Association of P450 Oxidoreductase Gene Polymorphism with Tacrolimus Pharmacokinetics in Renal Transplant Recipients: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
2.2. Data Extraction and Study Quality Assessment
2.3. Statistical Analysis
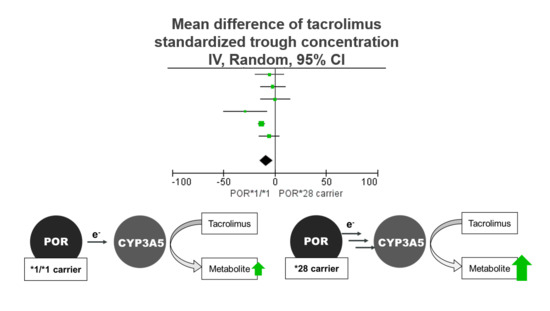
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lesley, S. Tacrolimus, A further update of its use in the management of organ transplantation. Drugs 2003, 63, 1247–1297. [Google Scholar]
- Felipe, C.R.; Silva, H.T.; Machado, P.G.; Garcia, R.; da Silva Moreira, S.R.; Pestana, J.O. The impact of ethnic miscegenation on tacrolimus clinical pharmacokinetics and therapeutic drug monitoring. Clin. Transplant. 2002, 16, 262–272. [Google Scholar] [CrossRef]
- Taylor, A.L.; Watson, C.J.; Bradley, J.A. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit. Rev. Oncol./Hematol. 2005, 56, 23–46. [Google Scholar] [CrossRef]
- Venkataramanan, R.; Swaminathan, A.; Prasad, T.; Jain, A.; Zuckerman, S.; Warty, V.; McMichael, J.; Lever, J.; Burckart, G.; Starzl, T. Clinical pharmacokinetics of tacrolimus. Clin. Pharmacokinet. 1995, 29, 404–430. [Google Scholar] [CrossRef] [PubMed]
- Laskow, D.A.; Vincenti, F.; Neylan, J.F.; Mendez, R.; Matas, A.J. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: A Report of the United States Multicenter FK506 Kidney Transplant Group1. Transplantation 1996, 62, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Anglicheau, D.; Verstuyft, C.; Laurent-Puig, P.; Becquemont, L.; Schlageter, M.-H.; Cassinat, B.; Beaune, P.; Legendre, C.; Thervet, E. Association of the Multidrug Resistance-1 Gene Single-Nucleotide Polymorphisms with the Tacrolimus Dose Requirements in Renal Transplant Recipients. J. Am. Soc. Nephrol. 2003, 14, 1889–1896. [Google Scholar] [CrossRef] [Green Version]
- Hustert, E.; Haberl, M.; Burk, O.; Wolbold, R.; He, Y.-Q.; Klein, K.; Nuessler, A.C.; Neuhaus, P.; Klattig, J.; Eiselt, R.; et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics 2001, 11, 773–779. [Google Scholar] [CrossRef]
- Kuehl, P.; Zhang, J.; Lin, Y.; Lamba, J.; Assem, M.; Schuetz, E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001, 27, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Mourad, M.; Mourad, G.; Wallemacq, P.; Garrigue, V.; Van Bellingen, C.; Van Kerckhove, V.; De Meyer, M.; Malaise, J.; Eddour, D.C.; Lison, D.; et al. Sirolimus and Tacrolimus Trough Concentrations and Dose Requirements after Kidney Transplantation in Relation to CYP3A5 and MDR1 Polymorphisms and Steroids. Transplantation 2005, 80, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Thervet, E.; Anglicheau, D.; King, B.; Schlageter, M.H.; Cassinat, B.; Beaune, P.; Christophe, L.; Daly, A.K. Impact of cytochrome P450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients12. Transplantation 2003, 76, 1233–1235. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Webber, S.; Zeevi, A.; Schuetz, E.; Zhang, J.; Bowman, P.; Boyle, G.; Law, Y.; Miller, S.; Lamba, J.; et al. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am. J. Transplant. 2003, 3, 477–483. [Google Scholar] [CrossRef]
