3D Spheroids of Human Primary Urine-Derived Stem Cells in the Assessment of Drug-Induced Mitochondrial Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs, Chemicals, and Assay Kits
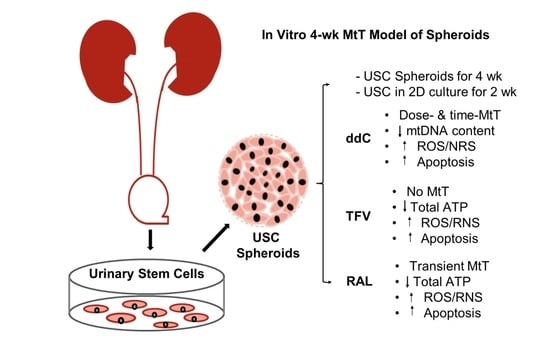
2.2. Isolation and Culture of USC
2.3. Preparation of 2D Culture and 3D Spheroids
2.4. Cytotoxicity and Viability
2.5. Hematoxylin and Eosin (H&E) Staining
2.6. Fluorescence Staining for Mitochondrial Mass
2.7. Quantitative Real-Time-PCR
2.8. Elisa
2.9. Colorimetric Assays
2.9.1. Total ATP Content
2.9.2. Caspase 3 Content
2.9.3. Total Glutathione Content
2.10. Fluorescence Assay
2.11. Statistics
3. Results
3.1. Morphology, Cell Number and Viability of 3D Spheroids and 2D Culture of USC
3.2. Cell Growth and Cytotoxicity in 3D Spheroids versus 2D Cultures after Drug Treatment
3.3. Oxidative Phosphorylation System (OXPHOS) Complex I, III, IV
3.4. Total ATP Content Decreased after Single ART Drug Administration
3.5. mt-DNA Content and Mass in Treated 3D Spheroids
3.6. Caspase 3 Activity
3.7. Measurement of ROS/RNS and Total GSSG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeni, P.G.; Hammer, S.M.; Hirsch, M.S.; Saag, M.S.; Schechter, M.; Carpenter, C.C.; Fischl, M.A.; Gatell, J.M.; Gazzard, B.G.; Jacobsen, D.M.; et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA 2004, 292, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Lenert, L.A.; Feddersen, M.; Sturley, A.; Lee, D. Adverse effects of medications and trade-offs between length of life and quality of life in human immunodeficiency virus infection. Am. J. Med. 2002, 113, 229–232. [Google Scholar] [CrossRef]
- Powderly, W.G. Long-term exposure to lifelong therapies. J. Acquir. Immune Defic. Syndr. 2002, 29, S28–S40. [Google Scholar] [CrossRef] [PubMed]
- Will, Y.; Shields, J.E.; Wallace, K.B. Drug-Induced Mitochondrial Toxicity in the Geriatric Population: Challenges and Future Directions. Biology 2019, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Kamalian, L.; Douglas, O.; Jolly, C.E.; Snoeys, J.; Simic, D.; Monshouwer, M.; Williams, D.P.; Kevin Park, B.; Chadwick, A.E. The utility of HepaRG cells for bioenergetic investigation and detection of drug-induced mitochondrial toxicity. Toxicol. In Vitro 2018, 53, 136–147. [Google Scholar] [CrossRef]
- Maria, B.; Ross, J.C.; Neil, R.C.; Stefan, A.P. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J. Anat. 2007, 211, 567–576. [Google Scholar]
- Ramaiahgari, S.C.; Ferguson, S.S. Organotypic 3D HepaRG Liver Model for Assessment of Drug-Induced Cholestasis. In Experimental Cholestasis Research; Vinken, M., Ed.; Springer: New York, NY, USA, 2019; pp. 313–323. [Google Scholar]
- Polo, M.; Alegre, F.; Funes, H.A.; Blas-Garcia, A.; Victor, V.M.; Esplugues, J.V.; Apostolova, N. Mitochondrial (dys)function—A factor underlying the variability of efavirenz-induced hepatotoxicity? Br. J. Pharmacol. 2015, 172, 1713–1727. [Google Scholar] [CrossRef] [Green Version]
- Jolly, C.E.; Douglas, O.; Kamalian, L.; Jenkins, R.E.; Beckett, A.J.; Penman, S.L.; Williams, D.P.; Monshouwer, M.; Simic, D.; Snoeys, J.; et al. The utility of a differentiated preclinical liver model, HepaRG cells, in investigating delayed toxicity via inhibition of mitochondrial-replication induced by fialuridine. Toxicol. Appl. Pharmacol. 2020, 403, 115163. [Google Scholar] [CrossRef]
- Honkoop, P.; Scholte, H.R.; de Man, R.A.; Schalm, S.W. Mitochondrial injury. Lessons from the fialuridine trial. Drug Saf. 1997, 17, 1–7. [Google Scholar] [CrossRef]
- Brown, A.N.; Drusano, G.L.; Adams, J.R.; Rodriquez, J.L.; Jambunathan, K.; Baluya, D.L.; Brown, D.L.; Kwara, A.; Mirsalis, J.C.; Hafner, R.; et al. Preclinical Evaluations To Identify Optimal Linezolid Regimens for Tuberculosis Therapy. mBio 2015, 6, e01741-15. [Google Scholar] [CrossRef] [Green Version]
