PAMAM-Calix-Dendrimers: Second Generation Synthesis, Fluorescent Properties and Catecholamines Binding
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Information
2.2. General Procedure for the Synthesis of the Compounds G1.5
2.2.1. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N,N-di(N-(2-(N,N-di(methoxycarbonylethyl)amino)ethyl)carbamoylethyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene, [G1.5-cone]. Viscous Yellowish Oil, Yield: 1.52 g (95%)
2.2.2. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N,N-di(N-(2-(N,N-di(methoxycarbonylethyl)amino)ethyl)carbamoylethyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene, [G1.5-paco]. Viscous Yellowish Oil, Yield: 1.57 g (98%)
2.2.3. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N,N-di(N-(2-(N,N-di(methoxycarbonylethyl)amino)ethyl)carbamoylethyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene, [G1.5-alt]. Viscous Yellowish Oil, Yield: 1.58 g (99%)
2.3. General Procedure for the Synthesis of the Compounds G2
2.3.1. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N,N-di(N-(2-(N,N-di(N-(2-aminoethyl)carbamoylethyl)amino)ethyl)carbamoylethyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene, [G2-cone]. White Solid Foam, m.p. 68 °C, Yield: 1.01 g (90%)
2.3.2. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N,N-di(N-(2-(N,N-di(N-(2-aminoethyl)carbamoylethyl)amino)ethyl)carbamoylethyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene, [G2-paco]. White Solid Foam, m.p. 69 °C, Yield: 0.96 g (85%)
2.3.3. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis[N-(6-(N,N-di(N-(2-(N,N-di(N-(2-aminoethyl)carbamoylethyl)amino)ethyl)carbamoylethyl)amino)hexyl)carbamoylmethoxy]-2,8,14,20-tetrathiacalix[4]arene [G2-alt]. White Solid Foam, m.p. 75 °C, Yield: 0.99 g (88%)
2.4. Procedure for the Synthesis of the Compound G0.5-Monomer
N-(6-(N,N-di(methoxycarbonylethyl)amino)hexyl)-2-(4-(tert-butyl)phenoxy)acetamide, [G0.5-monomer]. Viscous Yellowish Oil, Yield: 2.70 g (97%)
2.5. Procedure for the Synthesis of the Compound G1-Monomer
N-(6-(N,N-di(N-(2-aminoethyl)carbamoylethyl)amino)hexyl)-2-(4-(tert-butyl)phenoxy)acetamide, [G1-monomer]. Viscous Yellowish Oil, Yield: 0.76 g (97%)
2.6. Preparation of the Compounds G1∙HCl, G2∙HCl and G1-Monomer∙HCl
2.7. Study of the PAMAM-Calix-Dendrimers Emission Properties
2.8. Study of Hemolysis Activity
3. Results and Discussion
3.1. Synthesis of the Second Generation of Poly(Amidoamine) Dendrimers Based on p-Tert-Butylthiacalixarene (PAMAM-Calix-Dendrimers) in Different Conformations
3.2. Spectral Properties of the First and Second Generation of PAMAM-Calix-Dendrimers
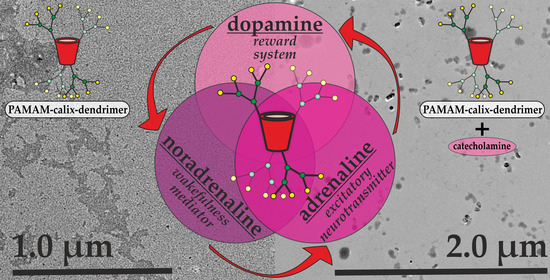
3.3. Binding of the Catecholamines by the G1 and G2 PAMAM-Calix-Dendrimers
3.4. Hemolytic Activity of the G1-alt and G2-alt PAMAM-Calix-Dendrimers
3.5. In Vitro Release Studies of Catecholamines from Their Complexes with PAMAM-Calix-Dendrimers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philippu, A. (Ed.) In Vivo Neuropharmacology and Neurophysiology; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Oberbeck, R. Catecholamines: Physiological Immunomodulators During Health and Illness. Curr. Med. Chem. 2006, 13, 1979–1989. [Google Scholar] [CrossRef]
- Khan, F.H.; Ahlberg, C.D.; Chow, C.A.; Shah, D.R.; Koo, B.B. Iron, Dopamine, Genetics, and Hormones in the Pathophysiology of Restless Legs Syndrome. J. Neurol. 2017, 264, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, K.; Noworyta-Sokołowska, K.; Jurczak, A.; Górska, A.; Rogóż, Z.; Gołembiowska, K. Risperidone and Escitalopram Co-Administration: A Potential Treatment of Schizophrenia Symptoms with Less Side Effects. Pharmacol. Rep. 2017, 69, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Post, M.R.; Lieberman, O.J.; Mosharov, E.V. Can Interactions Between α-Synuclein, Dopamine and Calcium Explain Selective Neurodegeneration in Parkinson’s Disease? Front. Neurosci. 2018, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Fact Sheet—Dementia. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 23 September 2022).
