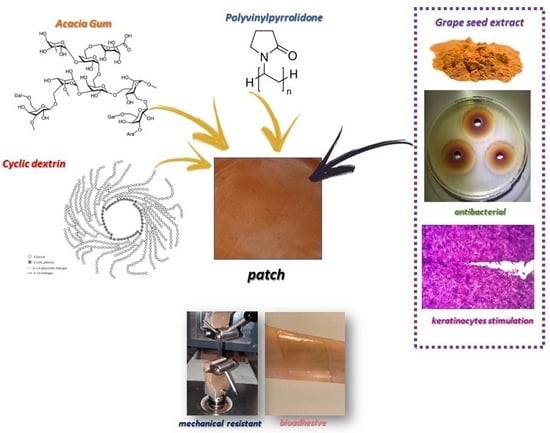
Wound Dressing: Combination of Acacia Gum/PVP/Cyclic Dextrin in Bioadhesive Patches Loaded with Grape Seed Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- -
- agar-well diffusion test medium: deionized water (containing agar 13%), meat extract (3%), sodium chloride (10%), glucose (4%), dibasic potassium phosphate (1%) and meat peptone (5%); after preparation the test medium was autoclaved.
- -
- Brain Heart Infusion (BHI) Broth; deionized water, BHI (3.7%, Biolife Italiana Srl, Milan, Italy).
- -
- Mueller Hinton Broth with 5% Blood; deionized water, Mueller Hinton Broth (2.2%, Biolife Italiana Srl, Milan, Italy), Horse Lysate Blood (5%, Allevamenti Blood di Fiastra Maddalena, Teramo, Italy).
- -
- 5% Sheep Blood Agar; deionized water, Columbia Agar Base (4.4%, Microbiol Srl, Macchiareddu, Cagliari, Italy), Defibrinated Sheep Blood (5%, Allevamenti Blood di Fiastra Maddalena). Bacterial suspension at concentrations of 1 × 105 CFU/mL was used for the antimicrobial test.
2.2. Methods
2.2.1. Patches Preparation
2.2.2. Thermogravimetric Analysis
2.2.3. Total Phenolic Content and Antioxidant Activity
2.2.4. Mechanical Characterization
2.2.5. Ex Vivo Adhesion Studies
2.2.6. Morphology and Thickness
2.2.7. FT-IR Analysis
2.2.8. Cytotoxicity Assay
2.2.9. Scratch Test
2.2.10. In Vitro Release Studies
2.2.11. Antibacterial Activity
3. Results and Discussions
3.1. Patches Preparation and Characterization
3.2. Thermal Stability of Patches
3.3. Mechanical Characterization
- -
- maximum tensile strength (σmax);
- -
- elongation at maximum strength (ε at σmax);
- -
- elastic modulus (E).
3.4. Ex Vivo Adhesion Studies
3.5. Loaded Films Preparation and Characterization
Scratch Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Liquid Prepolymer-Based in Situ Formation of Degradable Poly(ethylene glycol)-Linked-Poly(caprolactone)-Linked-Poly(2-dimethylaminoethyl)methacrylate Amphiphilic Conetwork Gels Showing Polarity Driven Gelation and Bioadhesion. ACS Appl. Bio Mater. 2018, 1, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Nutan, B.; Chandel, A.K.S.; Bhalani, D.V.; Jewrajka, S.K. Synthesis and tailoring the degradation of multi-responsive amphiphilic conetwork gels and hydrogels of poly(β-amino ester) and poly(amido amine). Polymer 2017, 111, 265–274. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Dalvi, Y.B.; Rehman, S.R.U.; Varghese, R.; Unni, R.N.; Yalcin, H.C.; Alfkey, R.; Thomas, S.; Al Moustafa, A.E. Growth factor loaded in situ photocrosslinkable poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/gelatin methacryloyl hybrid patch for diabetic wound healing. Mater. Sci. Eng. C 2021, 118, 111519. [Google Scholar] [CrossRef] [PubMed]
- Temirel, M.; Hawxhurst, C.; Tasoglu, S. Shape fidelity of 3D-bioprinted biodegradable patches. Micromachines 2021, 12, 195. [Google Scholar] [CrossRef]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric wound dressings, an insight into polysaccharide-based electrospun membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Bera, A.; Singh Chandel, A.K.; Uday Kumar, C.; Jewrajka, S.K. Degradable/cytocompatible and pH responsive amphiphilic conetwork gels based on agarose-graft copolymers and polycaprolactone. J. Mater. Chem. B 2015, 3, 8548–8557. [Google Scholar] [CrossRef]
