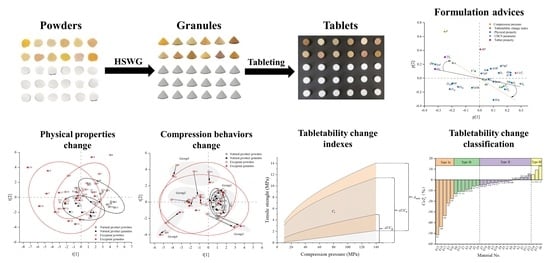
Using a Material Library to Understand the Change of Tabletability by High Shear Wet Granulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Powders
2.3. High Shear Wet Granulation Experiment
2.3.1. Experiment Design
2.3.2. Process Description
2.4. Tableting Process
2.5. Compression Models
2.5.1. The Kawakita Model
2.5.2. The Shapiro Model
2.5.3. The Heckel Model
2.5.4. The Gurnham Model
2.5.5. The Ryshkewitch-Duckworth Model
2.5.6. The Power Model
2.6. Evaluation of Change in Tabletability
2.6.1. The Reworking Potential
2.6.2. The Relative Change of Tabletability
2.7. Multivariate Data Analysis
3. Results and Discussions
3.1. Comparison of Physical Properties of Powders and Granules
3.1.1. Univariate Analysis
3.1.2. Principal Component Analysis
3.2. Comparison of Compression Behavior of Powders and Granules
3.2.1. Compression Model Fitting Results
3.2.2. Principal Component Analysis
3.3. Change of Tabletability from Powders to Granules
3.3.1. Qualitative Analysis
3.3.2. Quantitative Analysis
3.4. Comparsion of Critical Material Attributes for DC and HSWG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name |
| Amax | Maximum change of tabletability |
| AOR | Angle of repose |
| APIs | Active pharmaceutical ingredients |
| AUCg | The area under the tensile strength vs. compression pressure profile of granules |
| AUCmax | The maximum area under the curve |
| AUCmin | The minimum area under the curve |
| CBCS | Compression behavior classification system |
| CMAs | Critical material attributes |
| Cmax | Degree of compression at maximal pressure |
| CoTr | Relative change of tabletability |
| CPP | Critical process parameter |
| Da | Bulk density |
| Dc | Tapped density |
| DC | Direct compression |
| DG | Dry granulation |
| Dt | True density |
| HSWG | High shear wet granulation |
| HSWGT | High shear wet granulation tableting |
| %H | Hygroscopicity |
| %HR | Loss on drying |
| IC | Carr index |
| Icd | Cohesion index |
| Ie | Inter-particle porosity |
| IH | Hausner ratio |
| Iθ | Homogeneity index |
| LVs | Latent variables |
| MCS | Manufacturing classification system |
| NPGs | Natural product granules |
| NPPs | Natural product powders |
| OSD | Oral solid dosage |
| PC | Principal component |
| PCA | Principal component analysis |
| %pf | Percentage of particles measuring < 50 μm |
| PLS | Partial least squares |
| R2 | Determinant coefficient |
| RH | Relative humidity |
| RMSE | Root mean square error |
| RP | Reworking potential |
| SF | Solid fraction |
| SKH | Shapiro-Konopicky-Heckel |
| t’’ | Flowability |
| TCCS | Tabletability change classification system |
| TS | Tensile strength |
| WG | Wet granulation |
References
- Arshad, M.S.; Zafar, S.; Yousef, B.; Alyassin, Y.; Ali, R.; AlAsiri, A.; Chang, M.W.; Ahmad, Z.; Elkordy, A.A.; Faheem, A.; et al. A review of emerging technologies enabling improved solid oral dosage form manufacturing and processing. Adv. Drug Deliv. Rev. 2021, 178, 113840. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019 (accessed on 17 July 2022).
- CDER Report. Novel Drugs, 2020 Summary. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/new-drug-therapy-approvals-2020 (accessed on 17 July 2022).
- U.S. Food and Drug Administration (FDA). Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021 (accessed on 17 July 2022).
