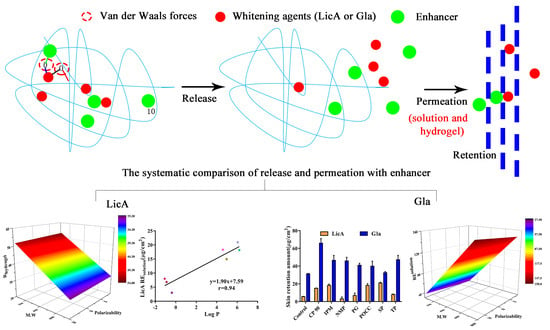
Quantitative Structure-Activity Relationship of Enhancers of Licochalcone A and Glabridin Release and Permeation Enhancement from Carbomer Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Hydrogels
2.3. Rheological Properties of Hydrogel
2.4. Determination of the Drug Solubility in the Donor Phase
2.5. In Vitro Release of Hydrogels
2.6. Mechanism of Enhancement Drug Release
2.6.1. Attenuated Total Reflection FT-IR (ATR-FT-IR) of the CP Hydrogels
2.6.2. Raman Spectroscopy
2.6.3. X-ray Diffraction (XRD)
2.6.4. Polarized Light Microscopy (PLM)
2.6.5. Differential Scanning Calorimetry (DSC)
2.6.6. Molecular Interaction Study: Molecular Docking
2.6.7. Molecular Dynamic Simulation
2.7. Correlation Analysis 1
2.8. In Vitro Skin Permeation of Drug Solution and Hydrogel
2.9. Drug Retention
2.10. Mechanism of Enhancement Drug Permeation
2.10.1. ATR-FT-IR Spectra of the Porcine Skin
2.10.2. Confocal Laser Microscope (CLSM)
2.10.3. Molecular Docking and Molecular Dynamic Simulation
2.11. Correlation Analysis 2
2.12. Statistical Analysis
3. Results
3.1. Preparation of the CP–Gla and CP–LicA Hydrogel
3.2. In Vitro Release of Gla–CP and LicA–CP Hydrogels
3.3. In Vitro Release of Drug in the Presence of Enhancers
3.4. Molecular Modeling and Correlation Analysis 1
3.5. The Release Mechanism of the Drug from the Drug–Enhancers–CP System
3.6. In Vitro Skin Permeation and Drug Retention of Drug Solution
3.7. The Enhancement Mechanism of the LicA and Gla
3.7.1. ATR-FT-IR of the Skin
3.7.2. CLSM
3.7.3. Molecular Modeling and Correlation Analysis 2
3.8. In Vitro Skin Permeation and Drug Retention of Drug Hydrogel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, Q.; Chen, G.; Zhang, Y.-H.; Chen, F.-Q.; Weng, H.-F.; Xiao, A.-F. Agarose Stearate-Carbomer940 as Stabilizer and Rheology Modifier for Surfactant-Free Cosmetic Formulations. Mar. Drugs 2021, 19, 344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, C.; Yang, D.G.; Ruan, J.H.; Luo, Z.; Quan, P.; Fang, L. Mechanism insight on drug skin delivery from polyurethane hydrogels: Roles of molecular mobility and intermolecular interaction. Eur. J. Pharm. Sci. 2021, 161, 105783. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, C.; Quan, P.; Yang, D.G.; Zhao, H.Q.; Wan, X.C.; Fang, L. Mechanistic insights of the controlled release capacity of polar functional group in transdermal drug delivery system: The relationship of hydrogen bonding strength and controlled release capacity. Acta Pharm. Sin. B 2020, 10, 928–945. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Meacham, R.; Liu, M.; Guo, J.; Zehnder, A.T.; Hui, C.Y. Effect of Hydration on Tensile Response of a Dual Cross-linked PVA Hydrogel. Exp. Mech. 2020, 60, 1161–1165. [Google Scholar] [CrossRef]
- Censi, R.; Vermonden, T.; van Steenbergen, M.J.; Deschout, H.; Braeckmans, K.; De Smedt, S.C.; van Nostrum, C.F.; di Martino, P.; Hennink, W.E. Photopolymerized thermosensitive hydrogels for tailorable diffusion-controlled protein delivery. J. Control. Release 2009, 140, 230–236. [Google Scholar] [CrossRef]