- Pagano, C.; Puglia, D.; Luzi, F.; Michele, A.D.; Scuota, S.; Primavilla, S.; Ceccarini, M.R.; Beccari, T.; Iborra, C.A.V.; Ramella, D.; et al. Development and characterization of xanthan gum and alginate based bioadhesive film for pycnogenol topical use in wound treatment. Pharmaceutics 2021, 13, 324. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine polysaccharides for wound dressings application: An overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Tudoroiu, E.E.; Dinu-Pîrvu, C.E.; Kaya, M.G.A.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An overview of cellulose derivatives-based dressings for wound-healing management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Sanchez, C.; Nigen, M.; Mejia Tamayo, V.; Doco, T.; Williams, P.; Amine, C.; Renard, D. Acacia gum: History of the future. Food Hydrocoll. 2018, 78, 140–160. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Generally recognized as safe (GRAS): History and description. Toxicol. Lett. 2004, 150, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate gums: Occurrence, production, and applications. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, S.; Dhiman, A. Acacia gum polysaccharide based hydrogel wound dressings: Synthesis, characterization, drug delivery and biomedical properties. Carbohydr. Polym. 2017, 165, 294–303. [Google Scholar] [CrossRef]
- Montenegro, M.A.; Boiero, M.L.; Valle, L.; Borsarelli, C.D. Gum Arabic: More Than an Edible Emulsifier. In 600 Products and Applications of Biopolymers Casparus; Verbeek, J.R., Ed.; Intechopen: London, UK, 2012. [Google Scholar]
- Singh, B.; Dhiman, A. Design of Acacia Gum-Carbopol-Cross-Linked-Polyvinylimidazole Hydrogel Wound Dressings for Antibiotic/Anesthetic Drug Delivery. Ind. Eng. Chem. Res. 2016, 55, 9176–9188. [Google Scholar] [CrossRef]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef]
- Fathollahiopur, S.; Maziarfar, S.; Tavakoli, J. Characterization and evaluation of acacia gum loaded PVA hybrid wound dressing. In Proceedings of the 2013 20th Iranian Conference on Biomedical Engineering, ICBME 2013, Tehran, Iran, 18–20 December 2013. [Google Scholar]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Preparation and characterization of Calendula officinalis-loaded PCL/gum arabic nanocomposite scaffolds for wound healing applications. Iran. Polym. J. 2019, 28, 51–63. [Google Scholar] [CrossRef]
- Luo, Y.; Hong, Y.; Shen, L.; Wu, F.; Lin, X. Multifunctional Role of Polyvinylpyrrolidone in Pharmaceutical Formulations. AAPS PharmSciTech 2021, 2222, 34. [Google Scholar] [CrossRef]
- Gupta, M.; Dey, S.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Grape seed extract: Having a potential health benefits. J. Food Sci. Technol. 2020, 57, 1205–1215. [Google Scholar] [CrossRef]
- Ajit, A.; Vishnu, A.G.; Varkey, P. Incorporation of grape seed extract towards wound care product development. 3 Biotech 2021, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fan, Q.; Hong, J.; Chen, Z.; Zhou, X.; Zhang, J.; Dai, Y.; Jiang, H.; Gu, Z.; Cheng, Y.; et al. Therapeutic Nanoparticles from Grape Seed for Modulating Oxidative Stress. Small 2021, 17, 2102485. [Google Scholar] [CrossRef] [PubMed]
- Borges-Vilches, J.; Figueroa, T.; Guajardo, S.; Aguayo, C.; Fernández, K. Improved hemocompatibility for gelatin-graphene oxide composite aerogels reinforced with proanthocyanidins for wound dressing applications. Colloids Surf. B Biointerfaces 2021, 206, 111941. [Google Scholar] [CrossRef]