- Kása, P.; Bajdik, J.; Zsigmond, Z.; Pintye-Hódi, K. Study of the compaction behaviour and compressibility of binary mixtures of some pharmaceutical excipients during direct compression. Chem. Eng. Process. Process Intensif. 2009, 48, 859–863. [Google Scholar] [CrossRef]
- Leane, M.; Pitt, K.; Reynolds, G.K.; Dawson, N.; Ziegler, I.; Szepes, A.; Crean, A.M.; Agnol, R.D.; Sy, T.M.C. Manufacturing classification system in the real world: Factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm. Dev. Technol. 2018, 23, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.H.; Hallam, C.N.; Baker, N.A.; Murphy, D.S.; Gabbott, I.P. Understanding tablet defects in commercial manufacture and transfer. J. Drug Deliv. Sci. Technol. 2018, 46, 1–6. [Google Scholar] [CrossRef]
- Malkowska, S.; Khan, K.A.; Lentle, R.; Marchant, J.; Elger, G. Effect of Re-Compression on the Properties of Tablets Prepared by Moist Granulation. Drug Dev. Ind. Pharm. 1983, 9, 349–361. [Google Scholar] [CrossRef]
- Rajkumar, A.D.; Reynolds, G.K.; Wilson, D.; Wren, S.A.; Salman, A.D. The effect of roller compaction and tableting stresses on pharmaceutical tablet performance. Powder Technol. 2018, 341, 23–37. [Google Scholar] [CrossRef]
- Sun, C.C.; Kleinebudde, P. Mini review: Mechanisms to the loss of tabletability by dry granulation. Eur. J. Pharm. Biopharm. 2016, 106, 9–14. [Google Scholar] [CrossRef]
- Sun, C.; Himmelspach, M.W. Reduced tabletability of roller compacted granules as a result of granule size enlargement. J. Pharm. Sci. 2006, 95, 200–206. [Google Scholar] [CrossRef]
- Herting, M.G.; Kleinebudde, P. Roll compaction/dry granulation: Effect of raw material particle size on granule and tablet properties. Int. J. Pharm. 2007, 338, 110–118. [Google Scholar] [CrossRef]
- Patel, S.; Dahiya, S.; Sun, C.C.; Bansal, A.K. Understanding Size Enlargement and Hardening of Granules on Tabletability of Unlubricated Granules Prepared by Dry Granulation. J. Pharm. Sci. 2011, 100, 758–766. [Google Scholar] [CrossRef]
- Wu, S.-J.; Sun, C. Insensitivity of Compaction Properties of Brittle Granules to Size Enlargement by Roller Compaction. J. Pharm. Sci. 2007, 96, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Freitag, F.; Kleinebudde, P. How do roll compaction/dry granulation affect the tableting behaviour of inorganic materials? Comparison of four magnesium carbonates. Eur. J. Pharm. Sci. 2003, 19, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Freitag, F.; Reincke, K.; Runge, J.; Grellmann, W.; Kleinebudde, P. How do roll compaction/dry granulation affect the tableting behaviour of inorganic materials?: Microhardness of ribbons and mercury porosimetry measurements of tablets. Eur. J. Pharm. Sci. 2004, 22, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C. On the mechanism of reduced tabletability of granules prepared by roller compaction. Int. J. Pharm. 2008, 347, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Grote, S.; Kleinebudde, P. Roll Compaction/Dry Granulation of Dibasic Calcium Phosphate Anhydrous-Does the Morphology of the Raw Material Influence the Tabletability of Dry Granules? J. Pharm. Sci. 2018, 107, 1104–1111. [Google Scholar] [CrossRef]
- Nordström, J.; Alderborn, G. Degree of compression as a potential process control tool of tablet tensile strength. Pharm. Dev. Technol. 2010, 16, 599–608. [Google Scholar] [CrossRef]
- Osborne, J.D.; Althaus, T.; Forny, L.; Niederreiter, G.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Investigating the influence of moisture content and pressure on the bonding mechanisms during roller compaction of an amorphous material. Chem. Eng. Sci. 2013, 86, 61–69. [Google Scholar] [CrossRef]