- Kammerer, S. Three-Dimensional Liver Culture Systems to Maintain Primary Hepatic Properties for Toxicological Analysis In Vitro. Int. J. Mol. Sci. 2021, 22, 10214. [Google Scholar] [CrossRef] [PubMed]
- Van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can animal models of disease reliably inform human studies? PLoS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; McNeill, E.; Tian, H.; Soker, S.; Andersson, K.-E.; Yoo, J.J.; Atala, A. Urine Derived Cells are a Potential Source for Urological Tissue Reconstruction. J. Urol. 2008, 180, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Long, T.; Deng, J.; Zhang, Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res. Ther. 2014, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Pei, M.; Li, J.; Zhang, Y.; Liu, G.; Wei, L. Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res. 2014, 356, 391–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, B.; Sun, X.; Han, D.; Chen, S.; Yao, B.; Gao, Y.; Bian, J.; Huang, Y.; Zhang, Y.; Wan, Z.; et al. Human urine-derived stem cells alone or genetically-modified with FGF2 Improve type 2 diabetic erectile dysfunction in a rat model. PLoS ONE 2014, 9, e92825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Wang, X.; Sun, X.; Deng, C.; Atala, A.; Zhang, Y. The effect of urine-derived stem cells expressing VEGF loaded in collagen hydrogels on myogenesis and innervation following after subcutaneous implantation in nude mice. Biomaterials 2013, 34, 8617–8629. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Wu, R.; Yang, B.; He, T.; Fan, Y.; Lu, X.; Zhou, X.; Liu, H.; et al. Multipotential differentiation of human urine-derived stem cells: Potential for therapeutic applications in urology. Stem Cells 2013, 31, 1840–1856. [Google Scholar] [CrossRef]
- Lang, R.; Liu, G.; Shi, Y.; Bharadwaj, S.; Leng, X.; Zhou, X.; Liu, H.; Atala, A.; Zhang, Y. Self-renewal and differentiation capacity of urine-derived stem cells after urine preservation for 24 hours. PLoS ONE 2013, 8, e53980. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Benda, C.; Dunzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y.; et al. Generation of human induced pluripotent stem cells from urine samples. Nat. Protoc. 2012, 7, 2080–2089. [Google Scholar] [CrossRef]
- Liu, G.; Pareta, R.A.; Wu, R.; Shi, Y.; Zhou, X.; Liu, H.; Deng, C.; Sun, X.; Atala, A.; Opara, E.C.; et al. Skeletal myogenic differentiation of urine-derived stem cells and angiogenesis using microbeads loaded with growth factors. Biomaterials 2013, 34, 1311–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Wang, Z.; Bharadwaj, S.; Hodges, S.J.; Atala, A.; Zhang, Y. Implantation of autologous urine derived stem cells expressing vascular endothelial growth factor for potential use in genitourinary reconstruction. J. Urol. 2011, 186, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Markert, C.; Andersson, K.E.; Atala, A.; Zhang, Y. Characterization of urine-derived stem cells obtained from upper urinary tract for use in cell-based urological tissue engineering. Tissue Eng. Part A 2011, 17, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Mack, D.L.; Moreno, C.M.; Strande, J.L.; Mathieu, J.; Shi, Y.; Markert, C.D.; Wang, Z.; Liu, G.; Lawlor, M.W.; et al. Dystrophin-deficient cardiomyocytes derived from human urine: New biologic reagents for drug discovery. Stem Cell Res. 2014, 12, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zheng, Y.; Li, S.; Xue, H.; Schmitt, K.; Hergenroeder, G.W.; Wu, J.; Zhang, Y.; Kim, D.H.; Cao, Q. Human neural progenitors derived from integration-free iPSCs for SCI therapy. Stem Cell Res. 2017, 19, 55–64. [Google Scholar] [CrossRef]
- Guo, H.; Deng, N.; Dou, L.; Ding, H.; Criswell, T.; Atala, A.; Furdui, C.M.; Zhang, Y. 3-D Human Renal Tubular Organoids Generated from Urine-Derived Stem Cells for Nephrotoxicity Screening. ACS Biomater. Sci. Eng. 2020, 6, 6701–6709. [Google Scholar] [CrossRef]