- Aslaner, G.; Sumnu, G.; Sahin, S. Encapsulation of Grape Seed Extract in Rye Flour and Whey Protein–Based Electrospun Nanofibers. Food Bioprocess Technol. 2021, 14, 1118–1131. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.M.; Rhim, J.W. Carboxymethyl cellulose-based multifunctional film combined with zinc oxide nanoparticles and grape seed extract for the preservation of high-fat meat products. Sustain. Mater. Technol. 2021, 29, e00325. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.W. Carrageenan-based functional hydrogel film reinforced with sulfur nanoparticles and grapefruit seed extract for wound healing application. Carbohydr. Polym. 2019, 224, 115191. [Google Scholar] [CrossRef] [PubMed]
- Koneru, A.; Dharmalingam, K.; Anandalakshmi, R. Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose–grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 2020, 148, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Marinozzi, M.; Baiocchi, C.; Beccari, T.; Calarco, P.; Ceccarini, M.R.; Chielli, M.; Orabona, C.; Orecchini, E.; Ortenzi, R.; et al. Bioadhesive Polymeric Films Based on Red Onion Skins Extract for Wound Treatment: An Innovative and Eco-Friendly Formulation. Molecules 2020, 25, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagano, C.; Perioli, L.; Blasi, F.; Bastianini, M.; Chiesi, C.; Cossignani, L. Optimisation of phenol extraction from wine using layered double hydroxides and technological evaluation of the bioactive-rich powder. Int. J. Food Sci. Technol. 2017, 52, 2582–2588. [Google Scholar] [CrossRef]
- Urbani, E.; Blasi, F.; Simonetti, M.S.; Chiesi, C.; Cossignani, L. Investigation on secondary metabolite content and antioxidant activity of commercial saffron powder. Eur. Food Res. Technol. 2016, 242, 987–993. [Google Scholar] [CrossRef]
- Pollini, L.; Rocchi, R.; Cossignani, L.; Mañes, J.; Compagnone, D.; Blasi, F. Phenol profiling and nutraceutical potential of lycium spp. Leaf extracts obtained with ultrasound and microwave assisted techniques. Antioxidants 2019, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Pagano, C.; Baiocchi, C.; Beccari, T.; Blasi, F.; Cossignani, L.; Ceccarini, M.R.; Orabona, C.; Orecchini, E.; Di Raimo, E.; Primavilla, S.; et al. Emulgel loaded with flaxseed extracts as new therapeutic approach in wound treatment. Pharmaceutics 2021, 13, 1107. [Google Scholar] [CrossRef]
- Pagano, C.; Calarco, P.; Di Michele, A.; Ceccarini, M.R.; Beccari, T.; Primavilla, S.; Scuota, S.; Marmottini, F.; Ramella, D.; Ricci, M.; et al. Development of sodium carboxymethyl cellulose based polymeric microparticles for in situ hydrogel wound dressing formation. Int. J. Pharm. 2021, 602, 120606. [Google Scholar] [CrossRef]
- Pagano, C.; Perioli, L.; Baiocchi, C.; Bartoccini, A.; Beccari, T.; Blasi, F.; Calarco, P.; Ceccarini, M.R.; Cossignani, L.; di Michele, A.; et al. Preparation and characterization of polymeric microparticles loaded with Moringa oleifera leaf extract for exuding wound treatment. Int. J. Pharm. 2020, 587, 119700. [Google Scholar] [CrossRef] [PubMed]
- Ouazib, F.; Bouslah Mokhnachi, N.; Haddadine, N.; Barille, R. Role of polymer/polymer and polymer/drug specific interactions in drug delivery systems. J. Polym. Eng. 2019, 39, 534–544. [Google Scholar] [CrossRef]
