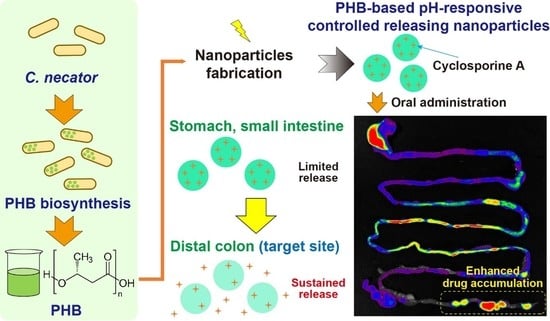
Cupriavidus necator-Produced Polyhydroxybutyrate/Eudragit FS Hybrid Nanoparticles Mitigates Ulcerative Colitis via Colon-Targeted Delivery of Cyclosporine A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PHB
2.2.1. Microorganism
2.2.2. PHB Biosynthesis, Extraction, and Purification
2.3. Preparation of CSA-PENPs
2.4. Drug Release Study
2.5. Development of the DSS-Induced Murine Colitis Model
2.6. In Vivo Distribution of CSA-PENPs
2.7. Evaluation of In Vivo Therapeutic Efficacy
2.7.1. Macroscopic Assessments
2.7.2. Histological Assessments
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation of PHB
3.2. Fabrication of CSA-PENPs
3.3. Drug Release Study
3.4. In Vivo Distribution Examination
3.5. In Vivo Therapeutic Efficacy of CSA-PENPs
3.5.1. Macroscopic Analysis of Colitis
3.5.2. Histological Analysis of Colitis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavan, F.A.; Junqueira, T.L.; Watanabe, M.D.B.; Bonomi, A.; Quines, L.K.; Schmidell, W.; de Aragao, G.M.F. Economic analysis of polyhydroxybutyrate production by Cupriavidus necator using different routes for product recovery. Biochem. Eng. J. 2019, 146, 97–104. [Google Scholar] [CrossRef]
- Akhlaq, S.; Singh, D.; Mittal, N.; Srivastava, G.; Siddiqui, S.; Faridi, S.A.; Siddiqui, M.H. Polyhydroxybutyrate biosynthesis from different waste materials, degradation, and analytic methods: A short review. Polym. Bull. 2022, 1–33. [Google Scholar] [CrossRef]
- Cho, H.-J. Recent progresses in the development of hyaluronic acid-based nanosystems for tumor-targeted drug delivery and cancer imaging. J. Pharm. Investig. 2020, 50, 115–129. [Google Scholar] [CrossRef]
- Lee, S.Y. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 1996, 49, 1–14. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Lezcano, M.F.; Álvarez, G.; Chuhuaicura, P.; Godoy, K.; Alarcón, J.; Acevedo, F.; Gareis, I.; Dias, F.J. Polyhydroxybutyrate (PHB) Scaffolds for Peripheral Nerve Regeneration: A Systematic Review of Animal Models. Biology 2022, 11, 706. [Google Scholar] [CrossRef]
- Riaz, S.; Raza, Z.A.; Majeed, M.I.; Jan, T. Synthesis of zinc sulfide nanoparticles and their incorporation into poly (hydroxybutyrate) matrix in the formation of a novel nanocomposite. Mater. Res. Express 2018, 5, 055027. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Kulkarni, A.R.; Aminabhavi, T.M. Blend microspheres of poly (3-hydroxybutyrate) and cellulose acetate phthalate for colon delivery of 5-fluorouracil. Ind. Eng. Chem. Res. 2011, 50, 10414–10423. [Google Scholar] [CrossRef]
- Naeem, M.; Choi, M.; Cao, J.; Lee, Y.; Ikram, M.; Yoon, S.; Lee, J.; Moon, H.R.; Kim, M.-S.; Jung, Y.; et al. Colon-targeted delivery of budesonide using dual pH- and time-dependent polymeric nanoparticles for colitis therapy. Drug Des. Dev. Ther. 2015, 9, 3789–3799. [Google Scholar] [CrossRef] [Green Version]
- Kucharzik, T.; Koletzko, S.; Kannengiesser, K.; Dignass, A. Ulcerative colitis—Diagnostic and therapeutic algorithms. Dtsch. Ärzteblatt. Int. 2020, 117, 564. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.F. miR-330 alleviates dextran sodium sulfate-induced ulcerative colitis through targeting IRAK1 in rats. Kaohsiung J. Med. Sci. 2021, 37, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Naeem, M.; Awan, U.A.; Subhan, F.; Cao, J.; Hlaing, S.P.; Lee, J.; Im, E.; Jung, Y.; Yoo, J.-W. Advances in colon-targeted nano-drug delivery systems: Challenges and solutions. Arch. Pharmacal Res. 2020, 43, 153–169. [Google Scholar] [CrossRef]