- Liu, G.; Wu, R.; Yang, B.; Deng, C.; Lu, X.; Walker, S.J.; Ma, P.X.; Mou, S.; Atala, A.; Zhang, Y. Human Urine-Derived Stem Cell Differentiation to Endothelial Cells with Barrier Function and Nitric Oxide Production. Stem Cells Transl. Med. 2018, 7, 686–698. [Google Scholar] [CrossRef] [Green Version]
- White, A.J. Mitochondrial toxicity and HIV therapy. Sex. Transm. Infect. 2001, 77, 158–173. [Google Scholar] [CrossRef] [Green Version]
- Birkus, G.; Hitchcock, M.J.; Cihlar, T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: Comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 2002, 46, 716–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brehm, T.T.; Franz, M.; Hufner, A.; Hertling, S.; Schmiedel, S.; Degen, O.; Kreuels, B.; Schulze Zur Wiesch, J. Safety and efficacy of elvitegravir, dolutegravir, and raltegravir in a real-world cohort of treatment-naive and -experienced patients. Medicine 2019, 98, e16721. [Google Scholar] [CrossRef] [PubMed]
- Gorwood, J.; Bourgeois, C.; Pourcher, V.; Pourcher, G.; Charlotte, F.; Mantecon, M.; Rose, C.; Morichon, R.; Atlan, M.; Le Grand, R.; et al. The Integrase Inhibitors Dolutegravir and Raltegravir Exert Proadipogenic and Profibrotic Effects and Induce Insulin Resistance in Human/Simian Adipose Tissue and Human Adipocytes. Clin. Infect. Dis. 2020, 71, e549–e560. [Google Scholar] [CrossRef]
- Varga, Z.V.; Ferdinandy, P.; Liaudet, L.; Pacher, P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1453–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnegelberger, R.D.; Lang, A.L.; Arteel, G.E.; Beier, J.I. Environmental toxicant-induced maladaptive mitochondrial changes: A potential unifying mechanism in fatty liver disease? Acta Pharm. Sin. B 2021, 11, 3756–3767. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Huang, R.; Sakamuru, S.; Witt, K.L.; Beeson, G.C.; Shou, L.; Schnellmann, R.G.; Beeson, C.C.; Tice, R.R.; Austin, C.P.; et al. Systematic Study of Mitochondrial Toxicity of Environmental Chemicals Using Quantitative High Throughput Screening. Chem. Res. Toxicol. 2013, 26, 1323–1332. [Google Scholar] [CrossRef] [Green Version]
- Wills, L.P. The use of high-throughput screening techniques to evaluate mitochondrial toxicity. Toxicology 2017, 391, 34–41. [Google Scholar] [CrossRef]
- Walker, U.A.; Auclair, M.; Lebrecht, D.; Kornprobst, M.; Capeau, J.; Caron, M. Uridine abrogates the adverse effects of antiretroviral pyrimidine analogues on adipose cell functions. Antivir. Ther. 2006, 11, 25–34. [Google Scholar] [CrossRef]
- Paintsil, E.; Cheng, Y.-C. Antiviral Agents. Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 223–257. [Google Scholar]
- Dobard, C.; Sharma, S.; Cong, M.; West, R.; Makarova, N.; Holder, A.; Pau Cp Hanson, D.; Novembre, F.; Garcia-lerma, J.; Heneine, W. Efficacy of topical tenofovir against transmission of a tenofovir-resistant SHIV in macaques. Retrovirology 2015, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cobb, D.A.; Smith, N.; Deodhar, S.; Bade, A.N.; Gautam, N.; Shetty, B.L.D.; McMillan, J.; Alnouti, Y.; Cohen, S.M.; Gendelman, H.E.; et al. Transformation of tenofovir into stable ProTide nanocrystals with long-acting pharmacokinetic profiles. Nat. Commun. 2021, 12, 5458. [Google Scholar] [CrossRef]
- Vidal, F.; Domingo, J.C.; Guallar, J.; Saumoy, M.; Cordobilla, B.; Sánchez de la Rosa, R.; Giralt, M.; Álvarez, M.L.; López-Dupla, M.; Torres, F.; et al. In Vitro Cytotoxicity and Mitochondrial Toxicity of Tenofovir Alone and in Combination with Other Antiretrovirals in Human Renal Proximal Tubule Cells. Antimicrob. Agents Chemother. 2006, 50, 3824–3832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, E.; Wang, T.; Park, Y.; Hao, J.; Lepist, E.-I.; Babusis, D.; Ray, A.S. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob. Agents Chemother. 