- Wang, E.; Klauda, J.B. Models for the Stratum corneum Lipid Matrix: Effects of Ceramide Concentration, Ceramide Hydroxylation, and Free Fatty Acid Protonation. J. Phys. Chem. B 2018, 122, 11996–12008. [Google Scholar] [CrossRef]
- Wang, Z.X.; Liu, L.; Xiang, S.J.; Jiang, C.P.; Wu, W.F.; Ruan, S.F.; Du, Q.Q.; Chen, T.T.; Xue, Y.Q.; Chen, H.J.; et al. Formulation and Characterization of a 3D-Printed Cryptotanshinone-Loaded Niosomal Hydrogel for Topical Therapy of Acne. AAPS Pharm. 2020, 21, 159. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.; Luo, Z.; Wan, X.C.; Fang, L. Investigation of molecular mobility of pressure-sensitive-adhesive in oxybutynin patch in vitro and in vivo: Effect of sorbitan monooleate on drug release and patch mechanical property. Eur. J. Pharm. Sci. 2018, 122, 116–124. [Google Scholar] [CrossRef]
- Song, W.T.; Quan, P.; Li, S.S.; Liu, C.; Lv, S.; Zhao, Y.S.; Fang, L. Probing the role of chemical enhancers in facilitating drug release from patches: Mechanistic insights based on FT-IR spectroscopy, molecular modeling and thermal analysis. J. Control. Release 2016, 227, 13–22. [Google Scholar] [CrossRef]
- Ruan, S.F.; Wang, Z.X.; Xiang, S.J.; Chen, H.J.; Liu, Q. Mechanisms of white mustard seed (Sinapis alba L.) volatile oils as transdermal penetration enhancers. Fitoterapia 2019, 138, 104195. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Quan, P.; Li, S.S.; Liu, C.; Zhao, Y.; Zhao, Y.S.; Fang, L. Time dependence of the enhancement effect of chemical enhancers: Molecular mechanisms of enhancing kinetics. J. Control. Release 2017, 248, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Witting, M.; Boreham, A.; Brodwolf, R.; Vavrova, K.; Alexiev, U.; Friess, W.; Hedtrich, S. Interactions of Hyaluronic Acid with the Skin and Implications for the Dermal Delivery of Biomacromolecules. Mol. Pharm. 2015, 12, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.Y.; Liu, C.; Li, Q.Y.; Wan, X.C.; Guo, J.P.; Quan, P.; Fang, L. Investigation of the enhancement effect of the natural transdermal permeation enhancers from Ledum palustre L. var. angustum N. Busch: Mechanistic insight based on interaction among drug, enhancers and skin. Eur. J. Pharm. Sci. 2018, 124, 105–113. [Google Scholar] [CrossRef]
- Calatayud-Pascual, M.A.; Sebastian-Morelló, M.; Balaguer-Fernández, C.; Delgado-Charro, M.B.; López-Castellano, A.; Merino, V. Influence of Chemical Enhancers and Iontophoresis on the In Vitro Transdermal Permeation of Propranolol: Evaluation by Dermatopharmacokinetics. Pharmaceutics 2018, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.W.; Liu, C.; Zhang, Y.; Quan, P.; Yang, D.G.; Fang, L. An investigation on the effect of drug physicochemical properties on the enhancement strength of enhancer: The role of drug-skin-enhancer interactions. In. J. Pharm. 2021, 607, 120945. [Google Scholar] [CrossRef]