- Sharma, A.; Bhushette, P.R.; Annapure, U.S. Physicochemical and rheological properties of Acacia Catechu exudate gum. Carbohydr. Polym. Technol. Appl. 2021, 2, 100127. [Google Scholar] [CrossRef]
- Delgado Adámez, J.; Gamero Samino, E.; Valdés Sánchez, E.; González-Gómez, D. In vitro estimation of the antibacterial activity and antioxidant capacity of aqueous extracts from grape-seeds (Vitis vinifera L.). Food Control 2012, 24, 136–141. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Aghel, N.; Rashidi, I.; Gholampur-Aghdami, A. Topical grape (Vitis vinifera) seed extract promotes repair of full thickness wound in rabbit. Int. Wound J. 2011, 8, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, A.A.; Foroozan, M.; Houshmand, G.; Moosavi, Z.B.; Bahadoram, M.; Maram, N.S. The topical effect of grape seed extract 2% cream on surgery wound healing. Glob. J. Health Sci. 2015, 7, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Roy, S.; Bagchi, D.; Bagchi, M.; Sen, C.K. Upregulation of oxidant-induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Radic. Biol. Med. 2001, 31, 38–42. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Gordillo, G.; Bagchi, D.; Bagchi, M.; Roy, S. Oxygen, oxidants, and antioxidants in wound healing: An emerging paradigm. In Proceedings of the Annals of the New York Academy of Sciences, New York, NY, USA, 16–18 November 2002; Volume 957. [Google Scholar]
- Izadpanah, A.; Soorgi, S.; Geraminejad, N.; Hosseini, M. Effect of grape seed extract ointment on cesarean section wound healing: A double-blind, randomized, controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 35, 323–328. [Google Scholar] [CrossRef]
- Rodríguez-Meizoso, I.; Cifuentes, A.; Román, J.S.; Ibáñez, E.; Elvira, C. A systematic study on the interactions between carnosic acid and ethylpyrrolidine methacrylate-methyl methacrylate copolymer in supercritical media. J. Supercrit. Fluids 2007, 41, 452–460. [Google Scholar] [CrossRef]
- Chen, W.; Wang, C.; Yan, L.; Huang, L.; Zhu, X.; Chen, B.; Sant, H.J.; Niu, X.; Zhu, G.; Yu, K.N.; et al. Improved polyvinylpyrrolidone microneedle arrays with non-stoichiometric cyclodextrin. J. Mater. Chem. B 2014, 2, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Han, J.H.; Tauscher, B. Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. Int. J. Food Sci. Technol. 2009, 44, 425–433. [Google Scholar] [CrossRef]
- Memar, M.Y.; Adibkia, K.; Farajnia, S.; Kafil, H.S.; Yekani, M.; Alizadeh, N.; Ghotaslou, R. The grape seed extract: A natural antimicrobial agent against different pathogens. Rev. Med. Microbiol. 2019, 30, 173–182. [Google Scholar] [CrossRef]











| Patch | AG/PVP (w/w) | AG (% wt) | PVP (%) | Glycerol (% wt) | Water (% wt) |
|---|---|---|---|---|---|
| A | 9.5:0.5 | 4.75 | 0.50 | 3.00 | 91.75 |
| B | 9.0:1.0 | 4.50 | 1.00 | 3.00 | 91.50 |
| C | 8.5:1.5 | 4.25 | 1.50 | 3.00 | 91.25 |
| D | 7.5:2.5 | 3.75 | 2.50 | 3.00 | 90.75 |
| Bacterial Strains | Growth Conditions | |
|---|---|---|
| Gram+ | Staphylococcus epidermidis WDCM 00036 | 37 °C for 24 ± 2 h |
| Bacillus subtilis WDCM 00003 | 30 °C for 24 ± 2 h | |
| Staphylococcus aureus WDCM 00034 | 37 °C for 24 ± 2 h | |
| Streptococcus pyogenes ATCC 19615 | 37 °C for 24–48 h | |
