google Scholar]
- Zuben, E.D.S.V.; Eloy, J.O.; Araujo, V.H.S.; Gremião, M.P.D.; Chorilli, M. Rapid and Sensitive Analytical Method for the Determination of Insulin in Liposomes by Reversed-Phase HPLC. Acta Chim. Slov. 2020, 67, 1273–1280. [Google Scholar] [CrossRef]
- Keck, T.; Leiacker, R.; Riechelmann, H.; Rettinger, G. Temperature profile in the nasal cavity. Laryngoscope 2000, 110, 651–654. [Google Scholar] [CrossRef]
- Lindemann, J.; Leiacker, R.; Rettinger, G.; Keck, T. Nasal Mucosal Temperature during Respiration. Clin. Otolaryngol. Allied Sci. 2002, 27, 135–139. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In Vitro Release Kinetics Model Fitting of Liposomes: An Insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the Use of the Weibull Function for the Discernment of Drug Release Mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Juhász, Á.; Ungor, D.; Berta, K.; Seres, L.; Csapó, E. Spreadsheet-Based Nonlinear Analysis of in Vitro Release Properties of a Model Drug from Colloidal Carriers. J. Mol. Liquids 2021, 328, 115405. [Google Scholar] [CrossRef]
- Dyer, A.M.; Hinchcliffe, M.; Watts, P.; Castile, J.; Jabbal-Gill, I.; Nankervis, R.; Smith, A.; Illum, L. Nasal Delivery of Insulin Using Novel Chitosan Based Formulations: A Comparative Study in Two Animal Models between Simple Chitosan Formulations and Chitosan Nanoparticles. Pharm. Res. 2002, 19, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Mainardes, R.M.; Khalil, N.M.; Gremiao, M.P.D. Intranasal Delivery of Zidovudine by PLA and PLA-PEG Blend Nanoparticles. Int. J. Pharm. 2010, 395, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, I.D.; Lima, T.F.O.; Inácio, M.D.; Costa, M.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Lycopene Improves the Metformin Effects on Glycemic Control and Decreases Biomarkers of Glycoxidative Stress in Diabetic Rats. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3117–3135. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelint, T.; Gordon, J. Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [Green Version]
- Hosny, K.M. Ciprofloxacin as Ocular Liposomal Hydrogel. AAPS PharmSciTech 2010, 11, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Ritthidej, G.C. Nasal Delivery of Peptides and Proteins with Chitosan and Related Mucoadhesive Polymers. Pept. Protein Deliv. 2011, 47–48. [Google Scholar] [CrossRef]
- Smistad, G.; Nyström, B.; Zhu, K.; Grønvold, M.K.; Røv-Johnsen, A.; Hiorth, M. Liposomes Coated with Hydrophobically Modified Hydroxyethyl Cellulose: Influence of Hydrophobic Chain Length and Degree of Modification. Colloids Surf. B Biointerfaces 2017, 156, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Mastropietro, D.J.; Nimroozi, R.; Omidian, H. Rheology in pharmaceutical formulations-a perspective. J. Dev. Drugs. 2013, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Braun, D.B.; Rosen, M.R. Rheology Modifiers Handbook: Practical Use and Application; William Andrew Publishing: New York, NY, USA, 2000. [Google Scholar]
- Chieng, Y.Y.; Chen, S.B. Rheological Study of Hydrophobically Modified Hydroxyethyl Cellulose and Phospholipid Vesicles. J. Colloid Interface Sci. 2010, 349, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Morais, G.G.; Santos, O.D.H.; Masson, D.S.; Oliveira, W.P.; Filho, P.A.R. Development of O/W emulsions with annato oil (Bixa orellana) containing liquid crystal. J. Dispers. Sci. Technol. 2005, 26, 591–596. [Google Scholar] [CrossRef]