- Yang, D.G.; Liu, C.; Quan, P.; Fang, L. A systematic approach to determination of permeation enhancer action efficacy and sites: Molecular mechanism investigated by quantitative. J. Control. Release 2020, 322, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, Y.; Chen, T.; Du, Q.; Zhu, Z.; Wang, Y.; Wu, Y.; Zeng, Q.; Shen, C.; Jiang, C.; et al. Glycyrrhiza acid micelles loaded with licochalcone A for topical delivery: Co-penetration and anti-melanogenic effect. Eur. J. Pharm. Sci. 2021, 68, 106029. [Google Scholar] [CrossRef]
- Du, Q.Q.; Liu, Q. ROS-responsive hollow mesoporous silica nanoparticles loaded with Glabridin for anti-pigmentation properties. Microporous Mesoporous Mater. 2021, 327, 111429. [Google Scholar] [CrossRef]
- Masukawa, Y.; Narita, H.; Shimizu, E.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; Kitahara, T.; et al. Characterization of overall ceramide species in human Stratum corneum. J. Lipid Res. 2008, 49, 1466–1476. [Google Scholar] [CrossRef] [Green Version]
- Quan, P.; Wan, X.C.; Tian, Q.; Liu, C.; Fang, L. Dicarboxylic acid as a linker to improve the content of amorphous drug in drug-in-polymer film: Effects of molecular mobility, electrical conductivity and intermolecular interactions. J. Control. Release 2020, 317, 142–153. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Tao, Y.Z.; Xu, W.L.; Xiao, S.L.; Du, S.M.; Zhou, Y.S.; Hasan, A. Rheological and controlled release properties of hydrogels based on mushroom hyperbranched polysaccharide and xanthan gum. Int. J. Biol. Macromol. 2018, 120, 2399–2409. [Google Scholar] [CrossRef]
- Islam, A.; Riaz, M.; Yasin, T. Structural and viscoelastic properties of chitosan-based hydrogel and its drug delivery application. Int. J. Biol. Macromol. 2013, 59, 119–124. [Google Scholar] [CrossRef]
- Tanriverdi, S.T.; Cheaburu-Yilmaz, C.N.; Carbone, S.; Ozer, O. Preparation and in vitro evaluation of melatonin-loaded HA/PVA gel formulations. Pharm. Dev. Technol. 2018, 23, 815–825. [Google Scholar] [CrossRef]
- Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. The Role of Polymer Concentration on the Molecular Mobility and Physical Stability of Nifedipine Solid Dispersions. Mol. Pharm. 2015, 12, 1477–1484. [Google Scholar] [CrossRef]
- Liu, C.; Quan, P.; Li, S.S.; Zhao, Y.S.; Fang, L. A systemic evaluation of drug in acrylic pressure sensitive adhesive patch in vitro and in vivo: The roles of intermolecular interaction and adhesive mobility variation in drug controlled release. J. Control. Release 2017, 252, 83–94. [Google Scholar] [CrossRef]
- Das, D.; Das, R.; Ghosh, P.; Dhara, S.; Panda, A.B.; Pal, S. Dextrin cross linked with poly(HEMA): A novel hydrogel for colon specific delivery of ornidazole. RSC Adv. 2013, 3, 25340–25350. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, J.; Yuan, Z.Y.; Han, H.Y.; Li, T.; Li, L.; Guo, X.H. Chitosan cross-linked poly(acrylic acid) hydrogels: Drug release control and mechanism. Coll. Surfaces B Biointerfaces 2017, 152, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.Y.; Quan, P.; Liu, C.; Li, Q.Y.; Fang, L. Effect of isopropyl myristate on the viscoelasticity and drug release of a drug-in-adhesive transdermal patch containing blonanserin. Acta Pharm. Sin. B 2016, 6, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browning, M.B.; Wilems, T.; Hahn, M.; Cosgriff-Hernandez, E. Compositional control of poly(ethylene glycol) hydrogel modulus independent of mesh size. J. Biomed. Mater. Res. Part A 2011, 98A, 268–273. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Fourier transform infrared spectroscopy for the analysis of neutralizer-carbomer and surfactant-carbomer interactions in aqueous, hydroalcoholic, and anhydrous gel formulations. AAPS J. 2004, 6, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Lee, S.M.; Bae, Y.C. Influence of hydroxyl group for thermoresponsive poly(N-isopropylacrylamide) gel particles in water/co-solvent (1,3-propanediol, glycerol) systems. Eur. Polym. J. 2014, 54, 151–159. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.; Liu, C.; Quan, P.; Zhao, Y.; Fang, L. An insight into the molecular mechanism of the temporary enhancement effect of isopulegol decanoate on the skin. Int. J. Pharm. 2017, 529, 161–167. [Google Scholar] [CrossRef]
- Smejkalova, D.; Muthny, T.; Nesporova, K.; Hermannova, M.; Achbergerova, E.; Huerta-Angeles, G.; Svoboda, M.; Cepa, M.; Machalova, V.; Luptakova, D.; et al. Hyaluronan polymeric micelles for topical drug delivery. Carbohydr. Polym. 2017, 156, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Quan, P.; Fang, L.; Xu, H.; Liu, C. A molecular mechanism investigation of the transdermal/topical absorption classification system on the basis of drug skin permeation and skin retention. Int. J. Pharm. 2021, 608, 121082. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]









| Drug/Enhancers | Molecular Weight (Da) | Log P | Solubility in 20% PEG400 (v/v, µg/mL) | H Bond Donor | H Bond Acceptor | Polarizability | Polar Surface Area (Å) |
|---|---|---|---|---|---|---|---|
| LicA | 338.40 | 4.95 | 25.15 | 2 | 4 | 39.8 | 66.8 |
| Gla | 324.40 | 4.26 | 121.47 | 2 | 4 | 36.1 | 58.9 |
| CP 90 | 203.30 | 4.97 | - | - | - | 33.5 | 47.0 |
| IPM | 270.40 | 4.61 | - | - | - | 32.7 | 26.0 |
| NMP | 99.10 | −0.38 | - | - | - | 10.6 | 20.0 |
| PG | 76.09 | −0.90 | - | - | - | 7.52 | 40.5 |
| POCC | 726.90 | 6.21 | - | - | - | 74.8 | 269.0 |
| SP | 428.60 | 6.06 | - | - | - | 47.3 | 96.0 |
| TP | 134.20 | −1.08 | - | - | - | 13.8 | 39.0 |
| χ (kcal/mol) | Emix (kcal/mol) | CED (kcal/mol) | |
|---|---|---|---|
| LicA-CP | 20.52 | 12.15 | - |
| Gla-CP | 13.00 | 7.70 | - |
| LicA-CP 90-CP | 6.97 | 4.13 | 2.57 × 109 |
| LicA-IPM-CP | 6.56 | 3.89 | 2.38 × 109 |
| LicA-NMP-CP | 10.87 | 6.44 | 2.21 × 109 |
| LicA-PG-CP | 19.48 | 11.54 | 2.15 × 109 |
| LicA-POCC-CP | −7.15 | −4.23 | 2.40 × 109 |
| LicA-SP-CP | −1.77 | −1.05 | 2.34 × 109 |
| LicA-TP-CP | 9.35 | 5.54 | 2.40 × 109 |
| Gla-CP 90-CP | 2.45 | 1.45 | 2.60 × 109 |
| Gla-IPM-CP | 3.01 | 1.78 | 2.64 × 109 |
| Gla-NMP-CP | 6.50 | 3.85 | 2.33 × 109 |
| Gla-PG-CP | 8.85 | 5.24 | 2.36 × 109 |
| Gla-POCC-CP | 2.57 | 1.52 | 2.68 × 109 |
| Gla-SP-CP | 6.37 | 3.77 | 2.38 × 109 |
| Gla-TP-CP | 6.34 | 3.76 | 2.43 × 109 |
| ERrelease | ERpermeation | ERcom | βR/P | ERsolution retention | ERhydrogel retention | FP/Q | |