- Herting, M.; Kleinebudde, P. Studies on the reduction of tensile strength of tablets after roll compaction/dry granulation. Eur. J. Pharm. Biopharm. 2008, 70, 372–379. [Google Scholar] [CrossRef]
- Leane, M.; Pitt, K.; Reynolds, G. The Manufacturing Classification System (MCS) Working Group A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm. Dev. Technol. 2014, 20, 12–21. [Google Scholar] [CrossRef]
- Yu, J.; Xu, B.; Zhang, K.; Shi, C.; Zhang, Z.; Fu, J.; Qiao, Y. Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction. Pharmaceutics 2019, 11, 662. [Google Scholar] [CrossRef]
- Van Snick, B.; Holman, J.; Vanhoorne, V.; Kumar, A.; De Beer, T.; Remon, J.; Vervaet, C. Development of a continuous direct compression platform for low-dose drug products. Int. J. Pharm. 2017, 529, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, B.; Wu, C.-Y.; Liu, L.X. The effects of screw-to-roll speed ratio on ribbon porosity during roll compaction. Int. J. Pharm. 2020, 588, 119770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shi, L.; Chattoraj, S.; Sun, C.C. Preparation and Characterization of Surface-Engineered Coarse Microcrystalline Cellulose Through Dry Coating with Silica Nanoparticles. J. Pharm. Sci. 2012, 101, 4258–4266. [Google Scholar] [CrossRef] [PubMed]
- Mosig, J.; Kleinebudde, P. Evaluation of lubrication methods: How to generate a comparable lubrication for dry granules and powder material for tableting processes. Powder Technol. 2014, 266, 156–166. [Google Scholar] [CrossRef]
- Mosig, J.; Kleinebudde, P. Critical Evaluation of Root Causes of the Reduced Compactability after Roll Compaction/Dry Granulation. J. Pharm. Sci. 2015, 104, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Tye, C.K.; Sun, C.C.; Amidon, G.E. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J. Pharm. Sci. 2015, 94, 465–472. [Google Scholar] [CrossRef]
- Kuntz, T.; Schubert, M.A.; Kleinebudde, P. Increased compactibility of acetames after roll compaction. Eur. J. Pharm. Biopharm. 2011, 77, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Tripathi, J.; Jeong, S.H. Recent trends and future perspective of pharmaceutical wet granulation for better process understanding and product development. Powder Technol. 2018, 344, 864–882. [Google Scholar] [CrossRef]
- Osei-Yeboah, F.; Zhang, M.; Feng, Y.; Sun, C.C. A Formulation Strategy for Solving the Overgranulation Problem in High Shear Wet Granulation. J. Pharm. Sci. 2014, 103, 2434–2440. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.I.F.; Gray, D.B.; Hussain, M.A. A Study on the Effect of Wet Granulation on Microcrystalline Cellulose Particle Structure and Performance. Pharm. Res. 2006, 23, 634–640. [Google Scholar] [CrossRef]
- Shi, L.; Feng, Y.; Sun, C.C. Roles of Granule Size in Over-Granulation During High Shear Wet Granulation. J. Pharm. Sci. 2010, 99, 3322–3325. [Google Scholar] [CrossRef] [PubMed]
- Khorsheed, B.; Gabbott, I.; Reynolds, G.K.; Taylor, S.C.; Roberts, R.J.; Salman, A.D. Twin-screw granulation: Understanding the mechanical properties from powder to tablets. Powder Technol. 2018, 341, 104–115. [Google Scholar] [CrossRef]
- Osei-Yeboah, F.; Feng, Y.; Sun, C.C. Evolution of Structure and Properties of Granules Containing Microcrystalline Cellulose and Polyvinylpyrrolidone During High-Shear Wet Granulation. J. Pharm. Sci. 2014, 103, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorne, V.; Bekaert, B.; Peeters, E.; De Beer, T.; Remon, J.-P.; Vervaet, C. Improved tabletability after a polymorphic transition of delta-mannitol during twin screw granulation. Int. J. Pharm. 2016, 506, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Záhonyi, P.; Szabó, E.; Domokos, A.; Haraszti, A.; Gyürkés, M.; Moharos, E.; Nagy, Z.K. Continuous integrated production of glucose granules with enhanced flowability and tabletability. Int. J. Pharm. 2022, 626, 122197. [Google Scholar] [CrossRef]