- Kim, E.R.; Chang, D.K. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. WJG 2014, 20, 9872. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.A.; Naeem, M.; Bae, J.; Kim, J.; Lee, J.; Hasan, N.; Kim, W.; Im, E.; Jung, Y.; Yoo, J.W. Colon-targeted dexamethasone microcrystals with pH-sensitive chitosan/alginate/Eudragit S multilayers for the treatment of inflammatory bowel disease. Carbohydr. Polym. 2018, 198, 434–442. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Jeong, S.; Ju, S.; Park, S.; Jung, Y. 5-[(3-Carboxy-4-hydroxyphenyl) diazenyl] nicotinic acid, an azo-linked mesalazine-nicotinic acid conjugate, is a colon-targeted mutual prodrug against dextran sulfate sodium-induced colitis in mice. J. Pharm. Investig. 2021, 51, 317–325. [Google Scholar] [CrossRef]
- Berends, S.E.; Strik, A.S.; Löwenberg, M.; D’Haens, G.R.; Mathôt, R.A.A. Clinical pharmacokinetic and pharmacodynamic considerations in the treatment of ulcerative colitis. Clin. Pharmacokinet. 2019, 58, 15–37. [Google Scholar] [CrossRef] [Green Version]
- Rutgeerts, P.; Vermeire, S.; Van Assche, G. Mucosal healing in inflammatory bowel disease: Impossible ideal or therapeutic target? Gut 2007, 56, 453–455. [Google Scholar] [CrossRef] [Green Version]
- Naeem, M.; Lee, J.; Oshi, M.A.; Cao, J.; Hlaing, S.P.; Im, E.; Jung, Y.; Yoo, J.-W. Colitis-targeted hybrid nanoparticles-in-microparticles system for the treatment of ulcerative colitis. Acta Biomater. 2020, 116, 368–382. [Google Scholar] [CrossRef]
- Burger, D.; Travis, S. Conventional medical management of inflammatory bowel disease. Gastroenterology 2011, 140, 1827–1837.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, E.L.; Nestor, M.; Onyewadume, L.; de Silva, P.S.; Korzenik, J.R.; Aguilar, H.; Bailen, L.; Berman, A.; Bhaskar, S.K.; Brown, M. High dietary intake of specific fatty acids increases risk of flares in patients with ulcerative colitis in remission during treatment with aminosalicylates. Clin. Gastroenterol. Hepatol. 2017, 15, 1390–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandborn, W.J. A critical review of cyclosporine therapy in inflammatory bowel disease. Inflamm. Bowel Dis. 1995, 1, 48–63. [Google Scholar] [CrossRef]
- Eun, C.S.; Han, D.S. Does the Cyclosporine Still Have a Potential Role in the Treatment of Acute Severe Steroid-Refractory Ulcerative Colitis? Gut Liver 2015, 9, 567. [Google Scholar] [CrossRef] [Green Version]
- Aberra, F.N.; Lichtenstein, G.R. Monitoring of immunomodulators in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2005, 21, 307–319. [Google Scholar] [CrossRef]
- Oshi, M.A.; Lee, J.; Kim, J.; Hasan, N.; Im, E.; Jung, Y.; Yoo, J.-W. pH-Responsive Alginate-Based Microparticles for Colon-Targeted Delivery of Pure Cyclosporine A Crystals to Treat Ulcerative Colitis. Pharmaceutics 2021, 13, 1412. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, M.; Wang, D.; Li, G.; Huang, J.; Tan, S.; Bao, L.; Li, Q.; Li, G.; Si, L. A PepT1 mediated medicinal nano-system for targeted delivery of cyclosporine A to alleviate acute severe ulcerative colitis. Biomater. Sci. 2019, 7, 4299–4309. [Google Scholar] [CrossRef]
- McConnell, E.L.; Short, M.D.; Basit, A.W. An in vivo comparison of intestinal pH and bacteria as physiological trigger mechanisms for colonic targeting in man. J. Control. Release 2008, 130, 154–160. [Google Scholar] [CrossRef]
- Chen, Q.; Gou, S.; Ma, P.; Song, H.; Zhou, X.; Huang, Y.; Han, M.K.; Wan, Y.; Kang, Y.; Xiao, B. Oral administration of colitis tissue-accumulating porous nanoparticles for ulcerative colitis therapy. Int. J. Pharm. 2019, 557, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chu, J.S.; Fix, J.A. Colon-specific drug delivery: New approaches and in vitro/in vivo evaluation. Int. J. Pharm. 2002, 235, 1–15. [Google Scholar] [CrossRef]
