Design of Nanoparticles in Cancer Therapy Based on Tumor Microenvironment Properties
Abstract
:1. Introduction
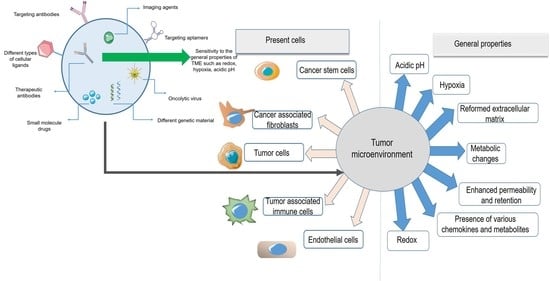
2. Tumor Microenvironment, a Limitation Turned into an Advantage
3. Nanoparticles for Targeting Physiological Conditions in the Tumor Microenvironment
3.1. Nanoparticles and Penetration into the Tumor Microenvironment
3.1.1. The Enhanced Permeability and Retention Effect
3.1.2. PEGylation of Nanoparticles
3.2. Nanoparticles Targeting the Acidic Tumor Microenvironment
3.3. Nanoparticles Targeting the Hypoxic Tumor Microenvironment
3.4. Nanoparticles Targeting the Reductive Tumor Microenvironment
3.5. Nanoparticles Targeting the Metabolic Changes in the Tumor Microenvironment
3.6. Nanoparticles Targeting the Extracellular Matrix of Tumor Microenvironment
3.6.1. Collagen
3.6.2. Lysophosphatidic Acid and Matrix Metalloproteinase Protein
4. Nanoparticles Targeting Cells in the Tumor Microenvironment
4.1. Nanoparticles Targeting Tumor-Associated Immune Cells
4.2. Nanoparticles Targeting Cancer Stem Cells
4.3. Nanoparticles Targeting Cancer-Associated Fibroblasts
4.4. Nanoparticles Targeting Endothelial Cells
| Ref. | In Vivo and In Vitro Studies | Specific Characteristics and Results | Target | Nanoparticle System |
|---|---|---|---|---|
| Kim et al. [51] | Human ovarian A2780 carcinoma cells | DOX (weak base positively charged) released in inferior pH values quicker than physiologic pH due to the change of electrostatic and hydrophobic forces in the polymeric complex | Acidic pH of TME | Polymeric micelles (PMAA attached to PEO) loaded with DOX |
| Ding et al. [55] | Human A431 squamous carcinoma tumor-bearing nude mice | The acid-responsive hydrazine bonds of the polymer of nanoparticle made it a promising system for drug delivery in the TME | Acidic pH of TME | Multiblock polyurethane nanoparticle loaded with paclitaxel |
| Zhang et al. [57] | HeLa and 3T3 cell lines HeLa cells subcutaneously injected into nude mice | After 36 h of incubation, the DOX release from PLNPs-PAMAM-AS1411/DOX at pH 5.0 was around 60%, compared with a 10% release at physiological conditions. | Acidic pH of TME | PLNPs-PAMAM modified with AS1411 aptamer and loaded with DOX |
| Dominski et al. [58] | Human colon adenocarcinoma cell line HCT-116, human cell line MCF-7, normal human dermal fibroblasts-neonatal (NHDF-Neo) | The drug was released much faster at a lower pH in comparison with normal pH conditions by in vitro studies | Acidic pH of TME | Nano micelles consisting of biodegradable triblock copolymer poly(ethylene glycol)-b-polycarbonate-b-oligo([R]-3-hydroxybutyrate) loaded with doxorubicin and 8-hydroxyquinoline glucose- and galactose conjugate |
| Huang et al. [59] | BEL-7402 cells | The nanoparticles demonstrated efficiency for the delivery of intact DNA for in vivo gene transfection. The nanoparticles were internalized into intra-tumoral cells due to the upregulation of CPP, suggesting these nanoparticles as an effective gene delivery system | Acidic pH of TME | PEG-DGL nanoparticles modified with activatable cell-penetrating peptide (designated as dtACPP) sensitive to lower pH and MMP2 present in the TME |
| Li et al. [60] | A549 tumor cells and tumor-bearing mice | The developed nanoparticles with a size around 113nm demonstrated significant MRI and photothermal properties and were capable of drug release with the assistance of exogenous NIR. | Acidic pH of TME | Mesoporous silica nano-system covered with polydopamine-Gd3+ (PDA–Gd) adjusted by poly (2-Ethyl-2-Oxazoline) (PEOz) and loaded with DOX |
| Son et al. [61] | SW620 and DU145 cells, SW620 cells injected into 6-week-old mice | The polymeric micelles formed by a series of mPEG-bPCHGE polymers showed higher stability, encapsulation efficiency, and manageable release kinetics. | Acidic pH of TME | Nano micelles of mPEG-b-PCHGE containing an acetal group as a pH-responsive acetal cleavable linkage loaded with paclitaxel and Nile red dye |
| Dominski et al. [63] | Normal human dermal fibroblasts-neonatal (NHDF-Neo), colon carcinoma (HCT-116), and breast cancer (MCF-7) | The developed micelles with a size of about 55 nm were stable in physiological pH but degraded in acidic pH and demonstrated pH-dependent drug release behavior in vitro | Acidic pH of TME | Polymeric micelles of a synthesized diblock copolymer poly(ethylene glycol)-hydrazone linkage-poly[R,S]-3-hydroxybutyrate loaded with hydroxyquinoline glucose, galactose conjugates, and DOX |
| Wang et al. [64] | MCF-7, BxPC-3, and NIH/3T3 cells Female Balb/c-nude mice | In normal conditions of pH 7.4, the ligand was hidden in the PEG layer, while in the pH of the TME (6.5), the ligand was exposed and targeted liposomes. These liposomes showed the highest cytotoxicity and cellular uptake in vitro, tumor site accumulation, and best antitumor effect in vivo in comparison with non-sensitive liposomes | Acidic pH of TME | Tyrosine-modified poly-ethylene glycol monostearate liposome system encapsulating irinotecan |
| Liu et al. [65] | 293 cells (a human renal epithelial cell line), HKC cells (a human renal tubular epithelial cell line), HeLa cells (a human epithelioid cervix carcinoma cell line), C6 cells (rat glioma cells), and Pc-12 cells (pheochromocytoma cells of the rat adrenal medulla) Glioma-bearing male CD-1 experimental mice | The developed nanoparticles simultaneously demonstrated efficiency in three types of therapy: chemodynamic treatment (CDT), chemotherapy, and photothermal therapy. This platform can be used as a multimodal synergistic cancer theranostic system | Acidic pH of TME | Fe–gallic acid (Fe–GA) nanospheres in combination with bovine serum albumin and encapsulating DOX |
| Du et al. [59] | MDA-MB-435s cells | This pH-responsive charge conversional nano-system promoted cellular uptake of Dox | Acidic pH of TME | PAMA–DMMA nanogels encapsulating Doxorubicin |
| Huo et al. [78] | Human epithelial HUVEC cell line cancerous HeLa cell line Breast and pancreatic tumor-bearing mice | The nanoparticles were degraded in the TME by MMP and enhanced the effect of radiation therapy | Hypoxia of TME | Tungsten oxide NPs (WO NPs) |
| as sensitizers for radiotherapy modified by CCL-28 chemokine ligand and a matrix metalloproteinase cleavable peptide | ||||
| Thambi et al. [79] | SCC7 cell line Nude mice bearing SCC7 tumor | A hydrophobically modified 2-nitroimidazole derivative was conjugated to the backbone of the nanoparticle responsible for the sustained release in normoxic conditions and burst release in hypoxia | Hypoxia of TME | Hypoxia-reactive carboxymethyl dextran nanoparticles containing doxurubicin |
| Son et al. [80] | SCC7 cells SCC7-bearing tumor athymic nude mice | The release degree of DOX increased by breakage of the azo bond in hypoxia conditions | Hypoxia of TME | Polymeric nanoparticles with carboxymethyl dextran and black hole quencher 3 encapsulating doxorubicin |
| Thambi et al. [81] | SCC7 cells | The hypoxia-sensitive polymeric micelles could preferentially release DOX under hypoxia conditions, proven by fluorescent imaging | Hypoxia of TME | Polymeric micelles with amphiphilic nature encapsulating DOX |
| Liu et al. [82] | K562 cell line (Leukemia cells) K562 tumor-bearing nude mice model | It was found that this nano-system can increase the sensitivity of the cells to the chemotherapeutic agent (danorubicin) and increase the intracellular density of DNR | Hypoxia of TME | PLGA-based nanoparticles modified with transferrin and loaded with danorubicin (DNR) |
| Zhu et al. [83] | EA.hy926 human umbilical vein cells HepG2 human hepatocellular carcinoma cells U87MG human glioma cells SGC-7901 gastric cancer cells MCF-7 human breast adenocarcinoma cells Nude mice bearing tumors | siHIF-1α cargo of the system was efficiently released in the hypoxic conditions of the TME. This system is also pH-responsive due to having hydrazone bonds. The intracellular delivery of siHIF-1α for gene silencing effects was enhanced significantly by this system | Hypoxia of TME | Hybrid quantum dots with a modified shell 2-deoxyglucose (DG)-polyethylene glycol (PEG) linked with the complex of lipoic acid, lysine, and 9-poly-d-arginine (LA-Lys-9R) by means of a hydrazone bond and a core of CdTe quantum dots |