- Hayashi, Y.; Nakano, Y.; Marumo, Y.; Kumada, S.; Okada, K.; Onuki, Y. Application of machine learning to a material library for modeling of relationships between material properties and tablet properties. Int. J. Pharm. 2021, 609, 121158. [Google Scholar] [CrossRef]
- Escotet-Espinoza, M.S.; Moghtadernejad, S.; Scicolone, J.; Wang, Y.; Pereira, G.; Schäfer, E.; Vigh, T.; Klingeleers, D.; Ierapetritou, M.; Muzzio, F.J. Using a material property library to find surrogate materials for pharmaceutical process development. Powder Technol. 2018, 339, 659–676. [Google Scholar] [CrossRef]
- Dhondt, J.; Bertels, J.; Kumar, A.; Van Hauwermeiren, D.; Ryckaert, A.; Van Snick, B.; Klingeleers, D.; Vervaet, C.; De Beer, T. A multivariate formulation and process development platform for direct compression. Int. J. Pharm. 2022, 623, 121962. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, B.; Dhondt, J.; Pandelaere, K.; Bertels, J.; Mertens, R.; Klingeleers, D.; Di Pretoro, G.; Remon, J.P.; Vervaet, C.; De Beer, T.; et al. A multivariate raw material property database to facilitate drug product development and enable in-silico design of pharmaceutical dry powder processes. Int. J. Pharm. 2018, 549, 415–435. [Google Scholar] [CrossRef]
- Dai, S.; Xu, B.; Zhang, Z.; Yu, J.; Wang, F.; Shi, X.; Qiao, Y. A compression behavior classification system of pharmaceutical powders for accelerating direct compression tablet formulation design. Int. J. Pharm. 2019, 572, 118742. [Google Scholar] [CrossRef]
- Casian, T.; Iurian, S.; Gâvan, A.; Porfire, A.; Pop, A.L.; Crișan, S.; Pușcaș, A.M.; Tomuță, I. In-Depth Understanding of Granule Compression Behavior under Variable Raw Material and Processing Conditions. Pharmaceutics 2022, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Oishi, T.; Shirotori, K.; Marumo, Y.; Kosugi, A.; Kumada, S.; Hirai, D.; Takayama, K.; Onuki, Y. Modeling of quantitative relationships between physicochemical properties of active pharmaceutical ingredients and tensile strength of tablets using a boosted tree. Drug Dev. Ind. Pharm. 2018, 44, 1090–1098. [Google Scholar] [CrossRef]
- Wang, Y.; O’Connor, T.; Li, T.; Ashraf, M.; Cruz, C.N. Development and applications of a material library for pharmaceutical continuous manufacturing of solid dosage forms. Int. J. Pharm. 2019, 569, 118551. [Google Scholar] [CrossRef] [PubMed]
- Arndt, O.-R.; Baggio, R.; Adam, A.K.; Harting, J.; Franceschinis, E.; Kleinebudde, P. Impact of Different Dry and Wet Granulation Techniques on Granule and Tablet Properties: A Comparative Study. J. Pharm. Sci. 2018, 107, 3143–3152. [Google Scholar] [CrossRef]
- Cai, L.; Farber, L.; Zhang, D.; Li, F.; Farabaugh, J. A new methodology for high drug loading wet granulation formulation development. Int. J. Pharm. 2013, 441, 790–800. [Google Scholar] [CrossRef]
- Di Martino, P.; Censi, R.; Malaj, L.; Martelli, S.; Joiris, E.; Barthélémy, C. Influence of Metronidazole Particle Properties on Granules Prepared in a High-Shear Mixer-Granulator. Drug Dev. Ind. Pharm. 2007, 33, 121–131. [Google Scholar] [CrossRef]
- Tan, L.; Carella, A.J.; Ren, Y.; Lo, J.B. Process optimization for continuous extrusion wet granulation. Pharm. Dev. Technol. 2010, 16, 302–315. [Google Scholar] [CrossRef]
- Rojas, J.; Ciro, Y.; Correa, L. Functionality of chitin as a direct compression excipient: An acetaminophen comparative study. Carbohydr. Polym. 2014, 103, 134–139. [Google Scholar] [CrossRef]