2015, 59, 3563–3569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishana, M.; Nilavar, N.M.; Kumari, R.; Pandey, M.; Raghavan, S.C. HIV integrase inhibitor, Elvitegravir, impairs RAG functions and inhibits V(D)J recombination. Cell Death Dis. 2017, 8, e2852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, D.F.; Wong, R.J.; Wenning, L.; Stevenson, D.K.; Mirochnick, M. Raltegravir in vitro effect on bilirubin binding. Pediatr. Infect. Dis. J. 2013, 32, 978–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, D.M.; Siccardi, M.; Murphy, M.; Piperakis, M.M.; Khoo, S.H.; Back, D.J.; Owen, A. Divalent metals and pH alter raltegravir disposition in vitro. Antimicrob. Agents chemother. 2012, 56, 3020–3026. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.-H.; Bednarczyk, E.M.; Catanzaro, L.M.; Shon, A.; Xu, J.-C.; Ma, Q. Pharmacokinetic drug interactions of integrase strand transfer inhibitors. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100044. [Google Scholar] [CrossRef]
- Mader, B.J.; Pivtoraiko, V.N.; Flippo, H.M.; Klocke, B.J.; Roth, K.A.; Mangieri, L.R.; Shacka, J.J. Rotenone inhibits autophagic flux prior to inducing cell death. ACS Chem. Neurosci. 2012, 3, 1063–1072. [Google Scholar] [CrossRef] [Green Version]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863–55889. [Google Scholar] [CrossRef] [Green Version]
- Nagiah, S.; Phulukdaree, A.; Chuturgoon, A. Mitochondrial and Oxidative Stress Response in HepG2 Cells Following Acute and Prolonged Exposure to Antiretroviral Drugs. J. Cell. Biochem. 2015, 116, 1939–1946. [Google Scholar] [CrossRef] [Green Version]
- Gureev, A.P.; Shaforostova, E.A.; Starkov, A.A.; Popov, V.N. Simplified qPCR method for detecting excessive mtDNA damage induced by exogenous factors. Toxicology 2017, 382, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Kyffin, J.; Sharma, P.; Leedale, J.; Colley, H.; Murdoch, C.; Harding, A.; Mistry, P.; Webb, S. Characterisation of a functional rat hepatocyte spheroid model. Toxicol. In Vitro 2018, 55, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.; Day, B.J.; Copeland, W.C. Mitochondrial toxicity of NRTI antiviral drugs: An integrated cellular perspective. Nat. Rev. Drug Discov. 2003, 2, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Van Dyke, R.B.; Mittleman, M.A.; Colan, S.D.; Oleske, J.M.; Seage, G.R. III. International Maternal Pediatric Adolescent ACTCST: The impact of HAART on cardiomyopathy among children and adolescents perinatally infected with HIV-1. AIDS 2012, 26, 2027–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, P.; Aleo, M.D.; Gosink, M.; Will, Y. Evaluation of in Vitro Mitochondrial Toxicity Assays and Physicochemical Properties for Prediction of Organ Toxicity Using 228 Pharmaceutical Drugs. Chem. Res. Toxicol. 2019, 32, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Arnaudo, E.; Dalakas, M.; Shanske, S.; Moraes, C.T.; DiMauro, S.; Schon, E.A. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet 1991, 337, 508–510. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Illa, I.; Pezeshkpour, G.H.; Laukaitis, J.P.; Cohen, B.; Griffin, J.L. Mitochondrial myopathy caused by long-term zidovudine therapy. N. Engl. J. Med. 1990, 322, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Semino-Mora, C.; Leon-Monzon, M. Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2’3’-dideoxycytidine (ddC). Lab. Investig. 2001, 81, 1537–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.D.; Wong, W.M.; Keruly, J.C.; McArthur, J.C. Incidence of neuropathy in HIV-infected patients on monotherapy versus those on combination therapy with didanosine, stavudine and hydroxyurea. AIDS 2000, 14, 273–278. [Google Scholar] [CrossRef]
- Cote, H.C.; Magil, A.B.; Harris, M.; Scarth, B.J.; Gadawski, I.; Wang, N.; Yu, E.; Yip, B.; Zalunardo, N.; Werb, R.; et al. Exploring mitochondrial nephrotoxicity as a potential mechanism of kidney dysfunction among HIV-infected patients on highly active antiretroviral therapy. Antivir. Ther. 2006, 11, 79–86. [Google Scholar] [CrossRef]