| Abbasi et al. [84] | EMT6 breast tumor cell MDA-MB-231 cells BALB/c and SCID mice | Both systems enhanced the effect of radiotherapy when administered before radiation and also modulated the hypoxia of tumors significantly. Median host survival enhanced 3–5 fold | Hypoxia of TME | hybrid manganese dioxide (MnO2) nanoparticles (MDNP) consisting of hydrophilic terpolymer-protein or hydrophobic polymer-lipid for reoxygenating the TME by means of endogenous H2O2 |
| Gao et al. [87] | Mice bearing 4T1 murine breast tumors | After IV injection, this nano-system oxygenates the whole TME and enhances the effect of radiotherapy | Hypoxia of TME | RBC-coated PLGA nanoparticles encapsulating PFC |
| Song et al. [89] | Murine breast cancer 4T1 cells Tumor-bearing Balb/c mice | Because of the high oxygen solubility of PFC, this nanoparticle can enhance the effect of DNA damage to cancer cells induced by X-ray | Hypoxia of TME | PEG nanoparticles containing PFC and decorated with TaOx (an Xray absorber) |
| Song et al. [90] | 4T1 murine breast cancer cells Tumor-bearing Balb/c mice | Due to the strong NIR absorbance of Bi2Se3. It can produce a strong photothermal effect as well as a radio-sensitizing effect. PFC is also responsible for releasing oxygen in the TME. | Hypoxia of TME | Bi2Se3 nanoparticles functionalized with PEG, encapsulating PFC and oxygen |
| Yin et al. [102] | MG63-osteosarcoma cells MG63 cell-bearing nude mice | The liposomes demonstrated high drug loading and stability under physiological conditions and degraded in the presence of reducing agents DTT and GSH. The disulfide bond containing liposomes showed high cellular uptake and internalization | Reductive environment of TME | Chotooligosaccharides (COS) Modified liposomes via a disulfide linker to cholesterol loaded with doxurubicin |
| Yin et al. [103] | MG63 osteosarcoma cells MG63 tumor-bearing nude mice | The liposomes with a size of around 110 nm demonstrated high cellular uptake in estrogen receptor-expressing osteosarcoma cells (MG63) and a rapid release of Dox due to the redox sensitivity | Reductive environment of TME | Estrogen-functionalized liposomes grafted with gluthathione-responsive chotooligosaccharides loaded with doxurubicin |
| Kumar et al. [104] | MCF-7, BT 474, and L929 cell line. Ehrlich’s ascites tumor cell line (EAT) (murine breast carcinoma) injected in Swiss albino mice | The nanoparticles demonstrated ~72% drug release at pH 5.5 in comparison with ~18% drug release at pH 7.4, and 91% tumor regression in Ehrlich ascites tumor (EAT) in comparison with free doxorubicin-treated mice | Reductive environment of TME | Folic acid and trastuzumab modified random multiblock copolymeric nanoparticles |
| Conte et al. [105] | A549 cells and spheroids | The nanoparticles were able to penetrate mucus and demonstrated high internalization ability in 2D and 3D models | Reductive environment of TME | PLGA-PEG nanoparticles containing disulfide bonds loaded with docetaxel |
| Wu et al. [106] | HeLa cells, human umbilical vein endothelial cells (HUVECs) Tumor-bearing female nude mice | MHPCNs−SS−PGA−FA nanoparticles demonstrated high drug loading capacity and efficient biodistribution in tumor sites as investigated with an MRI. This platform displayed synergistic photothermal/chemotherapy effects with decreased side effects | Reductive environment of TME | magnetic hollow and porous carbon nanoparticles (MHPCNs) covalently conjugated with cystamine dihydrochloride and capped with poly(γglutamic acid) (PGA) and Folic acid encapsulating DOX |
| Deng et al. [109] | HT-29 colorectal carcinoma cell line LNCaP metastatic prostate cancer cell line | The nanohydrogels demonstrated high cellular uptake and cytotoxicity due to the redox-responsive degradation and release of the oncolytic virus | Reductive environment of TME | Thiolated hyaluronic acid hydrogels encapsulating oncolytic viruses |
| Deng et al. [110] | RAW264.7 macrophage cell line | The nano-system demonstrated high internalization into macrophages, redox responsiveness, and high encapsulation efficiency for diverse proteins | Reductive environment of TME | Nanocapsules consisting of a triblock copolymer in the shell and thiolated hyaluronic acid in the core |
| Elgogari et al. [118] | P8, A6L, A32, P198, E3, P215, P10, JD13D patient-derived PDAC cell lines, and patient-derived pancreatic tumors | BPTES is a glutaminase inhibitor, and the nanoparticle system demonstrated a significant effect on pancreatic cancer models in combination with metformin therapy | Metabolic changes in TME | PLGA-PEG nanoparticles encapsulating BPTES |
| Gandham et al. [123] | human ovarian adenocarcinoma (SKOV-3) cell line | The surface-modified nanoparticles demonstrated increased permeability and cytotoxicity in the 3D multicellular model | Metabolic changes in TME | Liposomal nanoparticles encapsulating 3-BPA and modified with GE-11 |
| Zhang et al. [124] | mouse pancreatic cancer cell line Pan-02 C57BL/6 mice | The liposomes demonstrated high efficiency in delivering 3-BPA to tumor cells overexpressing MCT1 and | Metabolic changes in TME | Liposomal nanoparticles functionalized with a pentapeptide encapsulating 3-BPA |
| Murty et al. [132] | human alveolar epithelial adenocarcinoma cells (A549) 6-week-old female nu/nu nude mice | The collagenase-modified nanoparticles demonstrated 35% higher accumulation within the tumor area | ECM network of TME | Gold nanoparticles labeled with collagenase |
| Villegas et al. [133] | human osteosarcoma cells (HOS) 3D collagen matrices housing HOS | The nano system showed a high penetration rate into the tumoral tissue model and a homogenous distribution and pH-responsive release due to the properties of the applied polymer | ECM network of TME | Polymeric nanocapsules encapsulating collagenase |
| Zinger et al. [134] | LSLKrasG12D/+;LSL-Trp53R172H/+ of pancreatic carcinomas Tumor-bearing C57BL/6 mice | the collagen component of the pancreatic tumor stroma was digested by collagenase encapsulated in nanoparticles. These nanoparticles, along with the administration of paclitaxel micelles, decreased the size of tumors by up to 87% | ECM network of TME | Nanoliposomes containing collagenase |
| Liu et al. [135] | 4T1 tumor-bearing nude mice | Collagenase encapsulated nanoparticles contain acid-sensitive benzoic-imine organic linker that cleaves in hypoxic the TME and enhances the effect of chlorin e6 (Ce6)-loaded liposomes, which are applied for photodynamic therapy | ECM network of TME | Collagenase encapsulated Mn2+ based nanoparticles modified by PEG |
| Loskutov et al. [138] | Human astrocytes isolated from the human cortex Tumor-bearing immunodeficient male mice | LPA signaling was limited significantly by means of this nano-system, and tumor progression was inhibited. | ECM network of TME | PLGA-PEG nano-system encapsulating small molecule Ki16425 (an LPA signaling inhibitor) |
| Sun et al. [141] | HUVEC and A549 lung cancer cells J774A.1 macrophage cells Lung tumor-bearing BALB/c mice | It was observed that the encapsulated paclitaxel was efficiently released in the high concentration of MMP in the tumor microenvironment and this nano system was efficient in treating lung cancer | ECM network of TME | Methoxy-poly(ethylene glycol)-poly(lactic acid) (MPEG-PLA) nanoparticles modified with a multitargeting peptide-LinTT1(MMP sensitive) and a cellpenetrating peptide-TAT encapsulating paclitaxel |
| Anajafi et al. [142] | BxPC-3 and AsPC-1 cells (human pancreatic adenocarcinoma, ATTC) and the 3D cultures | The polymerosomes taking advantage of the redox sensitivity and active targeting by means of MMP-7, demonstrated high penetration and shrinkage of the spheroids up to 49% in comparison to the normal cells | ECM network of TME | Polymeric vesicles surface modified with matrix metalloproteinase-7 (MMP-7) encapsulating curcumin and doxorubicin |
| Chen et al. [147] | 4T1 murine breast cancer and CT26 colorectal cancer cell Tumor-bearing BALB/c mice | Great anti-tumor efficacy was absorbed by applying these nanoparticles, followed by anti-CTLA4 therapy. This nanoparticle combined therapy encapsulating both NIR heaters and immune-adjuvant TLR agonists can stimulate vaccine-like immune responses | Tumor-associated immune cells | PLGA nanoparticles encapsulating Indocyanine green (photothermal agent) and imiquimod (R837), a Toll-like-receptor-7 agonist |