- Paul, S.; Sun, C.C. Dependence of Friability on Tablet Mechanical Properties and a Predictive Approach for Binary Mixtures. Pharm. Res. 2017, 34, 2901–2909. [Google Scholar] [CrossRef]
- Yost, E.; Mazel, V.; Sluga, K.K.; Nagapudi, K.; Muliadi, A.R. Beyond Brittle/Ductile Classification: Applying Proper Constitutive Mechanical Metrics to Understand the Compression Characteristics of Pharmaceutical Materials. J. Pharm. Sci. 2022, 111, 1984–1991. [Google Scholar] [CrossRef]
- Schönfeld, B.V.; Westedt, U.; Wagner, K.G. Compression Modulus and Apparent Density of Polymeric Excipients during Compression—Impact on Tabletability. Pharmaceutics 2022, 14, 913. [Google Scholar] [CrossRef] [PubMed]
- Picker, K.M. The 3D Model: Explaining Densification and Deformation Mechanisms by Using 3D Parameter Plots. Drug Dev. Ind. Pharm. 2004, 30, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.; Antikainen, O.; Ehlers, H.; Blanco, D.; Yliruusi, J. Examining mechanical properties of various pharmaceutical excipients with the gravitation-based high-velocity compaction analysis method. Int. J. Pharm. 2018, 539, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.-H.; Kim, J.-Y.; Park, E.-S. Utilization of a compaction simulator to formulate mini-tablets containing high dose of acyclovir. J. Drug Deliv. Sci. Technol. 2021, 64, 102602. [Google Scholar] [CrossRef]
- Li, Z.; Wu, F.; Zhao, L.; Lin, X.; Shen, L.; Feng, Y. Evaluation of fundamental and functional properties of natural plant product powders for direct compaction based on multivariate statistical analysis. Adv. Powder Technol. 2018, 29, 2881–2894. [Google Scholar] [CrossRef]
- Tay, J.Y.S.; Liew, C.V.; Heng, P.W.S. Powder Flow Testing: Judicious Choice of Test Methods. AAPS PharmSciTech 2016, 18, 1843–1854. [Google Scholar] [CrossRef]
- Hamman, H.; Hamman, J.; Wessels, A.; Scholtz, J.; Steenekamp, J.H. Development of multiple-unit pellet system tablets by employing the SeDeM expert diagram system I: Pellets with different sizes. Pharm. Dev. Technol. 2017, 23, 706–714. [Google Scholar] [CrossRef]
- Hamman, H.; Hamman, J.; Wessels, A.; Scholtz, J.; Steenekamp, J. Development of multiple-unit pellet system tablets by employing the SeDeM expert diagram system II: Pellets containing different active pharmaceutical ingredients. Pharm. Dev. Technol. 2018, 24, 145–156. [Google Scholar] [CrossRef]
- Dai, S.; Xu, B.; Shi, G.; Liu, J.; Zhang, Z.; Shi, X.; Qiao, Y. SeDeM expert system for directly compressed tablet formulation: A review and new perspectives. Powder Technol. 2018, 342, 517–527. [Google Scholar] [CrossRef]
- Fayed, M.H.; Abdel-Rahman, S.I.; Alanazi, F.K.; Ahmed, M.O.; Tawfeek, H.M.; Al-Shdefat, R.I. New gentle-wing high-shear granulator: Impact of processing variables on granules and tablets characteristics of high-drug loading formulation using design of experiment approach. Drug Dev. Ind. Pharm. 2017, 43, 1584–1600. [Google Scholar] [CrossRef]
- Fayed, M.H.; Abdel-Rahman, S.I.; Alanazi, F.K.; Ahmed, M.O.; Tawfeek, H.M.; Ali, B.E. 2017 High Shear Granulation Process. Assessing Impact of Formulation Variables on Granules and Tablets Characteristics Using Design of Experiment Methodology. Acta Pol. Pharm. 2017, 74, 551–564. [Google Scholar] [PubMed]