- De Backer, D.; Foulon, P. Minimizing Catecholamines and Optimizing Perfusion. Crit. Care 2019, 23, 149. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Liu, C.; Pang, Z. Dendrimer-Based Drug Delivery Systems for Brain Targeting. Biomolecules 2019, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for SiRNA and MicroRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Pedziwiatr-Werbicka, E.; Milowska, K.; Dzmitruk, V.; Ionov, M.; Shcharbin, D.; Bryszewska, M. Dendrimers and Hyperbranched Structures for Biomedical Applications. Eur. Polym. J. 2019, 119, 61–73. [Google Scholar] [CrossRef]
- Nunes, D.; Loureiro, J.A.; Pereira, M.C. Drug Delivery Systems as a Strategy to Improve the Efficacy of FDA-Approved Alzheimer’s Drugs. Pharmaceutics 2022, 14, 2296. [Google Scholar] [CrossRef]
- Hao, M.; Chen, B.; Zhao, X.; Zhao, N.; Xu, F.-J. Organic/Inorganic Nanocomposites for Cancer Immunotherapy. Mater. Chem. Front. 2020, 4, 2571–2609. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in Vaccine Delivery: Recent Progress and Advances. Biomaterials 2022, 280, 121303. [Google Scholar] [CrossRef] [PubMed]
- Taghavi Pourianazar, N.; Mutlu, P.; Gunduz, U. Bioapplications of Poly(Amidoamine) (PAMAM) Dendrimers in Nanomedicine. J. Nanopart. Res. 2014, 16, 2342. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, S.; Abourehab, M.A.S.; Sahebkar, A.; Kesharwani, P. Exploring Dendrimer-Based Drug Delivery Systems and Their Potential Applications in Cancer Immunotherapy. Eur. Polym. J. 2022, 177, 111471. [Google Scholar] [CrossRef]
- Ahlawat, J.; Guillama Barroso, G.; Masoudi Asil, S.; Alvarado, M.; Armendariz, I.; Bernal, J.; Carabaza, X.; Chavez, S.; Cruz, P.; Escalante, V.; et al. Nanocarriers as Potential Drug Delivery Candidates for Overcoming the Blood–Brain Barrier: Challenges and Possibilities. ACS Omega 2020, 5, 12583–12595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shi, X. Dendrimer-Based Nanodevices for Targeted Drug Delivery Applications. J. Mater. Chem. B 2013, 1, 4199–4211. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In Vitro Cytotoxicity Testing of Polycations: Influence of Polymer Structure on Cell Viability and Hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Duncan, R.; Izzo, L. Dendrimer Biocompatibility and Toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef]
- Labieniec-Watala, M.; Watala, C. PAMAM Dendrimers: Destined for Success or Doomed to Fail? Plain and Modified PAMAM Dendrimers in the Context of Biomedical Applications. J. Pharm. Sci. 2015, 104, 2–14. [Google Scholar] [CrossRef]
- Iki, N. Thiacalixarenes. In Calixarenes and Beyond; Springer: Cham, Switzerland, 2016; pp. 335–362. [Google Scholar] [CrossRef]
- Solovieva, S.E.; Burilov, V.A.; Antipin, I.S. Thiacalix[4]Arene’s Lower Rim Derivatives: Synthesis and Supramolecular Properties. Macroheterocycles 2017, 10, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Shurpik, D.N.; Padnya, P.L.; Stoikov, I.I.; Cragg, P.J. Antimicrobial Activity of Calixarenes and Related Macrocycles. Molecules 2020, 25, 5145. [Google Scholar] [CrossRef]
- Fasting, C.; Schalley, C.A.; Weber, M.; Seitz, O.; Hecht, S.; Koksch, B.; Dernedde, J.; Graf, C.; Knapp, E.-W.; Haag, R. Multivalency as a Chemical Organization and Action Principle. Angew. Chem. Int. Ed. 2012, 51, 10472–10498. [Google Scholar] [CrossRef] [Green Version]