| Conde et al. [158] | A549-luciferase-C8 human lung adenocarcinoma cells lung cancer orthotopic murine model (BALB/c nude) | The nanoparticles demonstrated targeted delivery to murine lung TAMs and delivery of siRNA to those cells and to lung cancer cells. Due to the hybrid approach (silencing the VEGF gene), this system showed significant efficacy in inhibiting tumor progression. | Tumor-associated immune cells | RNA interference (RNAi)-peptide gold nanoparticles surface functionalized with M2 peptide and thiol-siRNA-Alexa Flour 488 |
| Schmid et al. [159] | Murine T cells B16 melanoma cells inoculated in six-to-ten-week-old C57BL/6 mice | The nanoparticles were capable of targeting T cells and delivery of the payload resulting in tumor growth delay and enhanced survival of tumor-bearing mice | Tumor-associated immune cells | PLGA-based nanoparticles surface modified with PD-1 antibodies encapsulating aTLR7/8 agonist or inhibitors of TGFBR1 |
| Yang et al. [160] | Primary CD8+ T cells Female C57Bl/6 mice 6–8 weeks | Targeted nanoparticles displayed a 40-fold increased uptake in CD8+ T cells in comparison with the non-targeted nanoparticles, and they showed high efficiency in a cancer vaccine model | Tumor-associated immune cells | Antibody-modified amphiphilic organic ligand-protected gold nanoparticle containing a small molecule TGF-β inhibitor |
| Sharma et al. [162] | RAW264.7 cells CCL-110 human fibroblast cell line 4T1 tumor-bearing female Balb/c mice | The nanoparticles demonstrated high encapsulation, targeting, and an eight-fold increase in cell death in comparison with free drug and blank nanoparticles | Tumor-associated immune cells | PLGA NPs functionalized with the LyP-1 peptide encapsulating clodronate |
| Zhu et al. [163] | murine J774A.1 macrophage cells | These nanoparticles can efficiently target TAMs in the tumor microenvironment. PEG shedding of the nano-system is also responsible for delivery in an acidic condition of the TME | Tumor-associated immune cells | PLGA-PEG nanoparticles modified with mannose |
| B16-F10 mouse melanoma tumors in C57BL/6 mice | ||||
| He et al. [164] | J774A.1 cells MCF-7 cells | By dual targeting with MCMC (for mannose ligands) and HA, this nano-system upregulates proinflammatory cytokines and shifts macrophages in M1 polarity | Tumor-associated immune cells | Mannosylated carboxymethyl chitosan (MCMC)/hyaluronan (HA) nanoparticles for delivery of CpG oligodeoxynucleotides (ODN) |
| Qiu et al. [165] | RAW264.7 macrophage S180 murine sarcoma cell line Kunming male mice | IBR is a Bruton’s tyrosine kinase (BTK) inhibitor, and BTK is overexpressed on TAMs. The nano-system delivered IBR to the tumor microenvironment efficiently and significantly reduced tumor growth. | Tumor-associated immune cells | Amphiphilic egg phosphatidylglycerol (EPG) nanoc-omplex modified with sialic acid (SA)–stearic acid conjugate encapsulating Ibrutinib (IBR) |
| Ordikhani et al. [166] | B16-F10 murine melanoma model Female C57BL/6, PD-1−/− LT-α−/− and BALB/c mice (7–9 weeks old) | Administration of high dose of anti–PD-1 NPs in mice resulted in increased mortality in comparison with those treated with free anti-PD-1 antibody because of the overactivation of T cells. Further modification of the anti-PD-1 NPs dosage resulted in less toxicity and higher antitumor effect of nanoparticles | Tumor-associated immune cells | Anti–PD-1 antibody encapsulated in PLGA nanoparticles |
| Ma et al. [170] | HeLa, MCF-7 and L929 cell lines | This nano-system demonstrated significant efficacy against CD44 overexpressing cancer cells | Cancer stem cells | Mesoporous silica linked with hyaluronic acid encapsulating camptothecin |
| Yi et al. [171] | Human breast cancer MDA-MB-231 cells Tumor-bearing NOD/SCID mice or BALB/c nude mice (female, eight weeks old) | The nanoparticles displayed high internalization into glucose transporter 1- overexpressing breast CSCs. These nano-systems stimulated gene silencing in a CSC-rich in vivo model | Cancer stem cells | Glucose-linked unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery |
| Kim et al. [172] | The human breast cancer cell line MCF-7 (MUC1/CD44 positive) human hepatocellular carcinoma cell line HepG2 (MUC1/CD44 negative) and their 3D cultures | Dual-aptamosomes had more cytotoxic effects on both CSCs and cancer cells in comparison to non-targeted liposomes and had shown an inhibitory effect against metastasis of breast CSCs and cancer cells in nude mice. | Cancer stem cells | Anti-MUC1/CD44 Dual-Aptamer-Conjugated Liposomes containing doxorubicin |
| Ning et al. [173] | Human colorectal cancer cell lines HT-29, SW620 and HCT116 cells HCT116-bearing female nude mice at the age of 4 weeks | The nanoparticles were efficiently targeted and internalized to CD133 overexpressing HCT116 cells and demonstrated high cytotoxicity. Immunohistochemistry results showed a reduction in CD133 expression in these cells after treatment with these nanoparticles. | Cancer stem cells | PEG−PCL-based nanoparticles modified with Anti-CD133 antibody encapsulating a topoisomerase inhibitor (SN-38) |
| Yu et al. [174] | HCT-116 | This nano-system significantly increased cellular uptake via HA receptor-mediated endocytosis in CD44 positive cell line (HCT-116 cells) | Cancer stem cells | Hyaluronic acid (HA) modified mesoporous silica nanoparticles encapsulating doxurubicin |
| Chen et al. [182] | LX-2, Hep G2 cells Tumor-bearing BALB/c nude mice | The antitumor efficacy of the nanoparticles was significantly higher than the free navitoclax and unmodified nanoliposomes | CAF | Navitoclax-loaded nanoliposomes modified with peptide FH (ligand of tenascin C, mainly expressed by CAFs) |
| Ji et al. [186] | CAFs, PC-3 (a prostate cancer cell line), and human umbilical endothelial cells (HUVECs) CAFs and PC-3 cells bearing nude mice | The mAb-modified PNPs demonstrated higher cellular uptake by CAFs, higher tumor penetration and less side effects of the encapsulated drug compared to non-modified PNPs | CAF | Polymeric nanoparticles (PNP), with a hydrophobic cholesterol core and a hydrophilic cationic R9 peptide shell modified with fibroblast activation protein-α (FAP-α) targeting antibody encapsulating doxurubicin |
| Ji et al. [187] | PC-3 (a prostate cancer cell line) Human umbilical endothelial cells (HUVECs), CAFs MCF-7 breast tumor Mia-paca-2 pancreatic tumor | The CAP-NPs showed promising antitumor efficacy for solid tumor models (breast and pancreatic tumors). | CAF | A nanoparticle consisting of cleavable amphiphilic peptide (CAP) responsive to fibroblast activation protein-a (FAP-a) expressed on CAFs encapsulating doxorubicin |
| Miao et al. [188] | Human bladder transitional cell line (UMUC3) Mouse embryonic fibroblast cell line (NIH 3T3) Bladder tumor Balb/C nude mice | It was observed that intravenous injection of these nanoparticles, along with cisplatin nanoparticles, inhibited tumor growth in the early and late stages of bladder cancer. | CAF | Liposome-protaminehyaluronic acid NP (LPH-NP) encapsulating siRNA against Wnt16 (siWnt) affecting cancer-associated fibroblasts |
| Du et al. [192] | Human hepatoma cells (HepG2) primary human umbilical vein endothelial cells (HUVECs) Tumor-bearing mice | The nanoparticles demonstrated normalizing the vascular structure and function of tumor blood cells. When loaded with Gem, the antitumor efficacy increased significantly. | Endothelial cells | Lipid derivative conjugates (LGCs) nanoparticles made of low molecular weight heparin (LMWH) and gemcitabine (Gem) |
| Cao et al. [193] | MDA-MB-231 and MCF-7 human breast cancer cell lines Tumor-bearing BALB/c nude mice | A7RC increased the targeting efficacy of nanoparticles significantly in high NRP-1 expressing cells of breast tumors, and nanoparticle accumulation and cellular uptake increased dramatically | Endothelial cells | Nanoliposomes modified with A7R-cysteine peptide (A7RC) encapsulating paclitaxel |