- Zhang, Y.; Binner, J.; Rielly, C.; Vaidhyanathan, B. Comparison of spray freeze dried nanozirconia granules using ultrasonication and twin-fluid atomisation. J. Eur. Ceram. Soc. 2014, 34, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Bowles, B.J.; Dziemidowicz, K.; Lopez, F.L.; Orlu, M.; Tuleu, C.; Edwards, A.J.; Ernest, T.B. Co-Processed Excipients for Dispersible Tablets–Part 1: Manufacturability. AAPS PharmSciTech 2018, 19, 2598–2609. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, L.; Meruva, S.; Donovan, M.D. Effect of Manufacturing Process on the Retention of Abuse-Deterrent Properties of PEO-Matrix Tablets. AAPS PharmSciTech 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Fell, J.; Newton, J. Determination of Tablet Strength by the Diametral-Compression Test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef]
- Kawakita, K.; Lüdde, K.-H. Some considerations on powder compression equations. Powder Technol. 1971, 4, 61–68. [Google Scholar] [CrossRef]
- Klevan, I.; Nordström, J.; Bauer-Brandl, A.; Alderborn, G. On the physical interpretation of the initial bending of a Shapiro–Konopicky–Heckel compression profile. Eur. J. Pharm. Biopharm. 2009, 71, 395–401. [Google Scholar] [CrossRef]
- Heckel, R.W. Density-pressure relationships in powder compaction. Trans. Metall. Soc. AIME 1961, 221, 671–675. [Google Scholar]
- Heckel, R.W. An analysis of powder compaction phenomena. Trans. Metall. Soc. AIME 1961, 221, 1001–1008. [Google Scholar]
- Hersey, J.; Rees, J. Particles-deformation of particles during briquetting. Nature 1971, 230, 96. [Google Scholar]
- Zhao, J.; Burt, H.; Miller, R. The Gurnham equation in characterizing the compressibility of pharmaceutical materials. Int. J. Pharm. 2006, 317, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Ryshkewitch, E. Compression Strength of Porous Sintered Alumina and Zirconia. J. Am. Ceram. Soc. 1953, 36, 65–68. [Google Scholar] [CrossRef]
- Duckworth, W. Discussion of Ryshkewitch paper. J. Am. Ceram. Soc. 1953, 36, 68. [Google Scholar]
- Wu, C.-Y.; Best, S.M.; Bentham, A.C.; Hancock, B.C.; Bonfield, W. A simple predictive model for the tensile strength of binary tablets. Eur. J. Pharm. Sci. 2005, 25, 331–336. [Google Scholar] [CrossRef]
- Jackson, J.E. Principal Components and Factor Analysis: Part II—Additional Topics Related to Principal Components. J. Qual. Technol. 1981, 13, 46–58. [Google Scholar] [CrossRef]
- Wold, S.; Trygg, J.; Berglund, A.; Antti, H. Some recent developments in PLS modeling. Chemom. Intell. Lab. Syst. 2001, 58, 131–150. [Google Scholar] [CrossRef]
- Faroongsarng, D.; Peck, G.E. Thermal porosity analysis of croscarmellose sodium and sodium starch glycolate by differential scanning calorimetry. AAPS PharmSciTech 2003, 4, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Mesnier, X.; Althaus, T.O.; Forny, L.; Niederreiter, G.; Palzer, S.; Hounslow, M.J.; Salman, A.D. A novel method to quantify tablet disintegration. Powder Technol. 2012, 238, 27–34. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Gajera, B.Y.; Xu, T.; Shah, H.; Dave, R.H. Influence of processing methods on physico-mechanical properties of Ibuprofen/HPC-SSL formulation. Pharm. Dev. Technol. 2018, 23, 1108–1116. [Google Scholar] [CrossRef]
- Cuevas, L.P.C.; Franco, M.A.; Baltazar, E.H. Indirect microwave heating to pharmaceutical excipients: Lactose hydrate. Powder Technol. 2012, 224, 57–68. [Google Scholar] [CrossRef]
- United States Pharmacopeia. General Chapter, 〈1174〉 Powder Flow. USP-NF; United States Pharmacopeia: Rockville, MD, USA, 2022. [Google Scholar]
- Juarez-Enriquez, E.; Olivas, G.; Zamudio-Flores, P.; Ortega-Rivas, E.; Perez-Vega, S.; Sepulveda, D. Effect of water content on the flowability of hygroscopic powders. J. Food Eng. 2017, 205, 12–17. [Google Scholar] [CrossRef]