- Padnya, P.L.; Terenteva, O.S.; Akhmedov, A.A.; Iksanova, A.G.; Shtyrlin, N.V.; Nikitina, E.V.; Krylova, E.S.; Shtyrlin, Y.G.; Stoikov, I.I. Thiacalixarene Based Quaternary Ammonium Salts as Promising Antibacterial Agents. Bioorg. Med. Chem. 2021, 29, 115905. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.F.P.; Mastelaro, V.R.; Vieira, E.G.; do Carmo, D.R. β-cyclodextrine PAMAM Dendrimer Surface Doped with Silver and Hexacyanoferrate (III) and its Applications for Dopamine Detection in Synthetic Samples. Electroanalysis 2022, 34, 1–13. [Google Scholar] [CrossRef]
- Mostovaya, O.; Padnya, P.; Shiabiev, I.; Mukhametzyanov, T.; Stoikov, I. PAMAM-Calix-Dendrimers: Synthesis and Thiacalixarene Conformation Effect on DNA Binding. Int. J. Mol. Sci. 2021, 22, 11901. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, O.A.; Padnya, P.L.; Shurpik, D.N.; Shiabiev, I.E.; Stoikov, I.I. Novel Lactide Derivatives of p-tert-Butylthiacalix[4]Arene: Directed Synthesis and Molecular Recognition of Catecholamines. J. Mol. Liq. 2021, 327, 114806. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Huang, A.Y.-T.; Kao, C.-L.; Peng, L. Poly(Amidoamine) Dendrimers: Covalent and Supramolecular Synthesis. Mater. Today Chem. 2019, 13, 34–48. [Google Scholar] [CrossRef]
- He, M.; McLuckey, S.A. Tandem Mass Spectrometry of Half-Generation PAMAM Dendrimer Anions. Rapid Commun. Mass Spectrom. 2004, 18, 960–972. [Google Scholar] [CrossRef]
- Tolić, L.P.; Anderson, G.A.; Smith, R.D.; Brothers, H.M., II; Spindler, R.; Tomalia, D.A. Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometric Characterization of High Molecular Mass Starburst™ Dendrimers. Int. J. Mass Spectrom. Ion Process. 1997, 165–166, 405–418. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Klajnert-Maculewicz, B.; Johnson, K.A.-M.; Brinkman, H.F.; Janaszewska, A.; Hedstrand, D.M. Non-Traditional Intrinsic Luminescence: Inexplicable Blue Fluorescence Observed for Dendrimers, Macromolecules and Small Molecular Structures Lacking Traditional/Conventional Luminophores. Prog. Polym. Sci. 2019, 90, 35–117. [Google Scholar] [CrossRef]
- Larson, C.L.; Tucker, S.A. Intrinsic Fluorescence of Carboxylate-Terminated Polyamido Amine Dendrimers. Appl. Spectrosc. 2001, 55, 679–683. [Google Scholar] [CrossRef]
- Jiang, N.; Zhu, D.; Su, Z.; Bryce, M.R. Recent Advances in Oligomers/Polymers with Unconventional Chromophores. Mater. Chem. Front. 2021, 5, 60–75. [Google Scholar] [CrossRef]
- Wang, D.; Imae, T. Fluorescence Emission from Dendrimers and Its PH Dependence. J. Am. Chem. Soc. 2004, 126, 13204–13205. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Imae, T.; Miki, M. Fluorescence Emission from PAMAM and PPI Dendrimers. J. Colloid Interface Sci. 2007, 306, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ficker, M.; Paolucci, V.; Christensen, J.B. Improved Large-Scale Synthesis and Characterization of Small and Medium Generation PAMAM Dendrimers. Can. J. Chem. 2017, 95, 954–964. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Mostovaya, O.A.; Sevastyanov, D.A.; Lenina, O.A.; Sapunova, A.S.; Voloshina, A.D.; Petrov, K.A.; Kovyazina, I.V.; Cragg, P.J.; Stoikov, I.I. Supramolecular Neuromuscular Blocker Inhibition by a Pillar[5]Arene through Aqueous Inclusion of Rocuronium Bromide. Org. Biomol. Chem. 2019, 17, 9951–9959. [Google Scholar] [CrossRef] [PubMed]
- Yavorovska, V.I.; Labyntseva, R.D.; Bevza, O.V.; Pugach, A.Y.; Drapailo, A.B.; Cherenok, S.O.; Kalchenko, V.I. Thiacalix[4]Arene Phosphonate C-800 as a Novel Fluorescent Probe for Zinc in Living Cells. Ukr. Biochem. J 2021, 93, 26–36. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; p. 954. [Google Scholar]