| Lu et al. [194] | Human brain microvascular endothelial cells (HBMECs) and C6 glioma cell line Wistar rats and New Zealand white rabbits. | The developed nanoparticles displayed high release (79.5%) in the reduced pH of the in vitro tests (pH = 5.5). Modification of the nanoparticles with RGD enhanced the cytotoxicity effect in in vitro BBB model due to increased uptake by C6 cells. | Endothelial cells | RGDyC/PEG co-modified PAMAM nanoparticles encapsulating arsenic trioxide |
| Murphy et al. [195] | Human umbilical vein endothelial cells (HUVECs) M21L-GFP mouse melanoma cells (integrin αvβ3 negative) iv injected mice | αvβ3 mediated drug delivery demonstrated a dramatic (15-fold) antimetastatic activity and a decrease in the side effects | Endothelial cells | RGD-modified polymeric nanoparticles encapsulating doxurubicin |
| Eloy et al. [196] | DU145 and PC3 cell lines | Modified nanoliposomes had more targeting efficacy on EGFR overexpressing cell line (DU145) in comparison with PC3 | EGFR | Nanoliposomes modified with anti-EGFR antibody containing docetaxel |
| McDaid et al. [197] | HCT116, A549, HKH-2, HCC827, PANC-1 cell lines | Cetuximab acts as a targeting agent for EGFR and demonstrated significant effects in pancreatic tumors | EGFR | PLGA nanoparticles modified with Cetuximab (CTX) encapsulating camptothecin |
| Aggarwal et al. [198] | MIA PaCa-2 (human pancreatic carcinoma) | This nano-system is a promising platform for EGFR- positive cancers therapy | EGFR | PLGA-PEG nanoparticles modified with EGFR antibody and loaded with Gemcitabine |
4.5. Nanoparticles for Targeting Epidermal Growth Factor Receptor
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATX | autotaxin enzyme |
| α-SMA | α smooth muscle actin |
| BDO | 1,4-butanediol |
| BHQ3 | black hole quencher 3 |
| BPTES | bis-2-(5-phenylacetamide-1,2,4-thiadiazol-2-yl) ethyl sulfide |
| CAF | cancer-associated fibroblasts |
| CAP | cleavable amphiphilic peptide |
| CART | chimeric antigen receptor T cell immunotherapy |
| CM-Dex | carboxymethyl dextran |
| CSC | cancer stem cells |
| CTLA-4 | cytotoxic T-lymphocyte-associated antigen-4 |
| CTX | cetuximab |
| DDS | drug delivery system |
| DG | deoxyglucose |
| DOX | doxorubicin |
| EC | endothelial cells |
| ECM | extracellular matrix |
| EPO | erythropoietin |
| EPR | enhanced permeability and retention |
| EMT | epithelial-mesenchymal transition |
| FAP | fibroblast activation protein |
| FGF | fibroblast growth factor |
| GLUT-1 | glucose transporter-1 |
| HR-NP | hypoxia responsive nano particles |
| HIF | hypoxia-inducible factor |
| LDI | L-lysine ethyl ester diisocyanate |
| LPA | Lysophosphatidic acid |
| LPC | lysophosphatidylcholine |
| MDSC | myeloid-derived suppressor cells |
| MMP2 | matrix metalloproteinase-2 |
| NK | natural killer |
| NP | nanoparticle |
| NIPAM | poly(N-isopropylacrylamide) |
| NIR | near-infrared |
| PAA | peroxy acetic acid |
| PBAA | poly(2-n-butylacrylic acid) |
| PbAE | poly beta-amino ester |
| PCL | polycaprolactone |
| PD-1 | programmed death-1 |
| PDAC | pancreatic ductal adenocarcinoma |
| PDL-1 | programmed death-ligand-1 |
| PEAA | polyethylacrylic acid |
| PEO | polyethylene oxide |
| PEG | polyethylen glycol |
| PFC | perfluorocarbon |
| PGA | poly(glycolic acid) |
| PMAA | poly methacrylic acid |
| PPAA | poly propylacrylic acid |
| PTT | photothermal therapy |
| PTX | paclitaxel |
| RB | retinoblastoma |
| QD | quantum dots |
| RBC | red blood cell |
| ROS | reactive oxygen species |
| RGD | Arginine-glycine-aspartic acid |
| SMA | smooth muscle actin |
| TAM | tumor-associated macrophages |
| Ta Ox | 13tantalum oxid |
| TIL | tumor-infiltrating lymphocytes |
| TME | tumor microenvironment |
| TP53 | tumor protein53 |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Jeong, J.H.; Chen, Z.; Chen, Z.; Luo, J.L. Targeting Tumor Microenvironment by Small-Molecule Inhibitors. Transl. Oncol. 2020, 13, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Hines, W.C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011, 17, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, F.; Schimming, A.; Jaeger, D.; Podar, K. Targeting the tumor microenvironment: Focus on angiogenesis. J. Oncol. 2012, 2012, 281261. [Google Scholar] [CrossRef] [Green Version]
- Benesch, M.G.K.; Yang, Z.; Tang, X.; Meng, G.; Brindley, D.N. Lysophosphatidate Signaling: The Tumor Microenvironment’s New Nemesis. Trends Cancer 2017, 3, 748–752. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Shan, X.; Chen, Z.; Gao, N.; Zeng, W.; Zeng, X.; Mei, L. Applications of surface modification technologies in nanomedicine for deep tumor penetration. Adv. Sci. 2021, 8, 2002589. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, Y.; Li, W.; Li, Y.; Lv, H.; Zhang, D.; Peng, J.; Cheng, W.; Mei, L.; Chen, H. Charge-reversal nanomedicines as a smart bullet for deep tumor penetration. Smart Mater. Med. 2022, 3, 243–253. [Google Scholar] [CrossRef]
- Huang, P.; Lian, D.; Ma, H.; Gao, N.; Zhao, L.; Luan, P.; Zeng, X. New advances in gated materials of mesoporous silica for drug controlled release. Chin. Chem. Lett. 2021, 32, 3696–3704. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.L.; Tan, W.; Ricono, J.M.; Korchynskyi, O.; Zhang, M.; Gonias, S.L.; Cheresh, D.A.; Karin, M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature 2007, 446, 690–694. [Google Scholar] [CrossRef]
- Haley, B.; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008, 26, 57–64. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Perrault, S.D.; Chan, W.C. In vivo assembly of nanoparticle components to improve targeted cancer imaging. Proc. Natl. Acad. Sci. USA 2010, 107, 11194–11199. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653. [Google Scholar] [CrossRef]
- Caldorera-Moore, M.; Guimard, N.; Shi, L.; Roy, K. Designer nanoparticles: Incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin. Drug Deliv. 2010, 7, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Pasqualini, R.; Arap, W.; Ferrari, M. Intravascular delivery of particulate systems: Does geometry really matter? Pharm. Res. 2009, 26, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natfji, A.A.; Ravishankar, D.; Osborn, H.M.I.; Greco, F. Parameters Affecting the Enhanced Permeability and Retention Effect: The Need for Patient Selection. J. Pharm. Sci. 2017, 106, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomed. 2011, 6, 715–728. [Google Scholar] [CrossRef] [Green Version]
- Devalapally, H.; Zhou, F.; McDade, J.; Goloverda, G.; Owen, A.; Hidalgo, I.J.; Silchenko, S. Optimization of PEGylated nanoemulsions for improved pharmacokinetics of BCS class II compounds. Drug Deliv. 2015, 22, 467–474. [Google Scholar] [CrossRef]
- Balguri, S.P.; Adelli, G.R.; Janga, K.Y.; Bhagav, P.; Majumdar, S. Ocular disposition of ciprofloxacin from topical, PEGylated nanostructured lipid carriers: Effect of molecular weight and density of poly (ethylene) glycol. Int. J. Pharm. 2017, 529, 32–43. [Google Scholar] [CrossRef]
- Dancy, J.G.; Wadajkar, A.S.; Schneider, C.S.; Mauban, J.R.; Goloubeva, O.G.; Woodworth, G.F.; Winkles, J.A.; Kim, A.J. Non-specific binding and steric hindrance thresholds for penetration of particulate drug carriers within tumor tissue. J. Control. Release 2016, 238, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.; Bentley, M.; Harris, J. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2002, 54, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q.J.N. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Tehrani, S.F.; Bernard-Patrzynski, F.; Puscas, I.; Leclair, G.; Hildgen, P.; Roullin, V.G.; Medicine. Length of surface PEG modulates nanocarrier transcytosis across brain vascular endothelial cells. Nanomed. Nanotechnol. Biol. 2019, 16, 185–194. [Google Scholar] [CrossRef]
- Jiang, Y.; Lodge, T.P.; Reineke, T.M. Packaging pDNA by polymeric ABC micelles simultaneously achieves colloidal stability and structural control. J. Am. Chem. Soc. 2018, 140, 11101–11111. [Google Scholar] [CrossRef]
- Jackson, M.A.; Werfel, T.A.; Curvino, E.J.; Yu, F.; Kavanaugh, T.E.; Sarett, S.M.; Dockery, M.D.; Kilchrist, K.V.; Jackson, A.N.; Giorgio, T.D. Zwitterionic nanocarrier surface chemistry improves siRNA tumor delivery and silencing activity relative to polyethylene glycol. ACS Nano 2017, 11, 5680–5696. [Google Scholar] [CrossRef]