- Ugurlu, T.; Halaçoğlu, M.D. Effects of some lubricants and evaluation of compression parameters on directly compressible powders. Pharm. Dev. Technol. 2013, 19, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Iwao, Y.; Miyagishima, A.; Itai, S. Application of principal component analysis enables to effectively find important physical variables for optimization of fluid bed granulator conditions. Int. J. Pharm. 2011, 409, 81–88. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, G.; Gao, Q. Preparation and properties of granular cold-water-soluble porous starch. Int. J. Biol. Macromol. 2020, 144, 656–662. [Google Scholar] [CrossRef]
- Hentzschel, C.M.; Sakmann, A.; Leopold, C.S. Comparison of traditional and novel tableting excipients: Physical and compaction properties. Pharm. Dev. Technol. 2011, 17, 649–653. [Google Scholar] [CrossRef]
- Nordström, J.; Klevan, I.; Alderborn, G. A Particle Rearrangement Index Based on the Kawakita Powder Compression Equation. J. Pharm. Sci. 2009, 98, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.C. A classification system for tableting behaviors of binary powder mixtures. Asian J. Pharm. Sci. 2016, 11, 486–491. [Google Scholar] [CrossRef] [Green Version]
- Diós, P.; Pernecker, T.; Nagy, S.; Pál, S.; DeVay, A. Influence of different types of low substituted hydroxypropyl cellulose on tableting, disintegration, and floating behaviour of floating drug delivery systems. Saudi Pharm. J. 2014, 23, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Zhao, L.; Lin, X.; Shen, L. An update on microcrystalline cellulose in direct compression: Functionality, critical material attributes, and co-processed excipients. Carbohydr. Polym. 2021, 278, 118968. [Google Scholar] [CrossRef]
- Solanki, N.G.; Kathawala, M.; Serajuddin, A.T. Effects of Surfactants on Itraconazole-Hydroxypropyl Methylcellulose Acetate Succinate Solid Dispersion Prepared by Hot Melt Extrusion III: Tableting of Extrudates and Drug Release from Tablets. J. Pharm. Sci. 2019, 108, 3859–3869. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Van Bockstal, P.-J.; Van Snick, B.; Peeters, E.; Monteyne, T.; Gomes, P.; De Beer, T.; Remon, J.; Vervaet, C. Continuous manufacturing of delta mannitol by cospray drying with PVP. Int. J. Pharm. 2016, 501, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyttä, K.M.; Lakio, S.; Wikström, H.; Sulemanji, A.; Fransson, M.; Ketolainen, J.; Tajarobi, P. Comparison between twin-screw and high-shear granulation—The effect of filler and active pharmaceutical ingredient on the granule and tablet properties. Powder Technol. 2020, 376, 187–198. [Google Scholar] [CrossRef]
- Ohrem, H.L.; Schornick, E.; Kalivoda, A.; Ognibene, R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm. Dev. Technol. 2014, 19, 257–262. [Google Scholar] [CrossRef]
- Yeniay, O.; Göktaş, A. A comparison of partial least squares regression with other prediction methods. Hacet. J. Math. Stat. 2002, 31, 99–111. [Google Scholar]
- Stocchero, M.; Locci, E.; d’Aloja, E.; Nioi, M.; Baraldi, E.; Giordano, G. PLS2 in Metabolomics. Metabolites 2019, 9, 51. [Google Scholar] [CrossRef]
- Dun, J.; Sun, C.C. Structures and Properties of Granules Prepared by High Shear Wet Granulation; Academic Press: Cambridge, MA, USA, 2019; pp. 119–147. [Google Scholar] [CrossRef]








| Run No. | Material No. | Wetting Agent | L/S Ratio (g·g−1) | Impeller Speed (rpm) | Addition Rate of Wetting Agent (mL·min−1) |
|---|---|---|---|---|---|
| 1 | Z1 | 95% ethanol | 0.3 | 600 | 50 |
| 2 | Z2 | 95% ethanol | 0.3 | 600 | 50 |
| 3 | Z3 | 95% ethanol | 0.3 | 600 | 50 |
| 4 | Z4 | 95% ethanol | 0.3 | 600 | 50 |
| 5 | Z5 | 95% ethanol | 0.3 | 600 | 50 |
| 6 | Z6 | 95% ethanol | 0.3 | 600 | 50 |
| 7 | Z7 | 95% ethanol | 0.3 | 600 | 50 |
| 8 | Z8 | 95% ethanol | 0.3 | 600 | 50 |
| 9 | Z9 | 95% ethanol | 0.3 | 600 | 50 |
| 10 | Z10 | 95% ethanol | 0.3 | 600 | 50 |