- Saumoy, M.; Vidal, F.; Peraire, J.; Sauleda, S.; Vea, A.M.; Vilades, C.; Ribera, E.; Richart, C. Proximal tubular kidney damage and tenofovir: A role for mitochondrial toxicity? AIDS 2004, 18, 1741–1742. [Google Scholar] [CrossRef]
- Samuels, R.; Bayerri, C.R.; Sayer, J.A.; Price, D.A.; Payne, B.A.I. Tenofovir disoproxil fumarate-associated renal tubular dysfunction: Noninvasive assessment of mitochondrial injury. AIDS 2017, 31, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, K.; ter Hofstede, H.J.; Burger, D.M.; Smeitink, J.A.; Koopmans, P.P. Adverse effects of reverse transcriptase inhibitors: Mitochondrial toxicity as common pathway. AIDS 1998, 12, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Moyle, G. Clinical manifestations and management of antiretroviral nucleoside analog-related mitochondrial toxicity. Clin. Ther. 2000, 22, 911–936, discussion 898. [Google Scholar] [CrossRef]
- Maagaard, A.; Kvale, D. Mitochondrial toxicity in HIV-infected patients both off and on antiretroviral treatment: A continuum or distinct underlying mechanisms? J. Antimicrob. Chemother. 2009, 64, 901–909. [Google Scholar] [CrossRef]
- Whitcomb, J.M.; Parkin, N.T.; Chappey, C.; Hellmann, N.S.; Petropoulos, C.J. Broad nucleoside reverse-transcriptase inhibitor cross-resistance in human immunodeficiency virus type 1 clinical isolates. J. Infect. Dis. 2003, 188, 992–1000. [Google Scholar] [CrossRef] [Green Version]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Sun, K.; Lan, Z.; Song, W.; Cheng, L.; Chi, W.; Chen, J.; Huo, Y.; Xu, L.; Liu, X.; et al. Tenofovir and adefovir down-regulate mitochondrial chaperone TRAP1 and succinate dehydrogenase subunit B to metabolically reprogram glucose metabolism and induce nephrotoxicity. Sci. Rep. 2017, 7, 46344. [Google Scholar] [CrossRef] [Green Version]
- Rooney, J.P.; Ryde, I.T.; Sanders, L.H.; Howlett, E.H.; Colton, M.D.; Germ, K.E.; Mayer, G.D.; Greenamyre, J.T.; Meyer, J.N. PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol. Biol. 2015, 1241, 23–38. [Google Scholar]
- Berger, A.R.; Arezzo, J.C.; Schaumburg, H.H.; Skowron, G.; Merigan, T.; Bozzette, S.; Richman, D.; Soo, W. 2’,3’-dideoxycytidine (ddC) toxic neuropathy: A study of 52 patients. Neurology 1993, 43, 358–362. [Google Scholar] [CrossRef]
- Young, M.J. Off-Target Effects of Drugs that Disrupt Human Mitochondrial DNA Maintenance. Front. Mol. Biosci. 2017, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Young, C.K.J.; Wheeler, J.H.; Rahman, M.M.; Young, M.J. The antiretroviral 2′,3′-dideoxycytidine causes mitochondrial dysfunction in proliferating and differentiated HepaRG human cell cultures. J. Biol. Chem. 2021, 296, 100206. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.; Auclairt, M.; Vissian, A.; Vigouroux, C.; Capeau, J. Contribution of mitochondrial dysfunction and oxidative stress to cellular premature senescence induced by antiretroviral thymidine analogues. Antivir. Ther. 2008, 13, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, T.; Morley, A. An evolutionary perspective on the Crabtree effect. Front. Mol. Biosci. 2014, 1, 17. [Google Scholar] [CrossRef] [PubMed]
- Dini, S.; Binder, B.J.; Fischer, S.C.; Mattheyer, C.; Schmitz, A.; Stelzer, E.H.; Bean, N.G.; Green, J.E. Identifying the necrotic zone boundary in tumour spheroids with pair-correlation functions. J. R. Soc. Interface 2016, 13, 20160649. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.M. Cell and environment interactions in tumor microregions: The multicell spheroid model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef] [Green Version]