- Williford, J.-M.; Archang, M.M.; Minn, I.; Ren, Y.; Wo, M.; Vandermark, J.; Fisher, P.B.; Pomper, M.G.; Mao, H.-Q. Critical length of PEG grafts on lPEI/DNA nanoparticles for efficient in vivo delivery. ACS Biomater. Sci. Eng. 2016, 2, 567–578. [Google Scholar] [CrossRef]
- Uz, M.; Bulmus, V.; Alsoy Altinkaya, S. Effect of PEG grafting density and hydrodynamic volume on gold nanoparticle–cell interactions: An investigation on cell cycle, apoptosis, and DNA damage. Langmuir 2016, 32, 5997–6009. [Google Scholar] [CrossRef] [Green Version]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Mozar, F.S.; Chowdhury, E.H. Impact of PEGylated Nanoparticles on Tumor Targeted Drug Delivery. Curr. Pharm. Des. 2018, 24, 3283–3296. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Gu, Z. Tumor microenvironment and intracellular signal-activated nanomaterials for anticancer drug delivery. Mater. Today 2016, 19, 274–283. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Milito, A.; Fais, S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005, 1, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Böhme, I.; Bosserhoff, A.K. Acidic tumor microenvironment in human melanoma. Pigment Cell Melanoma Res. 2016, 29, 508–523. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.; Spugnini, E.P.; Assaraf, Y.G.; Azzarito, T.; Rauch, C.; Fais, S. Microenvironment acidity as a major determinant of tumor chemoresistance: Proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist. Updat. 2015, 23, 69–78. [Google Scholar] [CrossRef]
- Tekade, R.K.; Dutta, T.; Gajbhiye, V.; Jain, N.K. Exploring dendrimer towards dual drug delivery: pH responsive simultaneous drug-release kinetics. J. Microencapsul. 2009, 26, 287–296. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef]
- Kim, J.O.; Kabanov, A.V.; Bronich, T.K. Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J. Control. Release Off. J. Control. Release Soc. 2009, 138, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.H.; Wang, C.H.; Hsiue, G.H. Polymeric micelles with a pH-responsive structure as intracellular drug carriers. J. Control. Release Off. J. Control. Release Soc. 2005, 108, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Tang, Z.; Li, M.; Lv, S.; Yu, H.; Ma, L.; Zhuang, X.; Huang, Y.; Chen, X. Tunable pH-sensitive poly(β-amino ester)s synthesized from primary amines and diacrylates for intracellular drug delivery. Macromol. Biosci. 2012, 12, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liang, D.; He, X.; Li, J.; Tan, H.; Li, J.; Fu, Q.; Gu, Q. The degradation and biocompatibility of pH-sensitive biodegradable polyurethanes for intracellular multifunctional antitumor drug delivery. Biomaterials 2012, 33, 2734–2745. [Google Scholar] [CrossRef]
- Ding, M.; Song, N.; He, X.; Li, J.; Zhou, L.; Tan, H.; Fu, Q.; Gu, Q. Toward the next-generation nanomedicines: Design of multifunctional multiblock polyurethanes for effective cancer treatment. ACS Nano 2013, 7, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Jiang, G.; Birrell, L.K.; El-Sayed, M.E. Degradable, pH-sensitive, membrane-destabilizing, comb-like polymers for intracellular delivery of nucleic acids. Biomaterials 2010, 31, 7150–7166. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhao, X.; Chen, L.J.; Yang, C.X.; Yan, X.P. Dendrimer grafted persistent luminescent nanoplatform for aptamer guided tumor imaging and acid-responsive drug delivery. Talanta 2020, 219, 121209. [Google Scholar] [CrossRef]
- Domiński, A.; Krawczyk, M.; Konieczny, T.; Kasprów, M.; Foryś, A.; Pastuch-Gawołek, G.; Kurcok, P. Biodegradable pH-responsive micelles loaded with 8-hydroxyquinoline glycoconjugates for Warburg effect based tumor targeting. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2020, 154, 317–329. [Google Scholar] [CrossRef]
- Huang, S.; Shao, K.; Kuang, Y.; Liu, Y.; Li, J.; An, S.; Guo, Y.; Ma, H.; He, X.; Jiang, C. Tumor targeting and microenvironment-responsive nanoparticles for gene delivery. Biomaterials 2013, 34, 5294–5302. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.; Wei, H.; Shan, X.; Wang, X.; Ou, M.; Liu, Q.; Gao, N.; Chen, H.; Mei, L. Charge-reversal biodegradable MSNs for tumor synergetic chemo/photothermal and visualized therapy. J. Control. Release 2021, 338, 719–730. [Google Scholar] [CrossRef]
- Son, I.; Lee, Y.; Baek, J.; Park, M.; Han, D.; Min, S.K.; Lee, D.; Kim, B.-S. pH-Responsive amphiphilic polyether micelles with superior stability for smart drug delivery. Biomacromolecules 2021, 22, 2043–2056. [Google Scholar] [CrossRef]
- Ren, Z.; Liao, T.; Li, C.; Kuang, Y. Drug Delivery Systems with a “Tumor-Triggered” Targeting or Intracellular Drug Release Property Based on DePEGylation. Mater. Today 2022, 15, 5290. [Google Scholar] [CrossRef] [PubMed]
- Domiński, A.; Domińska, M.; Skonieczna, M.; Pastuch-Gawołek, G.; Kurcok, P. Shell-Sheddable Micelles Based on Poly (ethylene glycol)-hydrazone-poly [R, S]-3-hydroxybutyrate Copolymer Loaded with 8-Hydroxyquinoline Glycoconjugates as a Dual Tumor-Targeting Drug Delivery System. Pharmaceutics 2022, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, X.; Chi, D.; Xu, Z.; Lin, G.; Liu, H.; Sun, J.; He, Z.; Wang, Y. Single-ligand dual-targeting irinotecan liposomes: Control of targeting ligand display by pH-responsive PEG-shedding strategy to enhance tumor-specific therapy and attenuate toxicity. Int. J. Pharm. 2020, 587, 119680. [Google Scholar] [CrossRef]
- Liu, C.; Li, C.; Jiang, S.; Zhang, C.; Tian, Y. pH-responsive hollow Fe–gallic acid coordination polymer for multimodal synergistic-therapy and MRI of cancer. Nanoscale Adv. 2022, 4, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Du, J.Z.; Sun, T.M.; Song, W.J.; Wu, J.; Wang, J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew. Chem. 2010, 49, 3621–3626. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A.; Höckel, M. Tumor hypoxia and malignant progression. Methods Enzymol. 2004, 381, 335–354. [Google Scholar] [CrossRef]
- Bailey, K.M.; Wojtkowiak, J.W.; Hashim, A.I.; Gillies, R.J. Targeting the metabolic microenvironment of tumors. Adv. Pharmacol. 2012, 65, 63–107. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.T.; Colgan, S.P. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef]
- Barsoum, I.B.; Smallwood, C.A.; Siemens, D.R.; Graham, C.H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014, 74, 665–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, V.L.; Al-Jamal, W.T. Exploiting the cancer niche: Tumor-associated macrophages and hypoxia as promising synergistic targets for nano-based therapy. J. Control. Release 2017, 253, 82–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casazza, A.; Di Conza, G.; Wenes, M.; Finisguerra, V.; Deschoemaeker, S.; Mazzone, M. Tumor stroma: A complexity dictated by the hypoxic tumor microenvironment. Oncogene 2014, 33, 1743–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bristow, R.G.; Hill, R.P. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jeon, J.; Park, J.H. Hypoxia-responsive nanoparticles for tumor-targeted drug delivery. Cancer Lett. 2020, 490, 31–43. [Google Scholar] [CrossRef]
- Huo, D.; Liu, S.; Zhang, C.; He, J.; Zhou, Z. Hypoxia-Targeting, Tumor Microenvironment Responsive Nanocluster Bomb for Radical-Enhanced Radiotherapy. ACS Nano 2017, 11, 10159–10174. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.G.; Yoon, H.Y.; Han, H.S.; Kim, S.H.; Son, S.; Jo, D.G.; Ahn, C.H.; Suh, Y.D.; Kim, K.; et al. Hypoxia-responsive polymeric nanoparticles for tumor-targeted drug delivery. Biomaterials 2014, 35, 1735–1743. [Google Scholar] [CrossRef]
- Son, S.; Rao, N.V.; Ko, H.; Shin, S.; Jeon, J.; Han, H.S.; Nguyen, V.Q.; Thambi, T.; Suh, Y.D.; Park, J.H. Carboxymethyl dextran-based hypoxia-responsive nanoparticles for doxorubicin delivery. Int. J. Biol. Macromol. 2018, 110, 399–405. [Google Scholar] [CrossRef]