| 11 | Z11 | 95% ethanol | 0.3 | 600 | 50 |
| 12 | Z12 | 95% ethanol | 0.3 | 600 | 50 |
| 13 | E1 | 95% ethanol | 0.2 | 300 | 25 |
| 14 | E2 | 95% ethanol | 0.2 | 300 | 25 |
| 15 | E3 | 95% ethanol | 0.2 | 300 | 25 |
| 16 | E4 | 95% ethanol | 0.2 | 300 | 25 |
| 17 | E5 | 95% ethanol | 0.2 | 300 | 25 |
| 18 | E6 | deionized water | 0.1 | 300 | 25 |
| 19 | E7 | deionized water | 0.1 | 300 | 25 |
| 20 | E8 | deionized water | 0.1 | 300 | 25 |
| 21 | E9 | deionized water | 0.1 | 300 | 25 |
| 22 | E10 | deionized water | 0.2 | 300 | 25 |
| 23 | E11 | deionized water | 0.2 | 300 | 25 |
| 24 | E12 | deionized water | 0.3 | 300 | 25 |
| 25 | E13 | deionized water | 0.3 | 300 | 25 |
| 26 | E14 | deionized water | 0.5 | 300 | 25 |
| 27 | E15 | deionized water | 0.5 | 300 | 25 |
| 28 | E16 | deionized water | 0.6 | 300 | 25 |
| 29 | E17 | deionized water | 0.9 | 300 | 25 |
| 30 | E18 | deionized water | 1.3 | 300 | 25 |
| Fitting Relationship | CBCS Parameter | Powders | Granules | ||
|---|---|---|---|---|---|
| The Lowest | The Highest | The Lowest | The Highest | ||
| Porosity-Pressure | a | 0.708 | 1.05 | 0.746 | 1.05 |
| ab | 0.0658 | 0.283 | 0.0386 | 0.288 | |
| f | 8.84 × 10−2 | 0.362 | 3.12 × 10−2 | 0.326 | |
| Py | 41.6 | 686 | 38.9 | 684 | |
| K | 6.35 | 24.5 | 5.87 | 24.9 | |
| Porosity-TS | kb | 4.54 | 22.8 | 5.58 | 24.9 |
| TS-Pressure | d | 9.25 × 10−4 | 1.00 | 1.07 × 10−4 | 0.517 |
| g | 0.287 | 1.68 | 0.398 | 1.79 | |
| Category | Criteria | Characteristics | No. of Powders | No. of Granules |
|---|---|---|---|---|
| 1 | d ≥ 0.2, 0 < g < 0.95 | Good tabletability at extreme low-pressure range (20–50 MPa) | 7 | 3 |
| 2 | 0.002 ≤ d < 0.2 or d < 0.002, g ≥ 1.6 | A: Acceptable tabletability at middle pressure (50–100 MPa) & Good tabletability at high pressure range (100–140 MPa) | 15 | 13 |
| 0.002 ≤ d < 0.05 or d < 0.002, g ≥ 1.6 | B: Acceptable tabletability at high pressure (100–140 MPa) | 6 | 10 | |
| 0.002 ≤ d < 0.005, g > 0.95 or d < 0.002, g ≥ 1.6 | C: Unacceptable tabletability over the full pressure range (10–140 MPa) | 1 | 3 |
| Variable Type | Variables | |
|---|---|---|
| Input | Physical property | D10, D50, D90, Span, %pf, Iθ, Da, Dc, Dt, SFp, εp, IH, IC, Ie, t″, AOR, %H, %HR, Icd |
| CBCS parameter | A, b−1, ab, f, Py, K, kb, d, g | |
| Tabletability change index | RP, CoTr | |
| Compression pressure | P | |
| Output | Tablet property | TS1, TS2 |
| LVs | R2Xcum | R2Ycum | Q2cum |
|---|---|---|---|
| 1 | 21.8% | 52.1% | 50.3% |
| 2 | 41.4% | 66.0% | 63.0% |
| 3 | 55.5% | 79.5% | 77.0% |
| 4 | 66.0% | 83.8% | 80.6% |
| 5 | 73.0% | 88.8% | 85.9% |
| 6 | 78.4% | 90.0% | 85.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, J.; Zhao, X.; Liang, Z.; Qiao, Y.; Luo, G.; Xu, B. Using a Material Library to Understand the Change of Tabletability by High Shear Wet Granulation. Pharmaceutics 2022, 14, 2631. https://doi.org/10.3390/pharmaceutics14122631
Wang Y, Cao J, Zhao X, Liang Z, Qiao Y, Luo G, Xu B. Using a Material Library to Understand the Change of Tabletability by High Shear Wet Granulation. Pharmaceutics. 2022; 14(12):2631. https://doi.org/10.3390/pharmaceutics14122631
Chicago/Turabian StyleWang, Yawen, Junjie Cao, Xiaoqing Zhao, Zichen Liang, Yanjiang Qiao, Gan Luo, and Bing Xu. 2022. "Using a Material Library to Understand the Change of Tabletability by High Shear Wet Granulation" Pharmaceutics 14, no. 12: 2631. https://doi.org/10.3390/pharmaceutics14122631
APA StyleWang, Y., Cao, J., Zhao, X., Liang, Z., Qiao, Y., Luo, G., & Xu, B. (2022). Using a Material Library to Understand the Change of Tabletability by High Shear Wet Granulation. Pharmaceutics, 14(12), 2631. https://doi.org/10.3390/pharmaceutics14122631