- Goto, M.; Masuda, S.; Kiuchi, T.; Ogura, Y.; Oike, F.; Okuda, M.; Tanaka, K.; Inui, K.-I. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics 2004, 14, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, P.A.; Shen, A.L.; Paschke, R.; Kasper, C.B.; Kim, J.J.P. NADPH-cytochrome P450 oxidoreductase: Structural basis for hydride and electron transfer. J. Biol. Chem. 2001, 276, 29163–29170. [Google Scholar] [CrossRef] [Green Version]
- Masters, B.S.S. The journey from NADPH-cytochrome P450 oxidoreductase to nitric oxide synthases. Biochem. Biophys. Res. Commun. 2005, 338, 507–519. [Google Scholar] [CrossRef]
- Agrawal, V.; Huang, N.; Miller, W.L. Pharmacogenetics of P450 oxidoreductase: Effect of sequence variants on activities of CYP1A2 and CYP2C19. Pharm. Genom. 2008, 18, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Lunde, I.; Bremer, S.; Midtvedt, K.; Mohebi, B.; Dahl, M.; Bergan, S.; Åsberg, A.; Christensen, H. The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients. Eur. J. Clin. Pharmacol. 2004, 70, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Madsen, M.J.; Bergmann, T.K.; Brøsen, K.; Thiesson, H.C. The pharmacogenetics of tacrolimus in corticosteroid-sparse pediatric and adult kidney transplant recipients. Drugs R&D 2017, 17, 279–286. [Google Scholar]
- De Jonge, H.; Metalidis, C.; Naesens, M.; Lambrechts, D.; Kuypers, D.R. The P450 oxidoreductase* 28 SNP is associated with low initial tacrolimus exposure and increased dose requirements in CYP3A5-expressing renal recipients. Pharmacogenomics 2011, 12, 1281–1291. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. 2002. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 4 January 2022).
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; White, I.R.; Anzures-Cabrera, J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat. Med. 2008, 27, 6072–6092. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Elens, L.; Hesselink, D.A.; Bouamar, R.; Budde, K.; De Fijter, J.W.; De Meyer, M.; Mourad, M.; Kuypers, D.R.; Haufroid, V.; Van Gelder, T.; et al. Impact of POR*28 on the Pharmacokinetics of Tacrolimus and Cyclosporine A in Renal Transplant Patients. Ther. Drug Monit. 2014, 36, 71–79. [Google Scholar] [CrossRef]
- Kurzawski, M.; Malinowski, D.; Dziewanowski, K.; Drozdzik, M. Impact of PPARA and POR polymorphisms on tacrolimus pharmacokinetics and new-onset diabetes in kidney transplant recipients. Pharm. Genom. 2014, 24, 397–400. [Google Scholar] [CrossRef]
- Li, C.-J.; Li, L.; Lin, L.; Jiang, H.-X.; Zhong, Z.-Y.; Li, W.-M.; Zhang, Y.-J.; Zheng, P.; Tan, X.-H.; Zhou, L. Impact of the CYP3A5, CYP3A4, COMT, IL-10 and POR Genetic Polymorphisms on Tacrolimus Metabolism in Chinese Renal Transplant Recipients. PLoS ONE 2014, 9, e86206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.J.; Liu, S.B.; Xue, L.; Ding, X.L.; Zhang, H.; Miao, L.Y. The genetic polymorphisms of POR* 28 and CYP3A5* 3 significantly influence the pharmacokinetics of tacrolimus in Chinese renal transplant recipients. Int. J. Clin. Pharmacol. Ther. 2015, 53, 728–736. [Google Scholar] [CrossRef]