- Naeem, M.; Kim, W.; Cao, J.; Jung, Y.; Yoo, J.-W. Enzyme/pH dual sensitive polymeric nanoparticles for targeted drug delivery to the inflamed colon. Colloids Surf. B Biointerfaces 2014, 123, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Lertpairod, J.; Tiyaboonchai, W. pH-sensitive beads containing curcumin loaded nanostructured lipid carriers for a colon targeted oral delivery system. J. Pharm. Investig. 2022, 52, 387–396. [Google Scholar] [CrossRef]
- Nygaard, D.; Yashchuk, O.; Hermida, É.B. Evaluation of culture medium on poly (3-hydroxybutyrate) production by Cupriavidus necator ATCC 17697: Application of the response surface methodology. Heliyon 2019, 5, e01374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsay, J.A.; Berger, E.; Voyer, R.; Chavarie, C.; Ramsay, B.A. Extraction of poly-3-hydroxybutyrate using chlorinated solvents. Biotechnol. Tech. 1994, 8, 589–594. [Google Scholar] [CrossRef]
- Mohidin Batcha, A.F.; Prasad, D.M.; Khan, M.R.; Abdullah, H. Biosynthesis of poly (3-hydroxybutyrate)(PHB) by Cupriavidus necator H16 from jatropha oil as carbon source. Bioprocess Biosyst. Eng. 2014, 37, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Bae, J.; Kwak, D.; Kim, H.; Kim, J.; Hlaing, S.P.; Saparbayeva, A.; Lee, E.H.; Yoon, I.-S.; Kim, M.-S. 5-Fluorouracil crystal-incorporated, pH-responsive, and release-modulating PLGA/Eudragit FS hybrid microparticles for local colorectal cancer-targeted chemotherapy. Int. J. Pharm. 2022, 630, 122443. [Google Scholar] [CrossRef]
- Hartmann, G.; Bidlingmaier, C.; Siegmund, B.; Albrich, S.; Schulze, J.; Tschoep, K.; Eigler, A.; Lehr, H.A.; Endres, S. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J. Pharmacol. Exp. Ther. 2000, 292, 22–30. [Google Scholar]
- Naeem, M.; Bae, J.; Oshi, M.A.; Kim, M.-S.; Moon, H.R.; Lee, B.L.; Im, E.; Jung, Y.; Yoo, J.-W. Colon-targeted delivery of cyclosporine A using dual-functional Eudragit(®) FS30D/PLGA nanoparticles ameliorates murine experimental colitis. Int. J. Nanomed. 2018, 13, 1225–1240. [Google Scholar] [CrossRef] [Green Version]
- Hasan, N.; Cao, J.; Lee, J.; Kim, H.; Yoo, J.-W. Development of clindamycin-loaded alginate/pectin/hyaluronic acid composite hydrogel film for the treatment of MRSA-infected wounds. J. Pharm. Investig. 2021, 51, 597–610. [Google Scholar] [CrossRef]
- Lee, J.; Hlaing, S.P.; Cao, J.; Hasan, N.; Yoo, J.-W. In vitro and in vivo evaluation of a novel nitric oxide-releasing ointment for the treatment of methicillin-resistant Staphylococcus aureus-infected wounds. J. Pharm. Investig. 2020, 50, 505–512. [Google Scholar] [CrossRef]
- Whittem, C.G.; Williams, A.D.; Williams, C.S. Murine colitis modeling using dextran sulfate sodium (DSS). JoVE J. Vis. Exp. 2010, e1652. [Google Scholar] [CrossRef]
- Ramakers, J.D.; Verstege, M.I.; Thuijls, G.; Te Velde, A.A.; Mensink, R.P.; Plat, J. The PPARγ agonist rosiglitazone impairs colonic inflammation in mice with experimental colitis. J. Clin. Immunol. 2007, 27, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigmann, B.; Lehr, H.A.; Yancopoulos, G.; Valenzuela, D.; Murphy, A.; Stevens, S.; Schmidt, J.; Galle, P.R.; Rose-John, S.; Neurath, M.F. The transcription factor NFATc2 controls IL-6–dependent T cell activation in experimental colitis. J. Exp. Med. 2008, 205, 2099–2110. [Google Scholar] [CrossRef] [Green Version]