- Callens, C.; Ceulemans, J.; Ludwig, A.; Foreman, P.; Remon, J.P. Rheological Study on Mucoadhesivity of Some Nasal Powder Formulations. Eur. J. Pharm. Biopharm. 2003, 55, 323–328. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Chen, H.; Bian, Z.; Zhang, G.; Riaz, M.K.; Tyagi, D.; Lin, G.; Zhang, Y.; Wang, J.; et al. Dual-Ligand Modified Liposomes Provide Effective Local Targeted Delivery of Lung-Cancer Drug by Antibody and Tumor Lineage-Homing Cell-Penetrating Peptide. Drug Deliv. 2018, 25, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Guo, S.; Wu, Y.; Chen, G.; Lai, J.; Xu, X. Behaviour of Cell Penetrating Peptide TAT-Modified Liposomes Loaded with Salvianolic Acid B on the Migration, Proliferation, and Survival of Human Skin Fibroblasts. J. Liposome Res. 2020, 30, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Kissel, T.; Werner, U. Nasal Delivery of Peptides: An in Vitro Cell Culture Model for the Investigation of Transport and Metabolism in Human Nasal Epithelium. J. Control. Release 1998, 53, 195–203. [Google Scholar] [CrossRef]
- Khafagy, E.S.; Morishita, M.; Onuki, Y.; Takayama, K. Current Challenges in Non-Invasive Insulin Delivery Systems: A Comparative Review. Adv. Drug Deliv. Rev. 2007, 59, 1521–1546. [Google Scholar] [CrossRef]
- Khafagy, E.; Kamei, N.; Juel, E.; Nielsen, B.; Nishio, R. European Journal of Pharmaceutics and Biopharmaceutics One-Month Subchronic Toxicity Study of Cell-Penetrating Peptides for Insulin Nasal Delivery in Rats. Eur. J. Pharm. Biopharm. 2013, 85, 736–743. [Google Scholar] [CrossRef]
- Khafagy, E.; Morishita, M.; Ida, N.; Nishio, R.; Isowa, K.; Takayama, K. Structural Requirements of Penetratin Absorption Enhancement Efficiency for Insulin Delivery. J. Control. Release 2010, 143, 302–310. [Google Scholar] [CrossRef]
- Souza, R.D.; Mutalik, S.; Venkatesh, M.; Vidyasagar, S.; Udupa, N. Nasal Insulin Gel as an Alternate to Parenteral Insulin: Formulation, Preclinical, and Clinical Studies. AAPS PharmSciTech 2005, 6, 184–189. [Google Scholar]
- Carvalheira, J.B.C.; Zecchin, H.G.; Saad, M.J.A. Vias de Sinalização Da Insulina. Arq. Bras. Endocrionol. Metabol. 2002, 46, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Cryer, P.E.; Davis, S.N.; Shamoon, H. Hypoglycemia in diabetes. Diabetes Care 2003, 26, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.D.; Balp, M.M.; Kulich, K.; Germain, N.; Rofail, D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer. Adherence 2012, 6, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, L.; Gibney, M.; Berube, J.; Manocchio, J. Impact of a Modified Needle Tip Geometry on Penetration Force as Well as Acceptability, Preference, and Perceived Pain in Subjects with Diabetes. J. Diabetes Sci. Technol. 2012, 6, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Nunes, K.M.; Teixeira, C.C.; Kaminski, R.C.; Sarmento, V.H.; Couto, R.O.; Pulcinelli, S.H.; Freitas, O. The monoglyceride content affects the self-assembly behavior, rheological properties, syringeability, and mucoadhesion of in situ–gelling liquid crystalline phase. J. Pharm. Sci. 2016, 105, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Bruschi, M.L.; Jones, D.S.; Panzeri, H.; Gremião MP, D.; Freitas, O.; Lara EH, G. Semisolid systems containing propolis for the treatment of periodontal disease: In vitro release kinetics, syringeability, rheological, textural, and mucoadhesive properties. J. Pharm. Sci. 2007, 96, 2074–2089. [Google Scholar] [CrossRef]
- Hägerström, H.; Edsman, K. Interpretation of Mucoadhesive Properties of Polymer. J. Pharm. Pharmacol. 2001, 53, 1589–1599. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Campos, M.L.; Peccinini, R.G.; Gremião, M.P.D. Nasal Administration of Liquid Crystal Precursor Mucoadhesive Vehicle as an Alternative Antiretroviral Therapy. Eur. J. Pharm. Biopharm. 2013, 84, 219–227. [Google Scholar] [CrossRef]