- Valeur, B. Molecular Fluorescence: Principles and Application; Wiley-VCH: Weinheim, Germany, 2001; p. 383. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Padnya, P.L.; Khripunova, I.A.; Mostovaya, O.A.; Mukhametzyanov, T.A.; Evtugyn, V.G.; Vorobev, V.V.; Osin, Y.N.; Stoikov, I.I. Self-Assembly of Chiral Fluorescent Nanoparticles Based on Water-Soluble L-Tryptophan Derivatives of p-tert-Butylthiacalix[4]Arene. Beilstein J. Nanotechnol. 2017, 8, 1825–1835. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, V.; Aggarwal, S.; Tiwari, A.K.; Chuttani, K.; Pratap, R.; Mishra, A.K. Design, Synthesis, and Biological Evaluation of Catecholamine Vehicle for Studying Dopaminergic System. Chem. Biol. Drug Des. 2013, 82, 226–232. [Google Scholar] [CrossRef]
- Lelou, E.; Corlu, A.; Nesseler, N.; Rauch, C.; Mallédant, Y.; Seguin, P.; Aninat, C. The Role of Catecholamines in Pathophysiological Liver Processes. Cells 2022, 11, 1021. [Google Scholar] [CrossRef]
- Kulikova, T.; Padnya, P.; Shiabiev, I.; Rogov, A.; Stoikov, I.; Evtugyn, G. Electrochemical Sensing of Interactions between DNA and Charged Macrocycles. Chemosensors 2021, 9, 347. [Google Scholar] [CrossRef]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers: Relationship Between Structure and Biocompatibility In Vitro, and Preliminary Studies on The Biodistribution of 125I-Labelled Polyamidoamine Dendrimers In Vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jeon, M.; Paeng, K.-J.; Paeng, I.R. Competitive Enzyme-Linked Immunosorbent Assay for the Determination of Catecholamine, Dopamine in Serum. Anal. Chim. Acta 2008, 619, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Barreto, W.J.; Ponzoni, S.; Sassi, P. A Raman and UV-Vis Study of Catecholamines Oxidized with Mn(III). Spectrochim. Acta Part A 1998, 55, 65–72. [Google Scholar] [CrossRef]
- Vavilova, A.A.; Nosov, R.V.; Mostovaya, O.A.; Stoikov, I.I. Synthesis of Three Stereoisomers of p-tert-Butylthiacalix[4]Arene Substituted with (Ethoxycarbonyl)Methoxy and Fluorescent 1-Amidoanthraquinone Fragments. Macroheterocycles 2016, 9, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Lang, K.; Prošková, P.; Kroupa, J.; Morávek, J.; Stibor, I.; Pojarová, M.; Lhoták, P. The Synthesis and Complexation of Novel Azosubstituted Calix[4]Arenes and Thiacalix[4]Arenes. Dyes Pigm. 2008, 77, 646–652. [Google Scholar] [CrossRef]
- Siva, S.; Venkatesh, G.; Prabhu, A.A.M.; Sankaranarayanan, R.K.; Rajendiran, N. Absorption and Fluorescence Spectral Characteristics of Norepinephrine, Epinephrine, Isoprenaline, Methyl Dopa, Terbutaline and Orciprenaline Drugs. Phys. Chem. Liq. 2012, 50, 434–452. [Google Scholar] [CrossRef]
- Mostovaya, O.A.; Gorbachuk, V.V.; Bazanova, O.B.; Gerasimov, A.V.; Evtugyn, V.G.; Osin, Y.N.; Myakushev, V.D.; Rizvanov, I.K.; Stoikov, I.I. Thiacalixarene “Knot” Effect on Protein Binding by Oligolactic Acid Particles. Mater. Chem. Front. 2019, 3, 292–300. [Google Scholar] [CrossRef]
- Mostovaya, O.A.; Padnya, P.L.; Vavilova, A.A.; Shurpik, D.N.; Khairutdinov, B.I.; Mukhametzyanov, T.A.; Khannanov, A.A.; Kutyreva, M.P.; Stoikov, I.I. Tetracarboxylic Acids on a Thiacalixarene Scaffold: Synthesis and Binding of Dopamine Hydrochloride. New J. Chem. 2018, 42, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Thordarson, P. Determining Association Constants from Titration Experiments in Supramolecular Chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
- Brynn Hibbert, D.; Thordarson, P. The Death of the Job Plot, Transparency, Open Science and Online Tools, Uncertainty Estimation Methods and Other Developments in Supramolecular Chemistry Data Analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bindfit v0.5, Open Data Fit. Available online: https://supramolecular.org/bindfit/ (accessed on 7 October 2022).