|---|---|---|---|---|---|---|---|
| LicA-CP 90 | 0.89 | 2.72 | 1.18 | 0.33 | 2.70 | 1.13 | 0.015 |
| LicA-IPM | 1.05 | 2.91 | 0.00 | 0.36 | 3.30 | 0.68 | 0.00 |
| LicA-NMP | 1.21 | 1.08 | 0.00 | 1.12 | 0.54 | 0.76 | 0.00 |
| LicA-PG | 1.25 | 0.55 | 1.10 | 2.28 | 1.23 | 1.82 | 0.0099 |
| LicA-POCC | 0.89 | 3.09 | 1.46 | 0.29 | 3.28 | 0.65 | 0.018 |
| LicA-SP | 1.02 | 2.77 | 1.12 | 0.37 | 3.78 | 0.99 | 0.012 |
| LicA-TP | 1.00 | 1.50 | 1.92 | 0.67 | 1.44 | 1.23 | 0.022 |
| Gla-CP 90 | 1.07 | 2.93 | 1.40 | 0.36 | 2.11 | 1.39 | 0.022 |
| Gla-IPM | 1.08 | 1.15 | 0.86 | 0.94 | 1.49 | 1.51 | 0.014 |
| Gla-NMP | 1.14 | 2.20 | 0.78 | 0.52 | 1.47 | 1.28 | 0.012 |
| Gla-PG | 1.11 | 0.68 | 1.28 | 1.63 | 1.31 | 1.11 | 0.020 |
| Gla-POCC | 1.06 | 2.16 | 2.05 | 0.49 | 1.28 | 1.79 | 0.033 |
| Gla-SP | 1.14 | 1.15 | 1.14 | 0.99 | 1.04 | 1.90 | 0.017 |
| Gla-TP | 1.15 | 1.38 | 0.72 | 0.83 | 1.51 | 1.14 | 0.011 |
| χ (kcal/mol) | Emix (kcal/mol) | CED (kcal/mol) | |
|---|---|---|---|
| LicA-CP 90-Skin | 27.54 | 16.31 | 1.49 × 109 |
| LicA-IPM-Skin | 24.82 | 14.70 | 1.37 × 109 |
| LicA-NMP-Skin | 39.04 | 23.12 | 1.59 × 109 |
| LicA-PG-Skin | 48.50 | 28.72 | 1.62 × 109 |
| LicA-POCC-Skin | 17.13 | 10.14 | 1.41 × 109 |
| LicA-SP-Skin | 16.74 | 9.92 | 1.35 × 109 |
| LicA-TP-Skin | 42.52 | 25.18 | 1.49 × 109 |
| Gla-CP 90-Skin | 10.82 | 6.41 | 1.30 × 109 |
| Gla-IPM-Skin | 6.97 | 4.13 | 1.39 × 109 |
| Gla-NMP-Skin | 28.00 | 16.58 | 1.46 × 109 |
| Gla-PG-Skin | 31.06 | 18.40 | 1.54 × 109 |
| Gla-POCC-Skin | 14.07 | 8.33 | 1.45 × 109 |
| Gla-SP-Skin | 1.44 | 0.86 | 1.54 × 109 |
| Gla-TP-Skin | 21.41 | 12.68 | 1.47 × 109 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xue, Y.; Zhu, Z.; Hu, Y.; Zeng, Q.; Wu, Y.; Wang, Y.; Shen, C.; Jiang, C.; Liu, L.; et al. Quantitative Structure-Activity Relationship of Enhancers of Licochalcone A and Glabridin Release and Permeation Enhancement from Carbomer Hydrogel. Pharmaceutics 2022, 14, 262. https://doi.org/10.3390/pharmaceutics14020262
Wang Z, Xue Y, Zhu Z, Hu Y, Zeng Q, Wu Y, Wang Y, Shen C, Jiang C, Liu L, et al. Quantitative Structure-Activity Relationship of Enhancers of Licochalcone A and Glabridin Release and Permeation Enhancement from Carbomer Hydrogel. Pharmaceutics. 2022; 14(2):262. https://doi.org/10.3390/pharmaceutics14020262
Chicago/Turabian StyleWang, Zhuxian, Yaqi Xue, Zhaoming Zhu, Yi Hu, Quanfu Zeng, Yufan Wu, Yuan Wang, Chunyan Shen, Cuiping Jiang, Li Liu, and et al. 2022. "Quantitative Structure-Activity Relationship of Enhancers of Licochalcone A and Glabridin Release and Permeation Enhancement from Carbomer Hydrogel" Pharmaceutics 14, no. 2: 262. https://doi.org/10.3390/pharmaceutics14020262
APA StyleWang, Z., Xue, Y., Zhu, Z., Hu, Y., Zeng, Q., Wu, Y., Wang, Y., Shen, C., Jiang, C., Liu, L., Zhu, H., & Liu, Q. (2022). Quantitative Structure-Activity Relationship of Enhancers of Licochalcone A and Glabridin Release and Permeation Enhancement from Carbomer Hydrogel. Pharmaceutics, 14(2), 262. https://doi.org/10.3390/pharmaceutics14020262