| Gram− | Pseudomonas aeruginosa WDCM 00025 | 25 °C for 24–48 h |
| Klebsiella pneumoniae WDCM 00097 | 37 °C for 24 ± 2 h | |
| Escherichia coli WDCM 00013 | 37 °C for 24 ± 2 h | |
| Yeast | Candida albicans WDCM 00054 | 25 °C for 24–72 h |
| Patch | σmax (MPa) | εat σmax (%) | E (MPa) |
|---|---|---|---|
| A | 0.13 ± 0.02 | 23 ± 2 | 4.00 ± 0.18 |
| B | 0.17 ± 0.04 | 19 ± 5 | 5.00 ± 1.59 |
| C | 0.03 ± 0.01 | 12 ± 2 | 2.17 ± 1.15 |
| D | 0.05 ± 0.03 | 19 ± 3 | 1.67 ± 0.66 |
| Patch | Bioadhesion Force (N) ± SD |
|---|---|
| A | 0.40 ± 0.01 |
| B | 0.37 ± 0.06 |
| C | 0.30 ± 0.01 |
| D | 0.42 ± 0.03 |
| Hydrogel | AG (g) | PVP (g) | Glycerol (g) | GSE (g) | CD (g) | Water (g) |
|---|---|---|---|---|---|---|
| A | 3.325 | 0.350 | 2.100 | 0.200 | 0.300 | 63.725 |
| D | 2.625 | 1.750 | 2.100 | 0.200 | 0.300 | 63.025 |
| Formulations | σmax (MPa) | εat σmax (%) | E (MPa) |
|---|---|---|---|
| Patch A | 0.13 ± 0.02 | 23.0 ± 2.0 | 4.0 ± 0.2 |
| Patch A + CD | 0.15 ± 0.05 | 9.0 ± 1.0 | 6.7 ± 0.2 |
| Patch A + GSE-CD | 0.15 ± 0.02 | 10.0 ± 1.0 | 4.6 ± 0.6 |
| Mt/M∞ = kt | Mt/M∞ = kt0.5 | Mt/M∞ = 1−e−kt |
|---|---|---|
| Zero-Order Kinetic | Higuchi Kinetic (Release 0–60%) | First Order Kinetic |
| y = 0.0081x + 11.836 R2 = 0.6926 | y = 0.5329x + 6.4844 R2 = 0.8844 | y = −4 × 10−5x − 0.0549 R2 = 0.7365 |
| Formulations | TPC (mg GAE/cm2 Patch) | ABTS (mg TE/cm2 Patch) | DPPH (mg TE/cm2 Patch) | FRAP (mg TE/cm2 Patch) |
|---|---|---|---|---|
| Patch A | 0.05 ± 0.00 | 0.08 ± 0.00 | n.d. | 0.02 ± 0.00 |
| Patch A + GSE-CD | 1.61 ± 0.03 | 4.90 ± 0.12 | 2.55 ± 0.08 | 0.25 ± 0.00 |
| Bacterial Strains | (mm) | |
|---|---|---|
| Gram+ | S. epidermidis | 27 |
| B. subtilis | 21 | |
| S. aureus | 27 | |
| S. pyogenes | 18 | |
| L. monocytogenes | 22 | |
| Gram− | P. aeruginosa | 15 |
| K. pneumoniae | 19 | |
| E. coli | 9 | |
| Yeast | C. albicans | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, C.; Luzi, F.; Ricci, M.; Michele, A.D.; Puglia, D.; Ceccarini, M.R.; Beccari, T.; Blasi, F.; Cossignani, L.; Schoubben, A.; et al. Wound Dressing: Combination of Acacia Gum/PVP/Cyclic Dextrin in Bioadhesive Patches Loaded with Grape Seed Extract. Pharmaceutics 2022, 14, 485. https://doi.org/10.3390/pharmaceutics14030485
Pagano C, Luzi F, Ricci M, Michele AD, Puglia D, Ceccarini MR, Beccari T, Blasi F, Cossignani L, Schoubben A, et al. Wound Dressing: Combination of Acacia Gum/PVP/Cyclic Dextrin in Bioadhesive Patches Loaded with Grape Seed Extract. Pharmaceutics. 2022; 14(3):485. https://doi.org/10.3390/pharmaceutics14030485
Chicago/Turabian StylePagano, Cinzia, Francesca Luzi, Maurizio Ricci, Alessandro Di Michele, Debora Puglia, Maria Rachele Ceccarini, Tommaso Beccari, Francesca Blasi, Lina Cossignani, Aurélie Schoubben, and et al. 2022. "Wound Dressing: Combination of Acacia Gum/PVP/Cyclic Dextrin in Bioadhesive Patches Loaded with Grape Seed Extract" Pharmaceutics 14, no. 3: 485. https://doi.org/10.3390/pharmaceutics14030485
APA StylePagano, C., Luzi, F., Ricci, M., Michele, A. D., Puglia, D., Ceccarini, M. R., Beccari, T., Blasi, F., Cossignani, L., Schoubben, A., Primavilla, S., Iborra, C. A. V., & Perioli, L. (2022). Wound Dressing: Combination of Acacia Gum/PVP/Cyclic Dextrin in Bioadhesive Patches Loaded with Grape Seed Extract. Pharmaceutics, 14(3), 485. https://doi.org/10.3390/pharmaceutics14030485