- Domnina, A.; Ivanova, J.; Alekseenko, L.; Kozhukharova, I.; Borodkina, A.; Pugovkina, N.; Smirnova, I.; Lyublinskaya, O.; Fridlyanskaya, I.; Nikolsky, N. Three-Dimensional Compaction Switches Stress Response Programs and Enhances Therapeutic Efficacy of Endometrial Mesenchymal Stem/Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 473. [Google Scholar] [CrossRef]
- Roper, S.J.; Coyle, B. Establishing an In Vitro 3D Spheroid Model to Study Medulloblastoma Drug Response and Tumor Dissemination. Curr. Protoc. 2022, 2, e357. [Google Scholar] [CrossRef]
- Will, Y.; Dykens, J. Mitochondrial toxicity assessment in industry--a decade of technology development and insight. Expert. Opin. Drug Metab. Toxicol. 2014, 10, 1061–1067. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc. Res. 2008, 79, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Yarchoan, R.; Mitsuya, H.; Broder, S. AIDS therapies. Sci. Am. 1988, 259, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-M.; Chen, P.-C.; Hsieh, H.-C.; Calkins, M.J. Mitochondrial defects arise from nucleoside/nucleotide reverse transcriptase inhibitors in neurons: Potential contribution to HIV-associated neurocognitive disorders. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T.; Gatanaga, H.; Oka, S. Tenofovir nephrotoxicity among Asians living with HIV: Review of the literature. Glob. Health. Med. 2019, 1, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marroquin, L.D.; Hynes, J.; Dykens, J.A.; Jamieson, J.D.; Will, Y. Circumventing the Crabtree Effect: Replacing Media Glucose with Galactose Increases Susceptibility of HepG2 Cells to Mitochondrial Toxicants. Toxicol. Sci. 2007, 97, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsythe, S.S.; McGreevey, W.; Whiteside, A.; Shah, M.; Cohen, J.; Hecht, R.; Bollinger, L.A.; Kinghorn, A. Twenty Years of Antiretroviral Therapy For People Living With HIV: Global Costs, Health Achievements, Economic Benefits. Health Aff. 2019, 38, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Llibre, J.M.; Montoliu, A.; Miro, J.M.; Domingo, P.; Riera, M.; Tiraboschi, J.; Curran, A.; Homar, F.; Ambrosioni, J.; Abdulghani, N.; et al. Discontinuation of dolutegravir, elvitegravir/cobicistat and raltegravir because of toxicity in a prospective cohort. HIV Med. 2019, 20, 237–247. [Google Scholar] [CrossRef]
- Li, M.; Foli, Y.; Liu, Z.; Wang, G.; Hu, Y.; Lu, Q.; Selvaraj, S.; Lam, W.; Paintsil, E. High frequency of mitochondrial DNA mutations in HIV-infected treatment-experienced individuals. HIV Med. 2017, 18, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Mouscadet, J.F.; Tchertanov, L. Raltegravir: Molecular basis of its mechanism of action. Eur. J. Med. Res. 2009, 14, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Eckard, A.R.; McComsey, G.A. Weight gain and integrase inhibitors. Curr. Opin. Infect. Dis. 2020, 33, 10–19. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.; Blanco, J.-R.; Muriel, A.; Pérez-Elías, M.J.; Rubio-Martín, R.; Berenguer, J.; Peraire, J.; Bernal, E.; Martínez, O.J.; Serrano-Villar, S.; et al. Weight changes after antiretroviral therapy initiation in CoRIS (Spain): A prospective multicentre cohort study. J. Int. AIDS Soc. 2021, 24, e25732. [Google Scholar] [CrossRef]
- Lake, J.E.; Wu, K.; Bares, S.H.; Debroy, P.; Godfrey, C.; Koethe, J.R.; McComsey, G.A.; Palella, F.J.; Tassiopoulos, K.; Erlandson, K.M. Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy. Clin. Infect. Dis. 2020, 71, e471–e477. [Google Scholar] [CrossRef] [PubMed]