- Thambi, T.; Son, S.; Lee, D.S.; Park, J.H. Poly(ethylene glycol)-b-poly(lysine) copolymer bearing nitroaromatics for hypoxia-sensitive drug delivery. Acta Biomater. 2016, 29, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, H.; Wu, X.; Guo, L.; Wang, F.; Xia, G.; Chen, B.; Yin, H.; Wang, Y.; Li, X. Tf-PEG-PLL-PLGA nanoparticles enhanced chemosensitivity for hypoxia-responsive tumor cells. OncoTargets Ther. 2016, 9, 5049–5059. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Zhang, S.; Ling, Y.; Meng, G.; Yang, Y.; Zhang, W. pH-responsive hybrid quantum dots for targeting hypoxic tumor siRNA delivery. J. Control. Release Off. J. Control. Release Soc. 2015, 220, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.Z.; Gordijo, C.R.; Amini, M.A.; Maeda, A.; Rauth, A.M.; DaCosta, R.S.; Wu, X.Y. Hybrid manganese dioxide nanoparticles potentiate radiation therapy by modulating tumor hypoxia. Cancer Res. 2016, 76, 6643–6656. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Usami, N.; Porcel, E.; Lacombe, S.; Le Sech, C. Enhancement of radiation effect by heavy elements. Mutat. Res. 2010, 704, 123–131. [Google Scholar] [CrossRef]
- Yoshimura, M.; Itasaka, S.; Harada, H.; Hiraoka, M. Microenvironment and radiation therapy. BioMed Res. Int. 2013, 2013, 685308. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29, 1701429. [Google Scholar] [CrossRef]
- Jägers, J.; Wrobeln, A.; Ferenz, K.B. Perfluorocarbon-based oxygen carriers: From physics to physiology. Pflug. Arch. Eur. J. Physiol. 2021, 473, 139–150. [Google Scholar] [CrossRef]
- Song, G.; Ji, C.; Liang, C.; Song, X.; Yi, X.; Dong, Z.; Yang, K.; Liu, Z. TaOx decorated perfluorocarbon nanodroplets as oxygen reservoirs to overcome tumor hypoxia and enhance cancer radiotherapy. Biomaterials 2017, 112, 257–263. [Google Scholar] [CrossRef]
- Song, G.; Liang, C.; Yi, X.; Zhao, Q.; Cheng, L.; Yang, K.; Liu, Z. Perfluorocarbon-Loaded Hollow Bi2Se3 Nanoparticles for Timely Supply of Oxygen under Near-Infrared Light to Enhance the Radiotherapy of Cancer. Adv. Mater. 2016, 28, 2716–2723. [Google Scholar] [CrossRef]
- Policastro, L.L.; Ibañez, I.L.; Notcovich, C.; Duran, H.A.; Podhajcer, O.L. The tumor microenvironment: Characterization, redox considerations, and novel approaches for reactive oxygen species-targeted gene therapy. Antioxid. Redox Signal. 2013, 19, 854–895. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.J.E.O. Role of tumour-associated macrophages in cancer-related inflammation. Exp. Oncol. 2010, 32, 153–158. [Google Scholar] [PubMed]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, Y.; Le, W.; Wang, K.; Kieffer, N.; Zhang, J. Redox control of the survival of healthy and diseased cells. Antioxid. Redox Signal. 2011, 15, 2867–2908. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [Green Version]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [Green Version]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxidative Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Bhasin, S.; Liu, Y.; Ayyash, D.; Rasmussen, J.; Huo, M. ROS-responsive polyprodrug nanoparticles for triggered drug delivery and effective cancer therapy. Adv. Mater. 2017, 29, 1700141. [Google Scholar] [CrossRef] [Green Version]
- Mirhadi, E.; Mashreghi, M.; Maleki, M.F.; Alavizadeh, S.H.; Arabi, L.; Badiee, A.; Jaafari, M.R. Redox-sensitive nanoscale drug delivery systems for cancer treatment. Int. J. Pharm. 2020, 589, 119882. [Google Scholar] [CrossRef]
- Li, D.; Zhang, R.; Liu, G.; Kang, Y.; Wu, J. Redox-Responsive Self-Assembled Nanoparticles for Cancer Therapy. Adv. Healthc. Mater. X 2020, 9, 2000605. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chi, Y.; Guo, C.; Feng, S.; Liu, J.; Sun, K.; Wu, Z. Chitooligosaccharides modified reduction-sensitive liposomes: Enhanced cytoplasmic drug delivery and osteosarcomas-tumor inhibition in animal models. Pharm. Res. 2017, 34, 2172–2184. [Google Scholar] [CrossRef]
- Yin, X.; Feng, S.; Chi, Y.; Liu, J.; Sun, K.; Guo, C.; Wu, Z. Estrogen-functionalized liposomes grafted with glutathione-responsive sheddable chotooligosaccharides for the therapy of osteosarcoma. Drug Deliv. 2018, 25, 900–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Lale, S.V.; Alex, M.A.; Choudhary, V.; Koul, V. Folic acid and trastuzumab conjugated redox responsive random multiblock copolymeric nanocarriers for breast cancer therapy: In-vitro and in-vivo studies. Colloids Surf. B Biointerfaces 2017, 149, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Mastrotto, F.; Taresco, V.; Tchoryk, A.; Quaglia, F.; Stolnik, S.; Alexander, C. Enhanced uptake in 2D-and 3D-lung cancer cell models of redox responsive PEGylated nanoparticles with sensitivity to reducing extra-and intracellular environments. J. Control. Release 2018, 277, 126–141. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, M.; Lu, H.; Liang, D.; Huang, Y.; Xia, Y.; Hu, Y.; Hu, S.; Wang, J.; Yi, X. Triple stimuli-responsive magnetic hollow porous carbon-based nanodrug delivery system for magnetic resonance imaging-guided synergistic photothermal/chemotherapy of cancer. ACS Appl. Mater. Interfaces 2018, 10, 21939–21949. [Google Scholar] [CrossRef]
- Guo, H.; Kim, J.-C. Reduction-sensitive poly (ethylenimine) nanogel bearing dithiodipropionic acid. Chem. Pharm. Bull. 2017, 65, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Achazi, K.; Schade, B.; Haag, R. Charge-conversional and reduction-sensitive poly (vinyl alcohol) nanogels for enhanced cell uptake and efficient intracellular doxorubicin release. J. Control. Release 2015, 205, 15–24. [Google Scholar] [CrossRef]
- Deng, S.; Iscaro, A.; Zambito, G.; Mijiti, Y.; Minicucci, M.; Essand, M.; Lowik, C.; Muthana, M.; Censi, R.; Mezzanotte, L. Development of a new hyaluronic acid based redox-responsive nanohydrogel for the encapsulation of oncolytic viruses for cancer immunotherapy. Nanomaterials 2021, 11, 144. [Google Scholar] [CrossRef]
- Deng, S.; Gigliobianco, M.R.; Mijiti, Y.; Minicucci, M.; Cortese, M.; Campisi, B.; Voinovich, D.; Battistelli, M.; Salucci, S.; Gobbi, P. Dually cross-linked core-shell structure nanohydrogel with redox–responsive degradability for intracellular delivery. Pharmaceutics 2021, 13, 2048. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Cairns, R.A.; Mak, T.W. The current state of cancer metabolism. Nat. Rev. Cancer 2016, 16, 613–614. [Google Scholar] [CrossRef]
- Curi, R.; Newsholme, P.; Newsholme, E.A. Metabolism of pyruvate by isolated rat mesenteric lymphocytes, lymphocyte mitochondria and isolated mouse macrophages. Biochem. J. 1988, 250, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Ristow, M.; Cuezva, J.M. Oxidative phosphorylation and cancer: The ongoing Warburg hypothesis. In Cellular Respiration and Carcinogenesis; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–18. [Google Scholar]
- Vander Heiden, M.G. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug Discov. 2011, 10, 671–684. [Google Scholar] [CrossRef] [Green Version]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef] [Green Version]
- Elgogary, A.; Xu, Q.; Poore, B.; Alt, J.; Zimmermann, S.C.; Zhao, L.; Fu, J.; Chen, B.; Xia, S.; Liu, Y. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E5328–E5336. [Google Scholar] [CrossRef] [Green Version]
- Lis, P.; Dyląg, M.; Niedźwiecka, K.; Ko, Y.H.; Pedersen, P.L.; Goffeau, A.; Ułaszewski, S. The HK2 dependent “Warburg effect” and mitochondrial oxidative phosphorylation in cancer: Targets for effective therapy with 3-bromopyruvate. Molecules 2016, 21, 1730. [Google Scholar] [CrossRef] [Green Version]
- Azevedo-Silva, J.; Queirós, O.; Baltazar, F.; Ułaszewski, S.; Goffeau, A.; Ko, Y.; Pedersen, P.; Preto, A.; Casal, M. The anticancer agent 3-bromopyruvate: A simple but powerful molecule taken from the lab to the bedside. J. Bioenerg. Biomembr. 2016, 48, 349–362. [Google Scholar] [CrossRef]
- Shoshan, M.C. 3-Bromopyruvate: Targets and outcomes. J. Bioenerg. Biomembr. 2012, 44, 7–15. [Google Scholar] [CrossRef]
- Allen, E.; Miéville, P.; Warren, C.M.; Saghafinia, S.; Li, L.; Peng, M.W.; Hanahan, D. Metabolic Symbiosis Enables Adaptive Resistance to Anti-angiogenic Therapy that Is Dependent on mTOR Signaling. Cell Rep. 2016, 15, 1144–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandham, S.K.; Talekar, M.; Singh, A.; Amiji, M.M. Inhibition of hexokinase-2 with targeted liposomal 3-bromopyruvate in an ovarian tumor spheroid model of aerobic glycolysis. Int. J. Nanomed. 2015, 10, 4405. [Google Scholar]