- Strange, P.G. The Energetics of Ligand Binding at Catecholamine Receptors. Trends Pharmacol. Sci. 1996, 17, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, O.A.; Padnya, P.L.; Shurpik, D.N.; Vavilova, A.A.; Evtugyn, V.G.; Osin, Y.N.; Stoikov, I.I. Iminodiacetic Derivatives of p-tert-Butylthiacalix[4]Arene: Synthesis and Influence of Conformation on the Aggregation with Bismarck Brown Y. Macroheterocycles 2017, 10, 154–163. [Google Scholar] [CrossRef]
- Domański, D.M.; Klajnert, B.; Bryszewska, M. Influence of PAMAM Dendrimers on Human Red Blood Cells. Bioelectrochemistry 2004, 63, 189–191. [Google Scholar] [CrossRef]
- Kesharwani, P.; Mishra, V.; Jain, N.K. Generation Dependent Hemolytic Profile of Folate Engineered Poly(Propyleneimine) Dendrimer. J. Drug Delivery Sci. Technol. 2015, 28, 1–6. [Google Scholar] [CrossRef]
- Serri, A.; Mahboubi, A.; Zarghi, A.; Moghimi, H.R. PAMAM-dendrimer Enhanced Antibacterial Effect of Vancomycin Hydrochloride Against Gram-Negative Bacteria. J. Pharm. Pharm. Sci. 2019, 22, 10–21. [Google Scholar] [CrossRef] [Green Version]









| Compound | C, M | d, nm | PDI |
|---|---|---|---|
| G1-monomer | 2 × 10−4 | 103.8 ± 1.3 | 0.26 ± 0.01 |
| 1 × 10−4 | 107.5 ± 1.4 | 0.26 ± 0.01 | |
| 5 × 10−5 | 109.7 ± 4.6 | 0.27 ± 0.02 | |
| 1 × 10−5 | 114.9 ± 2.3 | 0.24 ± 0.01 | |
| G1-cone | 1 × 10−4 | 22 ± 16 | 0.46 ± 0.14 |
| 5 × 10−5 | 165 ± 61 | 0.54 ± 0.25 | |
| 1 × 10−5 | 258 ± 3 | 0.21 ± 0.02 | |
| G1-paco | 1 × 10−4 | 111 ± 44 | 0.78 ± 0.13 |
| 5 × 10−5 | 332 ± 30 | 0.62 ± 0.13 | |
| 1 × 10−5 | 600 ± 64 | 0.37 ± 0.05 | |
| G1-alt | 1 × 10−4 | 1101 ± 47 | 0.38 ± 0.04 |
| 5 × 10−5 | 1041 ± 87 | 0.40 ± 0.07 | |
| 1 × 10−5 | 903 ± 44 | 0.46 ± 0.07 | |
| G2-cone | 1 × 10−4 | 574 ± 33 | 0.49 ± 0.08 |
| 5 × 10−5 | 985 ± 68 | 0.72 ± 0.07 | |
| 1 × 10−5 | 423 ± 39 | 0.62 ± 0.08 | |
| G2-paco | 1 × 10−4 | 1156 ± 78 | 0.51 ± 0.08 |
| 5 × 10−5 | 1274 ± 144 | 0.62 ± 0.11 | |
| 1 × 10−5 | 860 ± 185 | 0.82 ± 0.12 | |
| G2-alt | 1 × 10−4 | 2293 ± 704 | 0.46 ± 0.25 |
| 5 × 10−5 | 1632 ± 353 | 0.69 ± 0.22 | |
| 1 × 10−5 | 1208 ± 277 | 0.83 ± 0.15 |
| Compound | The Binding Constant Ka (% Error)/logKa | ||
|---|---|---|---|
| Dopamine HCl | L-Adrenaline HCl | L-Noradrenaline HCl | |
| G1-cone | 8550 (0.26)/3.93 | 10,188 (0.46)/4.00 | 17,014 (0.59)/4.23 |
| G1-paco | 7130 (0.45)/3.85 | 9236 (0.37)/3.97 | 17,208 (0.50)/4.24 |
| G1-alt | 32,800 (0.78)/4.52 | 28,356 (0.93)/4.45 | 52,435 (0.89)/4.72 |