- Zash, R.; Holmes, L.; Diseko, M.; Jacobson, D.L.; Brummel, S.; Mayondi, G.; Isaacson, A.; Davey, S.; Mabuta, J.; Mmalane, M.; et al. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N. Engl. J. Med. 2019, 381, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Gu, H.; Liu, D.; Ma, W.; Wei, X.-w.; Zhao, L.; Liu, Y.; Zhang, C.; Yuan, Z. Mitochondrial dysfunction is implicated in retinoic acid-induced spina bifida aperta in rat fetuses. Int. J. Dev. Neurosci. 2018, 68, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Korencak, M.; Byrne, M.; Richter, E.; Schultz, B.T.; Juszczak, P.; Ake, J.A.; Ganesan, A.; Okulicz, J.F.; Robb, M.L.; de Los Reyes, B.; et al. Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight 2019, 4, e126675. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.R.; Huhn, G.D. Excess Weight Gain with Integrase Inhibitors and Tenofovir Alafenamide: What Is the Mechanism and Does It Matter? Open Forum Infect Dis. 2021, 8, ofab542. [Google Scholar] [CrossRef]
- George, J.W.; Mattingly, J.E.; Roland, N.J.; Small, C.M.; Lamberty, B.G.; Fox, H.S.; Stauch, K.L. Physiologically Relevant Concentrations of Dolutegravir, Emtricitabine, and Efavirenz Induce Distinct Metabolic Alterations in HeLa Epithelial and BV2 Microglial Cells. Front. Immunol. 2021, 12, 639378. [Google Scholar] [CrossRef]
- Cuzin, L.; Pugliese, P.; Katlama, C.; Bani-Sadr, F.; Ferry, T.; Rey, D.; Lourenco, J.; Bregigeon, S.; Allavena, C.; Reynes, J.; et al. Integrase strand transfer inhibitors and neuropsychiatric adverse events in a large prospective cohort. J. Antimicrob. Chemother. 2019, 74, 754–760. [Google Scholar] [CrossRef]
- Kolakowska, A.; Maresca, A.F.; Collins, I.J.; Cailhol, J. Update on Adverse Effects of HIV Integrase Inhibitors. Curr. Treat. Options Infect. Dis. 2019, 11, 372–387. [Google Scholar] [CrossRef] [Green Version]
- Prats, A.; Martínez-Zalacaín, I.; Mothe, B.; Negredo, E.; Pérez-Álvarez, N.; Garolera, M.; Domènech-Puigcerver, S.; Coll, P.; Meulbroek, M.; Chamorro, A.; et al. Effects of integrase inhibitor-based antiretroviral therapy on brain outcomes according to time since acquisition of HIV-1 infection. Sci. Rep. 2021, 11, 11289. [Google Scholar] [CrossRef]
- Blas-García, A.; Polo, M.; Alegre, F.; Funes, H.A.; Martínez, E.; Apostolova, N.; Esplugues, J.V. Lack of mitochondrial toxicity of darunavir, raltegravir and rilpivirine in neurons and hepatocytes: A comparison with efavirenz. J. Antimicrob. Chemother. 2014, 69, 2995–3000. [Google Scholar] [CrossRef] [Green Version]
- Au-Coltman, N.J.; Au-Rochford, G.; Au-Hodges, N.J.; Au-Ali-Boucetta, H.; Au-Barlow, J.P. Exploring Mitochondrial Energy Metabolism of Single 3D Microtissue Spheroids using Extracellular Flux Analysis. JoVE 2022, 37, e63346. [Google Scholar]
- Rosso, F.; Quagliariello, V.; Tortora, C.; Di Lazzaro, A.; Barbarisi, A.; Iaffaioli, R.V. Cross-linked hyaluronic acid sub-micron particles: In vitro and in vivo biodistribution study in cancer xenograft model. J. Mater. Sci. Mater. Med. 2013, 24, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Hulgan, T.; Gerschenson, M. HIV and mitochondria: More than just drug toxicity. J. Infect. Dis. 2012, 205, 1769–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, C.; Suzuki, K.; Yanagi, K.; Satoh, H.; Niitani, M.; Aso, Y. Miglitol, an anti-diabetic drug, inhibits oxidative stress-induced apoptosis and mitochondrial ROS over-production in endothelial cells by enhancement of AMP-activated protein kinase. J. Pharmacol. Sci. 2012, 120, 121–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Culture Models 3D USC Spheroids vs. 2D Culture of USC | |
|---|---|
| Mit-Tox Positive control: | Canonical drug known to induce Mito-Tox: Rotenone (RTNN) |
| ART controls: | NRTI known to induce significant Mito-Tox: Zalcitabine (ddC) NRTI known to induce minimal Mito-Tox: Tenofovir (TFV) |
| Negative control: | Solvent: DMSO (0.1%) |
| New drug: | INSTI with unclear Mito-Tox [33,34]: Raltegravir (RAL) |
| Measurements: | Cell growth rate, cell viability, OXPHOS Complex I, III, and IV, total ATP, Mitochondrial DNA copy number/mass, Caspase 3 for apoptosis, ROS/RNS for oxidative stress, total GSSG for major intracellular antioxidant |
| Independent Measurements | DMSO | ddC | TFV | RAL | RTNN |
|---|---|---|---|---|---|