- Zhang, Y.; Wei, J.; Xu, J.; Leong, W.S.; Liu, G.; Ji, T.; Cheng, Z.; Wang, J.; Lang, J.; Zhao, Y. Suppression of tumor energy supply by liposomal nanoparticle-mediated inhibition of aerobic glycolysis. ACS Appl. Mater. Interfaces 2018, 10, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the "omics" era. Matrix Biol. J. Int. Soc. Matrix Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The matrisome: In silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteom. MCP 2012, 11, M111.014647. [Google Scholar] [CrossRef] [Green Version]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Raja, A.M.; Xu, S.; Zhuo, S.; Tai, D.C.; Sun, W.; So, P.T.; Welsch, R.E.; Chen, C.S.; Yu, H. Differential remodeling of extracellular matrices by breast cancer initiating cells. J. Biophotonics 2015, 8, 804–815. [Google Scholar] [CrossRef] [Green Version]
- Riegler, J.; Labyed, Y.; Rosenzweig, S.; Javinal, V.; Castiglioni, A. Tumor Elastography and Its Association with Collagen and the Tumor Microenvironment. Clin. Cancer Res. 2018, 24, 4455–4467. [Google Scholar] [CrossRef] [Green Version]
- Revert, F.; Revert-Ros, F.; Blasco, R.; Artigot, A.; López-Pascual, E.; Gozalbo-Rovira, R.; Ventura, I.; Gutiérrez-Carbonell, E.; Roda, N.; Ruíz-Sanchis, D.; et al. Selective targeting of collagen IV in the cancer cell microenvironment reduces tumor burden. Oncotarget 2018, 9, 11020–11045. [Google Scholar] [CrossRef] [Green Version]
- Murty, S.; Gilliland, T.; Qiao, P.; Tabtieng, T.; Higbee, E.; Zaki, A.A.; Puré, E.; Tsourkas, A. Nanoparticles functionalized with collagenase exhibit improved tumor accumulation in a murine xenograft model. Part. Part. Syst. Charact. 2014, 31, 1307–1312. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Hybrid collagenase nanocapsules for enhanced nanocarrier penetration in tumoral tissues. ACS Appl. Mater. Interfaces 2015, 7, 24075–24081. [Google Scholar] [CrossRef] [Green Version]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, L.; Zhang, R.; Dong, Z.; Wang, H.; Liu, Z. Collagenase-encapsulated pH-responsive nanoscale coordination polymers for tumor microenvironment modulation and enhanced photodynamic nanomedicine. ACS Appl. Mater. Interfaces 2018, 10, 43493–43502. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.; Zhao, Y.Y.; Curtis, J.M.; McMullen, T.P.; Brindley, D.N. Regulation of autotaxin expression and secretion by lysophosphatidate and sphingosine 1-phosphate. J. Lipid Res. 2015, 56, 1134–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rancoule, C.; Espenel, S.; Trone, J.C.; Langrand-Escure, J.; Vallard, A.; Rehailia-Blanchard, A.; El Meddeb Hamrouni, A.; Xia, Y.; Guy, J.B.; Ben-Mrad, M.; et al. Lysophosphatidic acid (LPA) as a pro-fibrotic and pro-oncogenic factor: A pivotal target to improve the radiotherapy therapeutic index. Oncotarget 2017, 8, 43543–43554. [Google Scholar] [CrossRef] [Green Version]
- Loskutov, Y.V.; Griffin, C.L.; Marinak, K.M.; Bobko, A.; Margaryan, N.V.; Geldenhuys, W.J.; Sarkaria, J.N.; Pugacheva, E.N. LPA signaling is regulated through the primary cilium: A novel target in glioblastoma. Oncogene 2018, 37, 1457–1471. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [Green Version]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Sun, Z.; Li, R.; Sun, J.; Peng, Y.; Xiao, L.; Zhang, X.; Xu, Y.; Wang, M. Matrix metalloproteinase cleavable nanoparticles for tumor microenvironment and tumor cell dual-targeting drug delivery. ACS Appl. Mater. Interfaces 2017, 9, 40614–40627. [Google Scholar] [CrossRef]
- Anajafi, T.; Yu, J.; Sedigh, A.; Haldar, M.K.; Muhonen, W.W.; Oberlander, S.; Wasness, H.; Froberg, J.; Molla, M.S.; Katti, K.S. Nuclear localizing peptide-conjugated, redox-sensitive polymersomes for delivering curcumin and doxorubicin to pancreatic cancer microtumors. Mol. Pharm. 2017, 14, 1916–1928. [Google Scholar] [CrossRef]
- Parcesepe, P.; Giordano, G. Cancer-Associated Immune Resistance and Evasion of Immune Surveillance in Colorectal Cancer. Gastroenterol. Res. Pract. 2016, 2016, 6261721. [Google Scholar] [CrossRef]
- Smith, H.A.; Kang, Y. The metastasis-promoting roles of tumor-associated immune cells. J. Mol. Med. 2013, 91, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Wang, Q.; Wang, X.; Ke, L.; Shi, K. Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics 2019, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Church, S.E.; Galon, J. Tumor microenvironment and immunotherapy: The whole picture is better than a glimpse. Immunity 2015, 43, 631–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès–Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Kim, J.; Bae, J.-S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat. Inflamm. 2016, 2016, 6058147. [Google Scholar] [CrossRef] [Green Version]
- Cuccarese, M.F.; Dubach, J.M.; Pfirschke, C.; Engblom, C.; Garris, C.; Miller, M.A.; Pittet, M.J.; Weissleder, R. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat. Commun. 2017, 8, 14293. [Google Scholar] [CrossRef] [Green Version]
- Ostuni, R.; Kratochvill, F.; Murray, P.J.; Natoli, G. Macrophages and cancer: From mechanisms to therapeutic implications. Trends Immunol. 2015, 36, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.; Hagemann, T. Tumour-associated macrophages and cancer. Curr. Opin. Pharmacol. 2013, 13, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Cieslewicz, M.; Tang, J.; Yu, J.L.; Cao, H.; Zavaljevski, M.; Motoyama, K.; Lieber, A.; Raines, E.W.; Pun, S.H. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc. Natl. Acad. Sci. USA 2013, 110, 15919–15924. [Google Scholar] [CrossRef] [Green Version]
- Conde, J.; Bao, C.; Tan, Y.; Cui, D.; Edelman, E.R.; Azevedo, H.S.; Byrne, H.J.; Artzi, N.; Tian, F. Dual targeted immunotherapy via in vivo delivery of biohybrid RNAi-peptide nanoparticles to tumor-associated macrophages and cancer cells. Adv. Funct. Mater. 2015, 25, 4183–4194. [Google Scholar] [CrossRef] [Green Version]
- Schmid, D.; Park, C.G.; Hartl, C.A.; Subedi, N.; Cartwright, A.N.; Puerto, R.B.; Zheng, Y.; Maiarana, J.; Freeman, G.J.; Wucherpfennig, K.W.J.N.c. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 2017, 8, 1747. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-S.S.; Moynihan, K.D.; Bekdemir, A.; Dichwalkar, T.M.; Noh, M.M.; Watson, N.; Melo, M.; Ingram, J.; Suh, H.; Ploegh, H. Targeting small molecule drugs to T cells with antibody-directed cell-penetrating gold nanoparticles. Biomater. Sci. 2019, 7, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Karmali, P.; Ramirez, M.; Xie, H.; Kotamraju, V.R.; Ruoslahti, E.; Smith, J.W. Targeting tumor associated macrophages using clodronate-loaded PLGA nanoparticles. In Proceedings of the NSTI Nanotech; NSTI Nanotech: Austin, TX, USA, 2010; Volume 3, pp. 382–385. [Google Scholar]
- Zhu, S.; Niu, M.; O’Mary, H.; Cui, Z. Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Mol. Pharm. 2013, 10, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Liu, B.-Y.; Wu, J.-L.; Ai, S.-L.; Zhuo, R.-X.; Cheng, S.-X. A dual macrophage targeting nanovector for delivery of oligodeoxynucleotides to overcome cancer-associated immunosuppression. ACS Appl. Mater. Interfaces 2017, 9, 42566–42576. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Li, C.; Song, Y.; Shi, T.; Luo, X.; Zhang, H.; Hu, L.; Yan, X.; Zheng, H.; Liu, M. Targeted delivery of ibrutinib to tumor-associated macrophages by sialic acid-stearic acid conjugate modified nanocomplexes for cancer immunotherapy. Acta Biomater. 2019, 92, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Uehara, M.; Kasinath, V.; Dai, L.; Eskandari, S.K.; Bahmani, B.; Yonar, M.; Azzi, J.R.; Haik, Y.; Sage, P.T.; et al. Targeting antigen-presenting cells by anti-PD-1 nanoparticles augments antitumor immunity. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kise, K.; Kinugasa-Katayama, Y.; Takakura, N. Tumor microenvironment for cancer stem cells. Adv. Drug Deliv. Rev. 2016, 99, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Bruno, A.; Gallo, C.; Pajardi, G.; Noonan, D.M.; Dallaglio, K. Cancer stem cells and the tumor microenvironment: Interplay in tumor heterogeneity. Connect. Tissue Res. 2015, 56, 414–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsten, U.; Goletz, S. What makes cancer stem cell markers different? Springerplus 2013, 2, 301. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Chen, H.; Chen, Y.; Zhang, K.; Wang, X.; Cui, X.; Shi, J. Hyaluronic acid-conjugated mesoporous silica nanoparticles: Excellent colloidal dispersity in physiological fluids and targeting efficacy. J. Mater. Chem. 2012, 22, 5615–5621. [Google Scholar] [CrossRef]