- Aramvash, A.; Akbari Shahabi, Z.; Dashti Aghjeh, S.; Ghafari, M.D. Statistical physical and nutrient optimization of bioplastic polyhydroxybutyrate production by Cupriavidus necator. Int. J. Environ. Sci. Technol. 2015, 12, 2307–2316. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Jiang, Y.; Wang, X.; Yang, J.; Gao, Y.; Zi, X.; Zhang, X.; Gao, H.; Hu, N. Psychrotrophic Pseudomonas mandelii CBS-1 produces high levels of poly-β-hydroxybutyrate. Springerplus 2013, 2, 335. [Google Scholar] [CrossRef] [Green Version]
- Verlinden, R.A.J.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Piotrowska-Seget, Z.; Radecka, I.K. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Express 2011, 1, 335. [Google Scholar] [CrossRef] [Green Version]
- De Rooy, S.L.; Wahyuni, E.T.; Wiratni, W.; Syamsiah, S.; Ismail, J. Purification and characterization of poly-hydroxybutyrate (PHB) in Cupriavidus necator. Indones. J. Chem. 2007, 7, 243–248. [Google Scholar] [CrossRef]
- Sawant, S.S.; Salunke, B.K.; Kim, B.S. A laboratory case study of efficient polyhydoxyalkonates production by Bacillus cereus, a contaminant in Saccharophagus degradans ATCC 43961 in minimal sea salt media. Curr. Microbiol. 2014, 69, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Kosugi, A.; Arai, T.; Sudesh, K.; Vaithanomsat, P. Enhanced polyhydroxybutyrate (PHB) production by newly isolated rare actinomycetes Rhodococcus sp. strain BSRT1-1 using response surface methodology. Sci. Rep. 2021, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Schäfer, U.; Lehr, C.-M. Size-dependent bioadhesion of micro-and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001, 18, 788–793. [Google Scholar] [CrossRef]
- Dünnhaupt, S.; Kammona, O.; Waldner, C.; Kiparissides, C.; Bernkop-Schnürch, A. Nano-carrier systems: Strategies to overcome the mucus gel barrier. Eur. J. Pharm. Biopharm. 2015, 96, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Young, T.J.; Sarkari, M.; Williams Iii, R.O.; Johnston, K.P. Preparation of cyclosporine A nanoparticles by evaporative precipitation into aqueous solution. Int. J. Pharm. 2002, 242, 3–14. [Google Scholar] [CrossRef] [PubMed]






| Size (nm) | PDI | Zeta Potential (mv) | CSA Loading (%) | |
|---|---|---|---|---|
| CSA-ENPs | 290.0 ± 26.3 | 0.119 ± 0.050 | −19.4 ± 0.4 | 7.36 ± 0.23 |
| CSA-PENPs | 311.4 ± 9.1 | 0.136 ± 0.010 | −18.4 ± 0.06 | 6.28 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Saparbayeva, A.; Hlaing, S.P.; Kwak, D.; Kim, H.; Kim, J.; Lee, E.H.; Yoo, J.-W. Cupriavidus necator-Produced Polyhydroxybutyrate/Eudragit FS Hybrid Nanoparticles Mitigates Ulcerative Colitis via Colon-Targeted Delivery of Cyclosporine A. Pharmaceutics 2022, 14, 2811. https://doi.org/10.3390/pharmaceutics14122811
Lee J, Saparbayeva A, Hlaing SP, Kwak D, Kim H, Kim J, Lee EH, Yoo J-W. Cupriavidus necator-Produced Polyhydroxybutyrate/Eudragit FS Hybrid Nanoparticles Mitigates Ulcerative Colitis via Colon-Targeted Delivery of Cyclosporine A. Pharmaceutics. 2022; 14(12):2811. https://doi.org/10.3390/pharmaceutics14122811
Chicago/Turabian StyleLee, Juho, Aruzhan Saparbayeva, Shwe Phyu Hlaing, Dongmin Kwak, Hyunwoo Kim, Jihyun Kim, Eun Hee Lee, and Jin-Wook Yoo. 2022. "Cupriavidus necator-Produced Polyhydroxybutyrate/Eudragit FS Hybrid Nanoparticles Mitigates Ulcerative Colitis via Colon-Targeted Delivery of Cyclosporine A" Pharmaceutics 14, no. 12: 2811. https://doi.org/10.3390/pharmaceutics14122811
APA StyleLee, J., Saparbayeva, A., Hlaing, S. P., Kwak, D., Kim, H., Kim, J., Lee, E. H., & Yoo, J. -W. (2022). Cupriavidus necator-Produced Polyhydroxybutyrate/Eudragit FS Hybrid Nanoparticles Mitigates Ulcerative Colitis via Colon-Targeted Delivery of Cyclosporine A. Pharmaceutics, 14(12), 2811. https://doi.org/10.3390/pharmaceutics14122811