| (Neg Ctr) | (NRTI) | (NRTI) | (INSTI) | (Pos Ctr) | |
| Size of spheroids | Stable | Drugs did not induce any effect on spheroid sizes | Reduced spheroid size at wk 4 (p < 0.01) | ||
| Cell number | Same or similar cell numbers were maintained during the 4-week culture | Decreased cell number at wk 2 and 4 (p < 0.01) | |||
| Percentage cell viability | ~99% | ~80% | ~95% | ~95% | ~60% |
| Complexes I, III, and IV | No effect | Time- or dose-dependent decrease Complexes I, III, and IV (p < 0.05, p < 0.01) | No significant effect | Transiently decrease in Complex IV At wk 2 (p < 0.05) | Time-dependent decrease Complex I (p < 0.01) |
| Total ATP | Stable | decrease at high dose (p < 0.05; p < 0.01) | decrease at high dose (p < 0.05; p < 0.01) | decrease at middle and high doses (p < 0.05; p < 0.01) | Time-dependent decrease (p < 0.01) |
| Mitochondria-DNA Content | Stable | Dose- and time-dependent Decrease (p < 0.01) | No negative effect | Slightly decreased (p < 0.05) | (Compensatory) increased (p < 0.01) |
| Mt-DNA mass | No effect | Significantly decrease (p < 0.01) | Normal | Slightly decreased (p > 0.05) | Slightly Increased (p > 0.05) |
| Caspase 3 Activity | Increase at day 3; stable at wk 2, 4 (p < 0.01) | Increase at middle and high doses at wk 2 (p < 0.05; p < 0.01) | Increase at low and middle doses at wk 2 (p < 0.05) | Increase at all doses at wk 2 (p < 0.05, p < 0.01) | Increase at wk 2 and 4 (p < 0.05; p < 0.01) |
| ROS/RNS levels | Increase at wk 4 (p < 0.05) | Increase at wk 2 (p < 0.01) | Increase at wk 2 (p < 0.01) | Slightly increased (p > 0.05) | Decreased at wk 4 (p < 0.05) |
| Total GSSG | Stable | Drugs had no effect on total GSSG | |||
| mtDNA Content (or mtDNA Copy Number) | ||
|---|---|---|
| Hu Mt | Forward primer | CACCCAAGAACAGGGTTTGT |
| Reverse primer | TGGCCATGGGTATGTTGTTA | |
| Hu Nu | Forward primer | TGCTGTCTCCATGTTTGATGTATCT |
| Reverse primer | TCTCTGCTCCCCACCTCTAAGT | |
| Independent Measures | 3D Spheroids | 2D Culture |
|---|---|---|
| Cell growth | Remain consistent cell number over 4 weeks | Rapid, continuing proliferation and detaching after cell reach over-confluence. |
| Doubling time | No sign for cell proliferation | 20 h at p4 |
| Population doubling | The number of cells remained nearly the same during the 4-week culture | 40–64 [19,20] |
| Cell viability/ cytotoxicity Assays | Both alive and dead cells can be identified by combined Mito-Tox with live/death kit |
|
| Mitochondrial function |
|
|
| DNA replication | Slow with no cell proliferation | Rapid cell proliferation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Jambunathan, K.; Jiang, G.; Margolis, D.M.; Leng, I.; Ihnat, M.; Ma, J.-X.; Mirsalis, J.; Zhang, Y. 3D Spheroids of Human Primary Urine-Derived Stem Cells in the Assessment of Drug-Induced Mitochondrial Toxicity. Pharmaceutics 2022, 14, 1042. https://doi.org/10.3390/pharmaceutics14051042
Ding H, Jambunathan K, Jiang G, Margolis DM, Leng I, Ihnat M, Ma J-X, Mirsalis J, Zhang Y. 3D Spheroids of Human Primary Urine-Derived Stem Cells in the Assessment of Drug-Induced Mitochondrial Toxicity. Pharmaceutics. 2022; 14(5):1042. https://doi.org/10.3390/pharmaceutics14051042
Chicago/Turabian StyleDing, Huifen, Kalyani Jambunathan, Guochun Jiang, David M. Margolis, Iris Leng, Michael Ihnat, Jian-Xing Ma, Jon Mirsalis, and Yuanyuan Zhang. 2022. "3D Spheroids of Human Primary Urine-Derived Stem Cells in the Assessment of Drug-Induced Mitochondrial Toxicity" Pharmaceutics 14, no. 5: 1042. https://doi.org/10.3390/pharmaceutics14051042
APA StyleDing, H., Jambunathan, K., Jiang, G., Margolis, D. M., Leng, I., Ihnat, M., Ma, J. -X., Mirsalis, J., & Zhang, Y. (2022). 3D Spheroids of Human Primary Urine-Derived Stem Cells in the Assessment of Drug-Induced Mitochondrial Toxicity. Pharmaceutics, 14(5), 1042. https://doi.org/10.3390/pharmaceutics14051042