- Yi, Y.; Kim, H.J.; Zheng, M.; Mi, P.; Naito, M.; Kim, B.S.; Min, H.S.; Hayashi, K.; Perche, F.; Toh, K. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J. Control. Release 2019, 295, 268–277. [Google Scholar] [CrossRef]
- Kim, D.-M.; Kim, M.; Park, H.-B.; Kim, K.-S.; Kim, D.-E. Anti-MUC1/CD44 dual-aptamer-conjugated liposomes for cotargeting breast cancer cells and cancer stem cells. ACS Appl. Bio Mater. 2019, 2, 4622–4633. [Google Scholar] [CrossRef]
- Ning, S.-T.; Lee, S.-Y.; Wei, M.-F.; Peng, C.-L.; Lin, S.Y.-F.; Tsai, M.-H.; Lee, P.-C.; Shih, Y.-H.; Lin, C.-Y.; Luo, T.-Y. Targeting colorectal cancer stem-like cells with anti-CD133 antibody-conjugated SN-38 nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 17793–17804. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Ireland, L.V.; Mielgo, A. Macrophages and fibroblasts, key players in cancer chemoresistance. Front. Cell Dev. Biol. 2018, 6, 131. [Google Scholar] [CrossRef] [Green Version]
- Kubo, N.; Araki, K.; Kuwano, H.; Shirabe, K. Cancer-associated fibroblasts in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 6841. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Ishii, G.; Ochiai, A.; Neri, S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev 2016, 99, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Kakarla, S.; Song, X.T.; Gottschalk, S. Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy 2012, 4, 1129–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tekade, R.K.; Dutta, T.; Tyagi, A.; Bharti, A.C.; Das, B.C.; Jain, N.K. Surface-engineered dendrimers for dual drug delivery: A receptor up-regulation and enhanced cancer targeting strategy. J. Drug Target. 2008, 16, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, Z.; Sun, J.; Song, Q.; He, B.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer-associated fibroblasts. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 131–141. [Google Scholar] [CrossRef]
- Wang, Y.; MacDonald, R.G.; Thinakaran, G.; Kar, S. Insulin-like growth factor-II/cation-independent mannose 6-phosphate receptor in neurodegenerative diseases. Mol. Neurobiol. 2017, 54, 2636–2658. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.I.; Conroy, K.P.; Henderson, N.C. Hepatic stellate cells: Central modulators of hepatic carcinogenesis. BMC Gastroenterol. 2015, 15, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beljaars, L.; Molema, G.; Weert, B.; Bonnema, H.; Olinga, P.; Groothuis, G.M.; Meijer, D.K.; Poelstra, K. Albumin modified with mannose 6-phosphate: A potential carrier for selective delivery of antifibrotic drugs to rat and human hepatic stellate cells. Hepatology 1999, 29, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Ding, Y.; Zhao, Y.; Wang, J.; Qin, H.; Liu, X.; Lang, J.; Zhao, R.; Zhang, Y.; Shi, J.J.A.m. Peptide assembly integration of fibroblast-targeting and cell-penetration features for enhanced antitumor drug delivery. Adv. Mater. 2015, 27, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Zhao, Y.; Ding, Y.; Wang, J.; Zhao, R.; Lang, J.; Qin, H.; Liu, X.; Shi, J.; Tao, N. Transformable peptide nanocarriers for expeditious drug release and effective cancer therapy via cancer-associated fibroblast activation. Angew. Chem. 2016, 128, 1062–1067. [Google Scholar] [CrossRef]
- Miao, L.; Wang, Y.; Lin, C.M.; Xiong, Y.; Chen, N.; Zhang, L.; Kim, W.Y.; Huang, L. Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. J. Control. Release 2015, 217, 27–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, C.; Heath, E.I. Angiogenesis inhibitors in the treatment of prostate cancer. J. Hematol. Oncol. 2010, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef]
- Lu, L.; Chen, H.; Hao, D.; Zhang, X.; Wang, F. The functions and applications of A7R in anti-angiogenic therapy, imaging and drug delivery systems. Asian J. Pharm. Sci. 2019, 14, 595–608. [Google Scholar] [CrossRef]
- Du, S.; Xiong, H.; Xu, C.; Lu, Y.; Yao, J. Attempts to strengthen and simplify the tumor vascular normalization strategy using tumor vessel normalization promoting nanomedicines. Biomater. Sci. 2019, 7, 1147–1160. [Google Scholar] [CrossRef]
- Cao, J.; Wang, R.; Gao, N.; Li, M.; Tian, X.; Yang, W.; Ruan, Y.; Zhou, C.; Wang, G.; Liu, X. A7RC peptide modified paclitaxel liposomes dually target breast cancer. Biomater. Sci. 2015, 3, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Han, S.; Zheng, H.; Ma, R.; Ping, Y.; Zou, J.; Tang, H.; Zhang, Y.; Xu, X.; Li, F. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int. J. Nanomed. 2018, 13, 5937. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.A.; Majeti, B.K.; Barnes, L.A.; Makale, M.; Weis, S.M.; Lutu-Fuga, K.; Wrasidlo, W.; Cheresh, D.A. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 9343–9348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eloy, J.O.; Ruiz, A.; de Lima, F.T.; Petrilli, R.; Raspantini, G.; Nogueira, K.A.B.; Santos, E.; de Oliveira, C.S.; Borges, J.C.; Marchetti, J.M. EGFR-targeted immunoliposomes efficiently deliver docetaxel to prostate cancer cells. Colloids Surf. B Biointerfaces 2020, 194, 111185. [Google Scholar] [CrossRef] [PubMed]
- McDaid, W.J.; Greene, M.K.; Johnston, M.C.; Pollheimer, E.; Smyth, P.; McLaughlin, K.; Van Schaeybroeck, S.; Straubinger, R.M.; Longley, D.B.; Scott, C.J. Repurposing of Cetuximab in antibody-directed chemotherapy-loaded nanoparticles in EGFR therapy-resistant pancreatic tumours. Nanoscale 2019, 11, 20261–20273. [Google Scholar] [CrossRef]
- Aggarwal, S.; Gupta, S.; Pabla, D.; Murthy, R. Gemcitabine-loaded PLGA-PEG immunonanoparticles for targeted chemotherapy of pancreatic cancer. Cancer Nanotechnol. 2013, 4, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef]
- Xu, W.; Luo, T.; Li, P.; Zhou, C.; Cui, D.; Pang, B.; Ren, Q.; Fu, S. RGD-conjugated gold nanorods induce radiosensitization in melanoma cancer cells by downregulating αvβ3 expression. Int. J. Nanomed. 2012, 7, 915. [Google Scholar]
- London, M.; Gallo, E. Epidermal growth factor receptor (EGFR) involvement in epithelial-derived cancers and its current antibody-based immunotherapies. Cell Biol. Int. 2020, 44, 1267–1282. [Google Scholar] [CrossRef]
- Jorissen, R.N.; Walker, F.; Pouliot, N.; Garrett, T.P.; Ward, C.W.; Burgess, A.W. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp. Cell Res. 2003, 284, 31–53. [Google Scholar] [CrossRef]
- Ayati, A.; Moghimi, S.; Salarinejad, S.; Safavi, M.; Pouramiri, B.; Foroumadi, A. A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorg. Chem. 2020, 99, 103811. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Santos, E.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; Reis, A.V.F.; Neto, J.d.B.V.; da Silva Júnior, I.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles. Int. J. Pharm. 2020, 592, 120082. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdavi Firouzabadi, B.; Gigliobianco, M.R.; Joseph, J.M.; Censi, R.; Di Martino, P. Design of Nanoparticles in Cancer Therapy Based on Tumor Microenvironment Properties. Pharmaceutics 2022, 14, 2708. https://doi.org/10.3390/pharmaceutics14122708
Mahdavi Firouzabadi B, Gigliobianco MR, Joseph JM, Censi R, Di Martino P. Design of Nanoparticles in Cancer Therapy Based on Tumor Microenvironment Properties. Pharmaceutics. 2022; 14(12):2708. https://doi.org/10.3390/pharmaceutics14122708
Chicago/Turabian StyleMahdavi Firouzabadi, Bita, Maria Rosa Gigliobianco, Joice Maria Joseph, Roberta Censi, and Piera Di Martino. 2022. "Design of Nanoparticles in Cancer Therapy Based on Tumor Microenvironment Properties" Pharmaceutics 14, no. 12: 2708. https://doi.org/10.3390/pharmaceutics14122708
APA StyleMahdavi Firouzabadi, B., Gigliobianco, M. R., Joseph, J. M., Censi, R., & Di Martino, P. (2022). Design of Nanoparticles in Cancer Therapy Based on Tumor Microenvironment Properties. Pharmaceutics, 14(12), 2708. https://doi.org/10.3390/pharmaceutics14122708