- Gupta, P.N.; Pattani, A.; Curran, R.M.; Kett, V.L.; Andrews, G.P.; Morrow, R.J.; Woolfson, A.D.; Malcolm, R.K. Development of Liposome Gel Based Formulations for Intravaginal Delivery of the Recombinant HIV-1 Envelope Protein CN54gp140. Eur. J. Pharm. Sci. 2012, 46, 315–322. [Google Scholar] [CrossRef]
- El Kechai, N.; Bochot, A.; Huang, N.; Nguyen, Y.; Ferrary, E.; Agnely, F. Effect of Liposomes on Rheological and Syringeability Properties of Hyaluronic Acid Hydrogels Intended for Local Injection of Drugs. Int. J. Pharm. 2015, 487, 187–196. [Google Scholar] [CrossRef]
- Karavasili, C.; Fatouros, D.G. Smart Materials: In Situ Gel-Forming Systems for Nasal Delivery. Drug Discov. Today 2016, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Bandyopadhyay, A.K. Development and characterization of mucoadhesive in situ nasal gel of midazolam prepared with Ficus carica mucilage. AAPS PharmSciTech 2010, 11, 1223–1231. [Google Scholar] [CrossRef] [PubMed]







| Formulations | 32 °C | 37 °C | ||||
|---|---|---|---|---|---|---|
| N | K | R | n | K | R | |
| Pure Hydrogel | 0.59146 Aa | 0.44693 Aa | 0.99785 Aa | 0.61356 Ab | 0.32128 Ab | 0.99791 Aa |
| HI | 0.60650 Aa | 0.46455 Aa | 0.99768 Aa | 0.61916 Aa | 0.39254 Bb | 0.99829 Aa |
| HLI | 0.56480 Ba | 0.62962 Ba | 0.99772 Aa | 0.58344 Bb | 0.51663 Cb | 0.99823 Aa |
| HLITAT | 0.55209 Ca | 0.75802 Ca | 0.99731 Aa | 0.56237 Cb | 0.68899 Db | 0.99670 Aa |
| HLIPNT | 0.56144 Ba | 0.66301 Ba | 0.99737 Aa | 0.56329 Ba | 0.55264 Eb | 0.99789 Aa |
| Formulations | 32 °C | 37 °C | ||
|---|---|---|---|---|
| G′ | G″ | G′ | G″ | |
| Pure Hydrogel | 0.61502 | 1.19306 | 0.53392 | 1.11020 |
| HI | 0.48396 | 1.09694 | 0.44103 | 1.04699 |
| HLI | 0.65501 | 1.28254 | 0.57007 | 1.15608 |
| HLITAT | 0.81508 | 1.55873 | 0.89033 | 1.57806 |
| HLIPNT | 0.67231 | 1.35616 | 0.69005 | 1.36092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Von Zuben, E.d.S.; Eloy, J.O.; Inácio, M.D.; Araujo, V.H.S.; Baviera, A.M.; Gremião, M.P.D.; Chorilli, M. Hydroxyethylcellulose-Based Hydrogels Containing Liposomes Functionalized with Cell-Penetrating Peptides for Nasal Delivery of Insulin in the Treatment of Diabetes. Pharmaceutics 2022, 14, 2492. https://doi.org/10.3390/pharmaceutics14112492
Von Zuben EdS, Eloy JO, Inácio MD, Araujo VHS, Baviera AM, Gremião MPD, Chorilli M. Hydroxyethylcellulose-Based Hydrogels Containing Liposomes Functionalized with Cell-Penetrating Peptides for Nasal Delivery of Insulin in the Treatment of Diabetes. Pharmaceutics. 2022; 14(11):2492. https://doi.org/10.3390/pharmaceutics14112492
Chicago/Turabian StyleVon Zuben, Eliete de Souza, Josimar Oliveira Eloy, Maiara Destro Inácio, Victor Hugo Sousa Araujo, Amanda Martins Baviera, Maria Palmira Daflon Gremião, and Marlus Chorilli. 2022. "Hydroxyethylcellulose-Based Hydrogels Containing Liposomes Functionalized with Cell-Penetrating Peptides for Nasal Delivery of Insulin in the Treatment of Diabetes" Pharmaceutics 14, no. 11: 2492. https://doi.org/10.3390/pharmaceutics14112492
APA StyleVon Zuben, E. d. S., Eloy, J. O., Inácio, M. D., Araujo, V. H. S., Baviera, A. M., Gremião, M. P. D., & Chorilli, M. (2022). Hydroxyethylcellulose-Based Hydrogels Containing Liposomes Functionalized with Cell-Penetrating Peptides for Nasal Delivery of Insulin in the Treatment of Diabetes. Pharmaceutics, 14(11), 2492. https://doi.org/10.3390/pharmaceutics14112492