- Liu, S.; Chen, R.X.; Li, J.; Zhang, Y.; Wang, X.D.; Fu, Q.; Chen, L.; Liu, X.; Huang, H.; Huang, M.; et al. The POR rs1057868–rs2868177 GC-GT diplotype is associated with high tacrolimus concentrations in early post-renal transplant recipients. Acta Pharmacol. Sin. 2016, 37, 1251–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phupradit, A.; Vadcharavivad, S.; Ingsathit, A.; Kantachuvesiri, S.; Areepium, N.; Sra-Ium, S.; Auamnoy, T.; Sukasem, C.; Sumethkul, V.; Kitiyakara, C. Impact of POR and CYP3A5 Polymorphisms on Trough Concentration to Dose Ratio of Tacrolimus in the Early Post-operative Period Following Kidney Transplantation. Ther. Drug Monit. 2018, 40, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Agrawal, V.; Giacomini, K.M.; Miller, W.L. Genetics of P450 oxidoreductase: Sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc. Natl. Acad. Sci. USA 2008, 105, 1733–1738. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.; Pandey, A.V.; Agrawal, V.; Reardon, W.; Lapunzina, P.D.; Mowat, D.; Jabs, E.W.; Van Vliet, G.; Sack, J.; Flück, C.E.; et al. Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am. J. Hum. Genet. 2005, 76, 729–749. [Google Scholar] [CrossRef] [Green Version]
- Fluck, C.E.; Mullis, P.E.; Pandey, A.V. Reduction in hepatic drug metabolizing CYP3A4 activities caused by P450 oxidoreductase mutations identified in patients with disordered steroid metabolism. Biochem. Biophys. Res. Commun. 2010, 401, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Choi, J.H.; Giacomini, K.M.; Miller, W.L. Substrate-specific modulation of CYP3A4 activity by genetic variants of cytochrome P450 oxidoreductase. Pharm. Genom. 2010, 20, 611–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oneda, B.; Crettol, S.; Sirot, E.J.; Bochud, M.; Ansermot, N.; Eap, C.B. The P450 oxidoreductase genotype is associated with CYP3A activity in vivo as measured by the midazolam phenotyping test. Pharm. Genom. 2009, 19, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fu, Z.; Chen, X.; Yuan, H.; Yang, H.; Huang, Y.; Ouyang, D.; Tan, Z.; Tan, H.; Huang, Z.; et al. Effects of the CYP Oxidoreductase Ala503Val Polymorphism on CYP3A Activity In Vivo: A Randomized, Open-Label, Crossover Study in Healthy Chinese Men. Clin. Ther. 2011, 33, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, D.R.; Claes, K.; Evenepoel, P.; Maes, B.; Vanrenterghem, Y. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin. Pharmacol. Ther. 2004, 75, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Borobia, A.M.; Romero, I.; Jimenez, C.; Gil, F.; Ramírez, E.; De Gracia, R.; Escuin, F.; González, E.; Sansuán, A.J.C. Trough Tacrolimus Concentrations in the First Week After Kidney Transplantation Are Related to Acute Rejection. Ther. Drug Monit. 2009, 31, 436–442. [Google Scholar] [CrossRef]
- Gijsen, V.M.; van Schaik, R.H.; Soldin, O.P.; Soldin, S.J.; Nulman, I.; Koren, G.; de Wildt, S.N. P450 oxidoreductase* 28 (POR* 28) and tacrolimus disposition in pediatric kidney transplant recipients—A pilot study. Ther. Drug Monit. 2014, 36, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [Green Version]




| No | Search Term | PubMed | Web of Science | Embase |
|---|---|---|---|---|
| #1 | (tacrolimus) OR (FK506) OR (FK-506) OR (calcineurin inhibitor) OR (Prograf) OR (immunosuppress*) | 481,508 | 153,419 | 391,339 |
| #2 | (kidney transplant*) OR (kidney graft*) OR (kidney allograft*) OR (renal transplant*) OR (renal graft*) OR (renal allograft*) | 190,248 | 202,994 | 324,655 |
| #3 | #1 and #2 | 48,448 | 31,892 | 78,405 |
| #4 | (POR) OR (P450 oxidoreductase) OR (cytochrome P450 oxidoreductase) OR (CYPOR) | 138,663 | 16,910 | 70,583 |