| G2-cone | 24,688 (0.54)/4.39 | 30,857 (1.08)/4.49 | 14,430 (0.55)/4.16 |
| G2-paco | 21,953 (0.61)/4.34 | 23,695 (0.62)/4.37 | 14,471 (0.40)/4.16 |
| G2-alt | 37,152 (0.72)/4.57 | 55,198 (1.01)/4.74 | 17,977 (0.50)/4.25 |
| System | d, nm | PDI |
|---|---|---|
| Dopamine HCl/G1-cone | 376 ± 11 | 0.22 ± 0.01 |
| Dopamine HCl/G1-paco | 745 ± 25 | 0.20 ± 0.05 |
| Dopamine HCl/G1-alt | 873 ± 63 | 0.51 ± 0.06 |
| Dopamine HCl/G2-cone | 444 ± 54 | 0.22 ± 0.18 |
| Dopamine HCl/G2-paco | 898 ± 125 | 0.50 ± 0.26 |
| Dopamine HCl/G2-alt | 1416 ± 367 | 0.84 ± 0.11 |
| Noradrenaline HCl/G1-cone | 516 ± 34 | 0.23 ± 0.02 |
| Noradrenaline HCl/G1-paco | 536 ± 7 | 0.21 ± 0.07 |
| Noradrenaline HCl/G1-alt | 1258 ± 78 | 0.49 ± 0.10 |
| Noradrenaline HCl/G2-cone | 423 ± 33 | 0.40 ± 0.04 |
| Noradrenaline HCl/G2-paco | 829 ± 99 | 0.52 ± 0.08 |
| Noradrenaline HCl/G2-alt | 1153 ± 146 | 0.53 ± 0.20 |
| Adrenaline HCl/G1-cone | 273 ± 10 | 0.25 ± 0.02 |
| Adrenaline HCl/G1-paco | 572 ± 23 | 0.30 ± 0.08 |
| Adrenaline HCl/G1-alt | 654 ± 23 | 0.25 ± 0.14 |
| Adrenaline HCl/G2-cone | 311 ± 27 | 0.28 ± 0.06 |
| Adrenaline HCl/G2-paco | 684 ± 99 | 0.63 ± 0.08 |
| Adrenaline HCl/G2-alt | 1446 ± 306 | 0.89 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostovaya, O.; Shiabiev, I.; Pysin, D.; Stanavaya, A.; Abashkin, V.; Shcharbin, D.; Padnya, P.; Stoikov, I. PAMAM-Calix-Dendrimers: Second Generation Synthesis, Fluorescent Properties and Catecholamines Binding. Pharmaceutics 2022, 14, 2748. https://doi.org/10.3390/pharmaceutics14122748
Mostovaya O, Shiabiev I, Pysin D, Stanavaya A, Abashkin V, Shcharbin D, Padnya P, Stoikov I. PAMAM-Calix-Dendrimers: Second Generation Synthesis, Fluorescent Properties and Catecholamines Binding. Pharmaceutics. 2022; 14(12):2748. https://doi.org/10.3390/pharmaceutics14122748
Chicago/Turabian StyleMostovaya, Olga, Igor Shiabiev, Dmitry Pysin, Alesia Stanavaya, Viktar Abashkin, Dzmitry Shcharbin, Pavel Padnya, and Ivan Stoikov. 2022. "PAMAM-Calix-Dendrimers: Second Generation Synthesis, Fluorescent Properties and Catecholamines Binding" Pharmaceutics 14, no. 12: 2748. https://doi.org/10.3390/pharmaceutics14122748
APA StyleMostovaya, O., Shiabiev, I., Pysin, D., Stanavaya, A., Abashkin, V., Shcharbin, D., Padnya, P., & Stoikov, I. (2022). PAMAM-Calix-Dendrimers: Second Generation Synthesis, Fluorescent Properties and Catecholamines Binding. Pharmaceutics, 14(12), 2748. https://doi.org/10.3390/pharmaceutics14122748