| #5 | (polymorph*) OR (variant*) OR (mutation*) OR (genotyp*) OR (phenotyp*) OR (haplotyp*) OR (SNP) OR (rs1057868) OR (Ala503Val) OR (A503V) | 2,146,909 | 2,144,583 | 2,821,213 |
| #6 | #4 and #5 | 25,468 | 1794 | 3279 |
| #7 | #3 and #6 | 460 | 53 | 73 |
| First Author, Year | Ethnic Background | N (Male %) | Age, Year (SD) | Weight, kg (SD) | POR*28 Allele Frequency (%) | Post-Transplantation Day | Initial Dose | Target Trough Level, ng/mL | Coadministration | Genotyping Methods | Quantification Methods | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elens et al., 2014 [26] | Caucasian, Asian, Africa-American, Others | 127 * (60.2) | 49.5 (15.3) | 72.6 (16.6) | 22.1 | 10 | NA | 5~15 | MMF or azathioprine, corticosteroids | TaqMan assay | MEIA | 9 |
| Kurzawski et al., 2014 [27] | Caucasian | 241 (55.6) | 45.8 (12.4) | 73.2 (13.9) | 26.4 | 7 | 100 ng/kg/day | 10~15 | MMF, corticosteroids | TaqMan assay | CMIA | 9 |
| Li et al., 2014 [28] | Asian | 240 (67.1) | 41.0 (12.2) | 57.9 (10.1) | 35.6 | 6~8 | 100 ng/kg, bid | 9~14 | MMF, steroids | SNaPshot assay | MEIA | 7 |
| Zhang et al., 2015 [29] | Asian | 83 (72.3) | 40.4 (11.3) | 62.0 (9.4) | 39.8 | 7 | NA | 10~15 | MMF, steroids | PCR-RFLP | Emit 2000 Tacrolimus assay | 9 |
| Liu et al., 2016 [30] | Asian | 154 (NA) | 40.0 (10.9) | 59.8 (10.7) | 34.1 | 7 | 50~75 ng/kg, bid | 5~8 | MMF, prednisolone | PCR-RFLP | MEIA | 8 |
| Phupradit et al., 2018 [31] | Asian | 216 (61.1) | 43.0 (14.6) | 57.1 (11.3) | 32.4 | 7 | 100 ng/kg/day | 4~8 | Mycophenolic acid, corticosteroids or basiliximab | TaqMan assay | CMIA | 9 |
| Excluded Study | Heterogeneity I2 (%) | Statistical Model | Mean Difference [95% CI] |
|---|---|---|---|
| None | 55 | Random | −11.67 [−14.16, −9.19] |
| Elens et al., 2014 [26] | 62 | Random | −8.68 [−15.95, −1.42] |
| Kurzawski et al., 2014 [27] | 53 | Random | −9.51 [−16.32, −2.70] |
| Li et al., 2014 [28] | 52 | Random | −9.61 [−16.04, −3.17] |
| Zhang et al., 2015 [29] | 54 | Random | −6.97 [−13.17, −0.76] |
| Liu et al., 2016 [30] | 29 | Fixed | −5.38 [−11.17, 0.40] |
| Phupradit et al., 2018 [31] | 58 | Random | −8.84 [−16.59, −1.09] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-H.; Lee, H.; Yoon, H.-Y.; Yee, J.; Gwak, H.-S. Association of P450 Oxidoreductase Gene Polymorphism with Tacrolimus Pharmacokinetics in Renal Transplant Recipients: A Systematic Review and Meta-Analysis. Pharmaceutics 2022, 14, 261. https://doi.org/10.3390/pharmaceutics14020261
Lee D-H, Lee H, Yoon H-Y, Yee J, Gwak H-S. Association of P450 Oxidoreductase Gene Polymorphism with Tacrolimus Pharmacokinetics in Renal Transplant Recipients: A Systematic Review and Meta-Analysis. Pharmaceutics. 2022; 14(2):261. https://doi.org/10.3390/pharmaceutics14020261
Chicago/Turabian StyleLee, Da-Hoon, Hana Lee, Ha-Young Yoon, Jeong Yee, and Hye-Sun Gwak. 2022. "Association of P450 Oxidoreductase Gene Polymorphism with Tacrolimus Pharmacokinetics in Renal Transplant Recipients: A Systematic Review and Meta-Analysis" Pharmaceutics 14, no. 2: 261. https://doi.org/10.3390/pharmaceutics14020261
APA StyleLee, D. -H., Lee, H., Yoon, H. -Y., Yee, J., & Gwak, H. -S. (2022). Association of P450 Oxidoreductase Gene Polymorphism with Tacrolimus Pharmacokinetics in Renal Transplant Recipients: A Systematic Review and Meta-Analysis. Pharmaceutics, 14(2), 261. https://doi.org/10.3390/pharmaceutics14020261